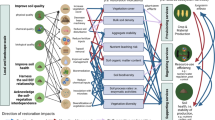
Abstract
Soil protists are key contributors to ecosystem functions. The impacts of transitions within agricultural systems on the soil biota and functionality receive less attention than that among distinct ecosystems including forest, grassland, and cropland. Here, we investigated the impacts of transitioning from open field (OF) to greenhouse (GH) agriculture on soil protist communities and multifunctionality across multiple paired sites. Our results revealed that the transition from OF to GH reduced protist phylogenetic diversity, with a greater impact on rare protists (42.1% reduction). Latitude was the strongest predictor of phagotrophic protist community variation in paired OF and GH soils across regions. The soil multifunctionality index declined by an average of 17.7%, mostly due to the loss of rare protists in GH soils. Reconstitution experiments confirmed that inoculating GH soils with rare protists from OF soils significantly restored multifunctionality. Our work highlights the critical role of low-abundant protists in modulating soil functioning.
Similar content being viewed by others
Introduction
The soil biota is responsible for a diverse array of metabolic functions, collectively termed multifunctionality1,2,3. Current studies have investigated the responses of the soil bacterial and fungal communities to land use conversion from forest and grassland to cropland4,5,6. These systems involve distinct plant types, chemical inputs, and intensities of human disturbance. However, within the farmland ecosystem, the soil is subject to highly heterogeneous utilization patterns. Considering the rapid development of modern agriculture, it is essential to further explore how transitions within agricultural systems impact soil microbial communities with implications for essential soil functions7. Besides, since most of these impacts can have a context-dependent effect that varies across regions, the integration of large-scale study sites can provide a more holistic view of these impacts6,8,9. This is of critical importance for better understanding local- and regional-scale impacts of systemic transitions on soil health with the aim of developing and maintaining sustainable agroecosystems.
Microbial taxa in soil are known to occur at uneven abundances, where a few abundant taxa coexist alongside a large number of low abundant (‘rare’) species10,11,12. While the most abundant taxa represent only a small fraction of the total microbial diversity in soils, rare taxa are essential components of soil biodiversity and play critical roles in maintaining ecosystem functioning and stability6,13,14. Often, abundant taxa exhibit broader metabolic versatility and have great abilities to adapt to environmental variations. On the other hand, rare taxa are usually species with narrow metabolic niches that are constantly present at low abundances or fluctuate in abundance depending on variations in environmental conditions10,15. Previous studies have demonstrated that the abundant and rare taxa often respond differently to land use conversion10,16. For instance, prevalent fungal taxa become even more abundant, whereas rare taxa became rarer or absent in arable lands in comparison to grassland systems6. Of key importance, evidence from the literature supports a disproportional role of rare microbial taxa (bacteria and fungi) in soil multifunctionality11,13,15. Notably, soil protists significantly contribute to the productivity of agroecosystems via the regulation of functions associated with carbon cycling, nutrient fluxes, modulation of the soil microbiota, and plant growth promotion17,18,19. However, this organismal group is often overlooked in soil studies with a focus steered mostly toward bacteria and fungi7,20,21.
Greenhouse (GH) cultivation systems (also termed ‘protected’ or ‘facility’ cultivation systems) have gained attraction by allowing year-round production via climate and the precise management of biotic and abiotic factors impacting cropping systems22,23,24. The implementation of this system has been fundamental in improving resource utilization efficiency to achieve increasing yields per unit area, thereby meeting the growing demand for nutritious and diverse food products24. Compared to open field (OF) agricultural systems, GH cultivation systems are characterized by often higher use of synthetic fertilizers and pesticides, and high-intensity planting25. Currently, China is a leading country in the adoption of intensive GH cultivation systems, with more than 3.8 million hectares of cultivation representing ~90% of the global GH cultivation area26,27. Despite the rapid adoption and broad-scale implementation of GH systems, the potential impacts of this farming system on soil health have recently started to raise concerns, in particular concerning the long-term impacts on soil degradation28,29,30.
The increasing adoption of GH cultivation system requires the continuous examination of the potential impact of this system on aspects of soil health. In this study, we report a large-scale assessment of the effects of systemic transition – specifically, from OF to GH agricultural systems – on soil protist communities and multifunctionality. This study includes multiple soil samples collected from 89 production sites spanning 1878 km in China. The site selection was based on their significance as important regions with GH system. The main objectives of this study were (i) to investigate the impacts of GH cultivation systems on soil protist communities as compared to adjacent OF systems; (ii) to identify important environmental factors structuring the biogeographical patterns of soil protists in this region; and (iii) to determine the importance of rare protist taxa for soil multifunctionality. The latter used a combination of comparative analysis between GH and OF systems across sites and a reconstitution experiment based on rare protist taxa inoculation in soil. Collectively, our findings contribute to a better understanding of the impact of transitions within agricultural systems on soil protists and their role in regulating multiple soil functions.
Results
Differences in protist communities between systems
GH systems exhibited significantly lower phylogenetic diversity of rare protists compared to OF systems (Wilcoxon test, p < 0.001, average decrease of 42.1%), while showing increased phylogenetic diversity of abundant protists (Wilcoxon test, p < 0.001, average increase of 12.4%; Fig. 1A). Similarly, Shannon index and evenness of abundant protists were significantly increased in GH systems (Wilcoxon test, p < 0.05, average increase of 5.1% and 5.2% in Shannon index and evenness, respectively), but Shannon index and richness of rare protists were significantly decreased in GH systems (Wilcoxon test, p < 0.001, average decrease of 10.2% and 43.8% in Shannon index and richness, respectively; Supplementary Fig. 2). However, richness of abundant (OF: 79.74, GH: 79.71) protists and evenness of rare (OF: 0.84, GH: 0.83) protists showed no significant differences between these two systems (Wilcoxon test, p > 0.05; Supplementary Fig. 2). Phylogenetic diversity and richness of abundant phagotrophic taxa were significantly higher in GH systems (Wilcoxon test, p < 0.001), while OF systems exhibited significantly higher alpha diversities of rare phagotrophic taxa (Wilcoxon test, p < 0.001; Supplementary Fig. 3). Phylogenetic diversity and richness of both abundant and rare phototrophic communities in GH systems were significantly lower than those in OF systems (Wilcoxon test, p < 0.001, average decrease of 16.2% and 19.7% in abundant, and average decrease of 50.9% and 66.3% in rare; Supplementary Fig. 3).
A Differences in phylogenetic diversity of abundant and rare protist taxa between the open field (OF) and greenhouse (GH) systems. Asterisks indicate significant differences between OF and GH soils (Wilcoxon rank-sum test, ***p < 0.001). B Non-metric multidimensional scaling (NMDS) analysis based on Bray–Curtis distances showing differences in community structure of abundant and rare protist taxa between OF and GH soils (cultivation system – CS), and latitude (La). P-values were determined using PERMANOVA. C Manhattan plot showing zOTUs that were significantly (Wilcoxon rank-sum test, p < 0.05) enriched in the OF or GH soils. Each dot or triangle represents a single zOTU. zOTUs are colored to indicate different protist phyla. Inset Venn diagrams display the number of zOTUs significantly enriched in the OF or GH soils.
NMDS analysis showed that OF and GH soils had distinct protist communities – based on the analysis of both abundant (PERMANOVA, p < 0.01), and rare taxa (PERMANOVA, p < 0.05; Fig. 1B). The latitude of the sample sites was significant in explaining the observed variation in abundant and rare protist taxa (PERMANOVA p < 0.001). Overall, edaphic properties, latitude, and the cultivation system accounted for 61.0% and 81.2% of the variation in abundant and rare protist taxa, respectively (Supplementary Fig. 4). We also analyzed protist zOTUs that exhibited obvious differences between GH and OF soils. For abundant taxa, most of the zOTUs affiliated with phagotrophs (including those in the phyla Ciliophora (average increase of 41.8%), Conosa (average increase of 50.0%), Lobosa (average increase of 273.6%), Centroheliozoa (average increase of 340.0%), and Cercozoa (average increase of 112.1%) had significantly higher relative abundances in GH soils (Wilcoxon test, p < 0.01; Fig. 1C and Supplementary Fig. 5). Conversely, the majority of rare zOTUs (i.e., 37.3%) had significantly lower relative abundances (or were absent) in GH soils (Wilcoxon test, p < 0.01). The analysis of zOTUs detected at an intermediate level of relative abundance showed a significantly higher Shannon index (Wilcoxon test, p < 0.05, average increase of 2.39%), and richness in OF soils when compared to GH soils (Wilcoxon test, p < 0.001, average increase of 18.9%; Supplementary Fig. 6A, B). Also, the community structure of protists occurring at the intermediate level was found to be different between OF and GH soils (PERMANOVA, p < 0.001), and latitude was the strongest variable associated with these observed differences (Supplementary Fig. 6C).
Ecological niche breadth of protist communities
We assessed differences in environmental adaptation of abundant and rare protist taxa between OF and GH soils. The values of habitat niche breath (Bcom) of abundant and rare protists in GH soils were significantly higher than those in OF soils (Wilcoxon test, p < 0.05 and average increase of 1.7% in abundant, p < 0.001 and average increase of 19.7% in rare; Fig. 2A). We developed predicted maps displaying the spatial distribution of the differences in Bcom values between pairwise OF and GH soils across a region (Fig. 2B). In brief, the difference in Bcom values of both abundant and rare protists were found to be significantly and positively correlated with latitude (p < 0.001; Fig. 2C).
A Comparative plot of mean Bcom of abundant and rare protist communities between the open field (OF) and greenhouse (GH) soils. Gray lines connect each pairwise OF and GH soils. Asterisks indicate significant differences between OF and GH soils (Wilcoxon rank-sum test, *p < 0.05; ***p < 0.001). B Predictive maps of spatial distributions of ΔBcom of abundant and rare protist communities between the pairwise OF and GH soils. Maps were created using Inverse Distance Weighted (IDW) interpolation. C Correlations between ΔBcom of protist communities and latitude. The error bands shaded in surrounding the regression lines indicate the 95% CI. ΔBcom is calculated by the difference of pairwise GH to OF soils.
Environmental constraints and drivers of protist communities
We found abundant and rare taxa distributions to be significantly and negatively correlated with geographic distance and edaphic dissimilarity for OF and GH soils (p < 0.001; Fig. 3A, B). Notably, the determination coefficients and the absolute values of slopes for GH (|Slopes| from 0.23 to 1.13) soils were significantly lower than those for OF (|Slopes| from 0.70 to 2.97) soils (Wilcoxon test, p < 0.001). We also tested for potential relationships of environmental variables and the zOTUs that differed significantly between the OF and GH soils (Fig. 3C, D). In brief, the relative abundances of zOTUs in OF soils were most strongly correlated with environmental variables, particularly latitude and soil pH. In addition, stochastic processes were the dominant assembly processes structuring abundant and rare taxa (|βNTI|<2) in both, OF and GH soils (68.6–95.4%). However, rare taxa (24.7–35.8%) were found to be relatively more influenced by deterministic processes when compared to abundant taxa (2.7–11.7%) (Supplementary Fig. 7).
A Distance-decay relationship between the similarity of protist communities and geographic distance. B The relationship between the similarity of protist communities and edaphic dissimilarity. The yellow and blue solid lines indicate the linear least-squares regression for the open field (OF) and greenhouse (GH) soils, respectively. Asterisks denote significant difference in slopes between OF and GH soils (***p < 0.001). Abundant (C) and rare (D) zOTUs that were significantly enriched in the OF or GH soils and their relationships with environmental variables. Only zOTUs that statistically varied between OF and GH systems (adjusted p < 0.05) are included in the phylogenetic trees. The inner ring (heatmap) displays the correlations between the relative abundances of zOTUs and environmental variables in the OF and GH soils, respectively.
Differences in phagotrophic protists between pairwise systems
Beta-diversity analyses revealed that the Repl process is dominant in determining the dissimilarities in phagotrophic taxa between pairwise OF and GH soils. It accounted for 28.4–57.1% of abundant and rare taxa, respectively (Fig. 4A). Notably, latitude and longitude were the most important factors explaining phagotrophic taxa dissimilarity between pairwise systems, followed by variations in soil EC and AP (Fig. 4B). CAP analysis corroborated these findings by showing that latitude was the most important variable associated with dissimilarities of abundant and rare phagotrophic taxa between pairwise systems (Fig. 4C). The warming effect is more pronounced at high latitudes, resulting in greater dissimilarities of phagotrophic taxa, especially for the abundant protists (Supplementary Fig. 8). In the GH system, a significantly lower relative abundance of rare phagotrophs was observed in sites located at high latitudes (Supplementary Fig. 9), including taxa within Ciliophora, Conosa, Centroheliozoa, and Cerozoa (Supplementary Fig. 10).
A Decomposition of the dissimilarity of the abundant and rare protist taxa between OF and GH soil pairs. Each point represents a pairwise comparison. The position of each point represents a triplet of values of the similarity (S), replacement (Repl), and richness difference (RichDiff). B Contributions of geographic variables and edaphic properties variations (Δ, GH–OF) to the dissimilarity of phagotrophic protists between OF and GH soil pairs. The circle size represents the importance of the variable calculated by random forest analysis. Colors represent the Spearman correlation coefficients. C Constrained analysis of principal coordinates (CAP) showing that latitude influenced the dissimilarity of phagotrophic protists between paired OF and GH soils. The points are colored according to latitude of paired OF and GH soils.
Differences in microbial functional genes between systems
NMDS analysis based on metagenomic reads revealed OF and GH soils to have significantly different functional compositions (PERMANOVA, p < 0.001; Fig. 5A). The soil multifunctionality index was found to be significantly lower in GH soils when compared to OF (Wilcoxon test, p < 0.05, ca. 17.7% reduction; Fig. 5B). Specifically, five functional categories associated with information storage and processing, and three functional categories related to metabolism were present at significantly lower abundances in GH soils (Wilcoxon test, p < 0.05; Fig. 5C).
A Non-metric multidimensional scaling (NMDS) analysis of soil microbial functions annotated using the eggNOG database based on Bray–Curtis distances. The p-value was determined using PERMANOVA. B Differences in soil multifunctionality index between the OF and GH soils (Wilcoxon rank-sum test, *p < 0.05). C Differences in functional gene abundances between the OF and GH soils.
Integrating environmental variables with protists and soil multifunctionality
SEM revealed the cultivation system to exert a direct positive effect on the phylogenetic diversity of abundant taxa and a negative effect on the rare taxa. Also, it showed climatic variables affect the soil pH with cascading effects on the structure of rare taxa (Fig. 6A). Most importantly, the phylogenetic diversity and community structure of rare protists were the most significant determinants of soil multifunctionality (Fig. 6A, B). We further explored the relationships between the phylogenetic diversity of abundant and rare taxa and soil multifunctionality using both the average and multiple-threshold methods. The results showed that the phylogenetic diversity of abundant taxa had a negative relationship with soil multifunctionality, while that of rare taxa was significantly positively correlated with soil multifunctionality (p < 0.001; Supplementary Fig. 11). The multiple-threshold analysis revealed a positive relationship between the phylogenetic diversity of abundant taxa and soil multifunctionality at lower thresholds (≤57%) but shifted to a negative relationship at higher thresholds (>57%) (Fig. 6C). In contrast, the phylogenetic diversity of rare taxa had a consistent and positive relationship with soil multifunctionality, with Tmax, Tmin, and Tmde of 80%, 30%, and 44%, respectively (Fig. 6D).
A Structural equation model (SEM) showing the effects of multiple factors on soil multifunctionality. The path widths represent the strength of path coefficient. The model parameters include χ2 = 29.61, df = 21, p = 0.100, CFI = 0.975, and RMSEA = 0.073. CS, cultivation system; NI, soil nutrient availability index; PD-A and PD-R, phylogenetic diversity of abundant and rare taxa, respectively; NMDS1-A and NMDS1-R, the scores in the first axis of non-metric multidimensional scaling (NMDS) of the abundant and rare zOTUs, respectively. B Standardized total effects of the variables used in the SEM. C, D Relationships between phylogenetic diversity and soil multifunctionality (based on multiple-thresholds, i.e., the number of soil functions above a series of sequential thresholds, ranging from 1 to 99% with 1% intervals) for abundant (C) and rare (D) protist taxa. Tmin and Tmax indicate the lowest and highest thresholds whose slopes are significantly different from zero, respectively. Tmde is the threshold with the steepest slope.
Reconstitution of soil multifunctionality via rare protist taxa amendment
The reconstitution experiment was performed to evaluate whether rare protist taxa from OF soils inoculated in GH soils can reconstitute the soil functional potential (Fig. 7A). The results showed average well color development (AWCD) to increase by 169.0% and 58.7% when local protists (Abundant + Rare, A + R) were inoculated in the soil, using north and south control GH soils, respectively (Tukey’s HSD test, p < 0.05; Fig. 7B, C). In contrast, when soils were inoculated with local protist taxa without rare species (A), the AWCD values increased by 57.1% and 13.9%, respectively (Tukey’s HSD test, p > 0.05; Fig. 7B, C). In addition, the inoculation of non-paired protist taxa resulted in AWCD value increases of 29.4–45.6% in the north GH soils, and in an AWCD value increase of 24.5% and decrease of 24.8% in the south GH soils (Fig. 7B, C).
A Diagram displaying the experimental design. Average well color development (AWCD) values of the carbon metabolism for GH soils from the north (B) and south (C) regions. Error bars indicate the standard errors. Gray dashed lines indicate the AWCD values of OF soils from the north and south regions. Different letters indicate significant differences at p < 0.05 based on the Tukey’s HSD test.
Discussion
Elucidating the mechanisms structuring and maintaining microbial diversity across divergent systems is a fundamental research topic in microbial ecology31,32. In this study, we investigated the extent to which changes in agroecosystem management impact soil protist communities with implications for essential soil functions. In brief, our findings revealed that GH systems had a significantly lower diversity of rare protists, while it increased the dominance of abundant taxa. Specifically in soils, microbes and several other soil biota organisms often display a long tail distribution of rare taxa, which comprise a large proportion of species diversity within distinct community types14,33. Rare taxa frequently occupy narrow ecological niches and are more vulnerable to disturbance and ecological drift, which collectively can result in local population extinction10,15. Here, a decline in the diversity of rare taxa was observed in the GH system, which is an agroecosystem subjected to the intensive use of chemical fertilizers and pesticides, monoculture practices, and intensive soil use25,34. In contrast, abundant taxa often have broader metabolic versatility associated with resource utilization, thus assumed to be more resilient to disturbance and environmental changes10,35. This is consistent with a previous observation showing that nutrient-rich environments promote the proliferation of abundant taxa while intensifying competition between rare taxa36. Furthermore, a previous study reported a 23.2% difference in abundant protist taxa between grassland and cropland, even though no significant difference was found in protist community richness37. Our results, however, revealed a significantly lower (32.8%) protist richness in GH soils compared to OF, and a 73.2% difference in abundant taxa, which notably exceeded the differences previously reported between land use types37.
Niche breadth is a key concept used to study mechanisms of ecological adaptation across species. In brief, species with wider niche breadth are assumed to have greater environmental tolerance to adverse edaphic conditions38,39. In this study, we assessed the difference in habitat niche breadth of protist communities from soils in the OF and GH systems. Abundant and rare protist communities in the GH systems exhibited significantly greater habitat niche breadth than those in OF systems, with this effect being particularly pronounced in rare taxa. These findings further demonstrate that GH systems exert stronger influence on soil rare protist taxa. It has been reported that environmental variables such as temperature, water availability, and pH directly impact the niche breadth of microbial communities by influencing species growth and propagation40. It aligns with our observation that the differences in habitat niche breadth of protists were primarily associated with latitude, reflecting intrinsic spatial variation across sites. The predicted spatial maps illustrated an increase in the difference in habitat niche breadth of protist communities between paired OF and GH systems with the increase in latitude, consistent with previous findings that higher temperatures resulted in wider niche width40. These patterns may likely be attributed to similar irrigation practices in both the northern and southern GH sites, with the more pronounced increase in temperature at sites in high latitudes for the GH system. Collectively, these results support a dynamic modulation of protist communities between agricultural systems due to variations in microclimate conditions.
Identifying the factors structuring microbial communities has direct implications for informing management practices – in this case in agroecosystems – with consequences for ecosystem functioning41. The importance of geographic distance in modulating bacterial, fungal, and archaeal communities in agricultural systems has been broadly studied8,42,43, with fewer studies focusing on soil protists. Here, we report protist communities in GH systems to exhibit a weaker geographic distance decay pattern in comparison to protist communities in the OF system. Commonly, weak distance decay relationships are interpreted as the lack of a clear spatial structure in the distribution of ecological communities42. The rate of distance decay also reflects the turnover rate of the species in a particular habitat44. In this sense, our results align with a previous study showing that intensive management reduces the influence of geographic distance on soil fungal communities, and lowers their spatial turnover and heterogeneity6. Besides, a study has also shown that the conversion of a natural ecosystem to agriculture results in taxonomic and functional homogenization of soil bacteria45. Similarly, land use change from natural systems to urban greenspaces was recently reported to cause the homogenization of soil microbial structure and functions at a global scale46. This phenomenon of biotic homogenization can lead to a long-term decline in biodiversity with a consequent reduction of the ecosystem functioning6,45.
A recent report on the global-scale distribution of soil protists also lacked a comprehensive representation of agricultural soils, which results in a limited understanding of the factors structuring soil protist communities in agroecosystems40. Previous studies on soil protists have largely focused on specific sites and a set of environmental conditions7,47,48. Here, we investigated the importance of several environmental factors affecting soil protists and tested whether these factors are consistent between OF and GH systems at a broad spatial scale (Fig. 3C, D). Our findings revealed the distribution of protist taxa in OF systems to be statistically more explained by edaphic properties than those in GH systems. One plausible explanation for this disparity is the lower effect of edaphic environmental factors in GH systems due to the increase in chemical fertilizer and pesticide, monoculture cultivation, and internal control of other environmental variables. Collectively, this also aligns with the weak distance-decay relationship of soil protist communities in GH systems49. Nevertheless, we still found geographic distribution and pH as important environmental variables affecting the soil protist communities in OF and GH systems, consistent with previous studies41,50,51.
Soil protist communities are dominated by phagotrophic taxa, which are predators associated with microbial species turnover. For instance, it has been shown that phagotrophic protists can enhance plant performance via induced changes in bacterial and fungal communities41,52,53. Our study revealed that dissimilarities of phagotrophic communities between all pairwise samples were primarily driven by species replacement rather than richness difference. This indicates that limited protist species were shared between the OF and GH systems. Species replacement typically occurs in communities with elevated speciation rates, dispersal limitation, ecological drift, or habitat heterogeneity54. Here, latitude was the most important factor explaining the dissimilarities of phagotrophic communities between paired GH and OF samples. Specifically, high dissimilarities in phagotrophic communities between OF and GH systems were observed in high-latitude sites. The average higher temperature increase induced in GH systems (when compared to OF) at high latitudes is likely associated with this pattern55.
Soil multifunctionality is an important factor associated with soil health and sustainability in agroecosystems1,56,57. Here, we report that GH systems had a significantly lower soil multifunctionality index than that of OF systems. Thus, corroborating the notion that agricultural intensification often leads to a reduction in the rate of essential soil ecosystem functions2,56. Specifically, we found a significantly lower abundance of functional genes associated with microbial information storage and processing and metabolism, which is consistent with previous studies2,4,58. For example, it was previously shown that agricultural intensification reduces soil multifunctionality, in particular functions associated with SOM decomposition, P mineralization, and symbiosis2. Similarly, the conversion of native forests to croplands was shown to result in significant reductions in carbon storage, nutrient cycling dynamics, and soil functions associated with organic matter decomposition17. These declines in soil multifunctionality are often attributed to the changes in bacterial and fungal communities. However, impacts on soil protists with consequences for soil multifunctionality have often been overlooked. For example, some soil saprophytic protists, such as oomycetes, can directly participate in the decomposition of organic matter15. Moreover, predatory soil protists feeding on bacteria or fungi, are well-known to affect species turnover and nutrient dynamics in soil, also altering the soil microbial diversity and composition48,59,60.
Mounting evidence from the literature supports the role of microbial communities modulating soil multifunctionality15,61,62,63. For example, a global observational study combined with an experimental microcosm provided strong evidence that soil biodiversity (bacteria, fungi, protists, and invertebrates) is significantly positively associated with multifunctionality57. In addition, it has been shown that protists are very responsive to environmental perturbations when compared to other eukaryotes, bacteria, and archaea in soils. Thus, protists have been recently proposed as effective bioindicators of soil health64. Here, we found that rare protist taxa were directly associated with soil multifunctionality. In line with this, a recent study showed that protist predation enhances the abundance of carbon and nitrogen cycle-related genes65. Also, it points to the fact that rare microbial taxa are important drivers of soil functional gene enrichment65, both in bacterial and fungal communities13,66. Rare species also constitute an important proportion of soil biodiversity, with fundamental value for maintaining ecosystem multifunctionality62,67,68. The functional importance of rare taxa is due to their over-proportional role in mediating diverse soil functions and by acting as an ‘ecological insurance’ in soils11. Last, rare taxa also contribute to the resilience of microbial communities as seed banks via the maintenance of microbial-mediated functions under distinct environmental conditions11,12,13. In line with these points, our results revealed a lower multifunctionality index in GH systems as compared to OF, which was directly associated with a reduction of rare protist taxa.
The reduction in soil biotic diversity has consequences for the ecology and functioning of soil systems. For example, it has been shown that the removal of rare taxa in soils increases the susceptibility of the system to pathogen invasion69. In addition, another study showed that a reduction of rare species in soils can lead to significant decreases in the ability of plants to uptake nutrients (in this case, ryegrass exhibited a significant decrease in Cd uptake by 52.34–73.71% once rare species were removed)70. Here we corroborate with findings from these studies by showing that rare protist taxa were directly associated with rates of soil multifunctionality in our systems. This was validated using a reconstitution experiment that successfully recovered soil functions via rare protist inoculation in soils. Specifically, we observed that only the local protist inoculations were able to reconstitute potential microbial functions in GH soils, as opposed to protists obtained from distant soil sites. This result points to an important aspect of local adaptation of protist communities, in line with a recent study on bacteria71, highlighting the home-field advantage of local microbes. In summary, we provide direct evidence that rare protists are critically important for maintaining ecosystem function in agricultural systems. Protists play major roles within the soil food web and influence soil ecosystem functioning by structuring the bacterial and fungal communities48,53,72. Thus, future research should delve into elucidating the complex interactions between soil protists, bacteria, fungi, and environmental factors in agricultural ecosystems and their impacts on diverse soil ecological functions.
Methods
Soil sampling
Soil samples were collected from 89 locations in 9 provinces located in mid-eastern China (105.21°−120.88°E and 23.02°−38.45°N, Supplementary Fig. S1A). This area is characterized by a range in mean annual temperature (MAT) of 8.2–22.3 °C and a range in mean annual precipitation (MAP) of 194.6–1852.5 mm (Supplementary Fig. S1B). At each location, several OF and GH pairs of cultivation systems were established. The studied GH systems were originally OF systems, which were developed from OF systems in recent decades, representing a typical modern and intensive agricultural system. The distance between each pair of OF and GH cultivation systems was set as <100 m (Supplementary Fig. S1C). Soil samples from each OF or GH were established by mixing nine subsamples (topsoil 0–20 cm), followed by removal of plant roots and other debris. Soil samples were sieved through a 2 mm mesh and divided into two parts. One part was stored at 4 °C for physicochemical analysis and the other was stored at −80 °C for further DNA extraction. Given the variation in management practices across GH systems (e.g., fertilization, irrigation), we collected a higher number of soil samples in GH than in OF systems. That is, for each OF soil sample, a total of 3 to 4 GH soil samples were collected in each paired system. This resulted in a total of 396 soil samples (nOF = 89 and nGH = 307).
Edaphic properties determination and climatic data collection
Soil pH and electric conductivity (EC) were measured using S220k and S230 meters (Mettler, Switzerland) at 1:2.5 and 1:5 (w/v) soil/water ratios, respectively. Soil organic matter (SOM) was determined by wet digestion with H2SO4-K2Cr2O7. The concentration of ammonium nitrogen (NH4+-N) and nitrate nitrogen (NO3−-N) were obtained via extraction using 1 mol/L of potassium chloride solution at a 1:5 (w/v) ratio and measured with a continuous flow analyzer (San++; Skalar, Netherlands). Soil available phosphorus (AP) and available potassium (AK) were determined using the ammonium molybdate and ammonium acetate methods, respectively. Climatic data (i.e., MAT and MAP) were obtained from the WorldClim database (www.worldclim.org) based on recorded geographic coordinates.
DNA extraction, amplicon sequencing and data processing
Soil DNA was extracted from 0.5 g of each soil sample using the FastDNA SPIN Kit (MP Biomedicals, USA), following the manufacture’s protocol. The DNA concentration and purity were assessed with a DS-11 spectrophotometer (Denovix, USA). The composition and diversity of protist communities were assessed using Illumina MiSeq sequencing. In brief, the V4 region of the 18S rRNA gene was amplified using the primer set TAReuk454F and TAReukREV3 (Supplementary Table 1). Following the PCR, amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using the QuantiFluor™ -ST (Promega, USA). The purified products were adjusted to equimolar concentrations and subjected to library preparation and sequencing on an Illumina MiSeq platform (Majorbio Bio-Pharm Technology Co. Ltd., China).
Raw sequencing data were processed using a combination of Quantitative Insights into Microbial Ecology (QIIME), USEARCH and UNOISE3. In brief, primer sequences and low quality read ends with quality scores (Q) < 30 were trimmed. Paired-end sequences were merged, subjected to quality-filtering (maximum expected error = 0.5), and singletons were removed using USEARCH. The UNOISE3 algorithm with default parameters in USEARCH was used to produce the zero-radius operational taxonomic units (zOTUs). zOTU sequences were taxonomically annotated using BLAST against the Protist Ribosomal Reference 4.5 (PR2) database73. The zOTUs belonging to Rhodophyta, Streptophyta, Metazoa, Opisthokonta_X, Fungi, and ambiguous taxa in Eukaryotes were removed from the final dataset. A total of 8191 protist zOTUs at 100% nucleotide identity were obtained. In addition, these protist zOTUs were categorized into different functional groups (i.e., phagotrophs, phototrophs, plant pathogens, parasites, and saprotrophs) based on their nutrient acquisition strategy18.
Metagenomics sequencing and data processing
A total of 78 soil samples (i.e., 27 from OF and 51 from GH) from nine provinces were subjected to metagenomics sequencing on an Illumina HiSeq 2500 platform (Magigene Co. Ltd, China). Raw sequencing reads were quality-filtered using TRIMMOMATIC v. 0.39 (LEADING:3, TRAILING:3, SLIDINGWINDOW:5:20, MINLEN:50), resulting in an average of 14 GB of reads per sample. Assembly of quality-filtered reads was performed using MEGAHIT v. 1.2.9. The obtained contigs >500 bp were subjected to open reading frames (ORFs) prediction using Prodigal. LINCLUST with the parameter -e 0.001 --min-seq-id 0.95 -c 0.90 was used to remove redundant genes and to obtain the non-redundant gene catalog. The gene abundance in each sample was calculated by mapping the raw reads to the non-redundant gene catalog using Salmon v. 1.8.0. Last, the protein-coding sequences were annotated against the evolutionary genealogy of genes: Non-supervised Orthologous Groups v. 5.0.2 (eggNOG) using DIAMOND. The relative abundance of functional genes was expressed as CPM (Counts Per Million-normalized).
Soil incubation experiments
Two pairs of OF and GH soils were collected from the northern (N) and southern (S) China, in the provinces of Shandong (118.86°E, 36.93°N) and Guangdong (113.54°E, 23.53°N), respectively (Fig. 7A). The isolation of protists from the OF soils was carried out according to a previous study74. Briefly, five grams of fresh OF soil was homogenized in 100 mL of sterile Page’s ameba saline solution. A volume of 100 µL of this suspension was transferred to 96-well plates (BKMAN, China) containing inactivated Escherichia coli DH5α. To maximize the diversity of protist species, we isolated a total of 960 wells (2 soils × 5 plates × 96 wells) from southern and northern OF soils. This high-throughput isolation method is similar to that isolating soil bacteria75, ensuring that the greatest variety of protists could be isolated under the current conditions. Plates were sealed and placed in the dark at 20 °C for 14 days. After that, individual wells were examined with an inverted microscope Nikon Eclipse TS100 (NIKON, Tokyo, Japan). The wells containing only protists were transferred to Petri dishes containing inactivated E. coli DH5α for further cultivation. After cultivation in the dark for 3 days, the protist suspensions within each well were pooled, washed, and re-suspended in 20 mL of sterile water. Following the method to dilute the suspensions in order to exclude rare species outlined by Hol et al.76, protist suspension with no rare species (A) was prepared using dilutions (A + R), that is 100× with sterile water.
The protist reconstitution experiment was performed by inoculating protist communities obtained from the OF soils into the GH soils. For that, 4 g of GH soil from each location was amended with 400 µL of protist inoculums (A + R) obtained from a paired OF site or a distant (non-paired) OF site, at a concentration of 105 mL−1. GH soils amended with 400 µL of sterile water were used as controls. These soil samples were incubated in sterile 6-well plates at 25 °C in the dark. All treatments were conducted using a total of three replicates. After 21 days of incubation, samples were collected and subjected to Biolog EcoPlates™ (Biolog, Inc., Hayward, CA, USA) analysis.
Statistical analysis
We categorized zOTUs as “abundant” based on relative abundance values >0.1% across all samples. Conversely, zOTUs with relative abundances <0.01% across all samples were defined as “rare”. This classification and threshold values are in line with previous studies26,36. In addition, zOTUs with cumulative counts <30 across all samples were removed to prevent random effects during the identification of rare taxa. The zOTUs with “intermediate” relative abundances (i.e., 0.01% <zOTUs <0.1%) were maintained in the analysis. Shannon index, richness, evenness, and phylogenetic diversity were calculated using the package “picante” (https://cran.r-project.org/web/packages/picante) in R v. 4.3.1 (http://www.r-project.org). Nonmetric multidimensional scaling (NMDS) analysis was performed using Bray–Curtis distances with the package “vegan” (http://vegan.r-forge.r-project.org). Permutational multivariate analysis of variance (PERMANOVA) was performed to test for significant differences in community structure across treatments and latitudes using the package “vegan”. Variance partitioning analysis (VPA) was used to explore the relative contributions of edaphic variables, geographic variables, and cultivation systems in explaining variation in protist beta-diversity based on abundant and rare taxa using the package “vegan”. Manhattan plot was used to visualize differences in taxa composition using the package “ggplot2” (https://cran.r-project.org/web/packages/ggplot2/index.html).
The Levins’ niche breadth (B) index was calculated to assess the diversity of resources utilized or environments tolerated by protist communities. The niche breadth (B) index was calculated using the formula Bj = 1 /\(\,{\sum }_{i=1}^{N}P\)ij2, where Bj is the habitat niche breadth of zOTUj in a metacommunity, Pij is the proportion of zOTUj in the community I, and N is the total number of communities in each metacommunity42. The habitat niche breadth (Bcom) of all taxa within each metacommunity was calculated to represent the community-level B value. This analysis was performed using the “niche.width” function in the package “spaa” (https://helixcn.r-universe.dev/spaa/doc/manual.html). The spatial distributions of niche breadth in OF and GH sample sites were mapped by Inverse Distance Weighted (IDW) interpolation using the package “gstat” (https://cran.r-project.org/web/packages/gstat/index.html) and visualized using the package “ggplot2”.
Distance-decay relationships (DDRs) were calculated using community similarity values (1 − Bray–Curtis dissimilarity) and geographic distances, as well as edaphic properties (dissimilarity) based on linear least-squares regression. The geographical distances among the sampling sites were calculated based on the sampling coordinates (latitude and longitude). The edaphic dissimilarity among samples were calculated using soil physiochemical properties based on Euclidean distance. The difference in slopes of DDRs was calculated using the “diffslope” function in the package “simba” (https://cran.r-project.org/web/packages/simba/index.html). The β-nearest taxon index (βNTI) and the Raup–Crick index (RC) were calculated to quantify the relative influence of ecological processes modulating protist community assembly. In brief, βNTI values > 2 or <−2 indicate significantly greater or lower turnover than expected, respectively; which suggests selection is a major process structuring community assembly (either variable selection >2 or homogeneous selection <−2). βNTI values between −2 and 2 indicate a greater influence of stochastic processes, including homogenizing dispersal (RC <–0.95), dispersal limitation (RC > 0.95), and undominated process (|RC| < 0.95).
Phagotrophic protist community dissimilarity between each OF and GH soil pair was decomposed into similarity (S), replacement (Repl), and richness difference (RichDiff) using the package “adespatial” (https://cran.r-project.org/web/packages/adespatial/). Spearman correlations were conducted between these parameters, climatic factors, and the differences in edaphic properties (Δedaphic properties, GH−OF) within soil pairs. Constrained analysis of principal coordinates (CAP) was used to explore the impacts of geographic variables and Δedaphic properties on protist community dissimilarity within soil pairs.
Soil multifunctionality and its relationship with phylogenetic diversity of protist communities were assessed using average values and multiple-threshold approaches. The average values approach consists of soil functions annotated with the eggNOG database and normalized to the scale of 0 to 1 (z-score transformation). These normalized values were averaged to obtain a multifunctionality index for each soil. The multiple-threshold approach consists of measuring several soil functions and categorizing these across a series of sequential thresholds (ranging from 1 to 99% with 1% intervals). Detailed information and indices used for assessing the relationship between biodiversity and multifunctionality were previously described13,61. Structural equation model (SEM) was used to explore potential variables associated with soil multifunctionality, using the package “lavaan”. All potential pathways were considered in the conceptual model, and non-significant pathways were sequentially removed unless they were biologically informative.
Data availability
The 18S rRNA gene sequences and the metagenomic sequences were submitted to the NCBI Sequence Read Archive (SRA) database under the accession number PRJNA1108194 and PRJNA1161440, respectively. The data supporting this study are provided online at https://doi.org/10.6084/m9.figshare.29539124.
Code availability
No custom code was generated for this study.
References
Delgado-Baquerizo, M. et al. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 7, 10541 (2016).
Xue, R. et al. Agricultural intensification weakens soil multifunctionality by reducing fungal diversity. Appl Soil Ecol. 189, 104900 (2023).
Bradford, M. A. et al. Discontinuity in the responses of ecosystem processes and multifunctionality to altered soil community composition. Proc. Natl. Acad. Sci. USA 111, 14478–14483 (2014).
Qu, X. et al. Deforestation impacts soil biodiversity and ecosystem services worldwide. Proc. Natl. Acad. Sci. USA 121, e2318475121 (2024).
Cornell, C. R. et al. Land use conversion increases network complexity and stability of soil microbial communities in a temperate grassland. ISME J. 17, 2210–2220 (2023).
Banerjee, S. et al. Biotic homogenization, lower soil fungal diversity and fewer rare taxa in arable soils across Europe. Nat. Commun. 15, 327 (2024).
Xiong, W. et al. Soil protist communities form a dynamic hub in the soil microbiome. ISME J. 12, 634–638 (2018).
Jiao, S., Xu, Y., Zhang, J. & Lu, Y. Environmental filtering drives distinct continental atlases of soil archaea between dryland and wetland agricultural ecosystems. Microbiome 7, 15 (2019).
Labouyrie, M. et al. Patterns in soil microbial diversity across Europe. Nat. Commun. 14, 3311 (2023).
Jiao, S. & Lu, Y. Abundant fungi adapt to broader environmental gradients than rare fungi in agricultural fields. Glob. Change Biol. 26, 4506–4520 (2020).
Jousset, A. et al. Where less may be more: how the rare biosphere pulls ecosystems strings. ISME J. 11, 853–862 (2017).
Ziegler, M., Eguiluz, V. M., Duarte, C. M. & Voolstra, C. R. Rare symbionts may contribute to the resilience of coral-algal assemblages. ISME J. 12, 161–172 (2018).
Chen, Q. L. et al. Rare microbial taxa as the major drivers of ecosystem multifunctionality in long-term fertilized soils. Soil Biol. Biochem. 141, 107686 (2020).
Jiao, S., Chen, W. & Wei, G. Biogeography and ecological diversity patterns of rare and abundant bacteria in oil-contaminated soils. Mol. Ecol. 26, 5305–5317 (2017).
Xiong, C. et al. Rare taxa maintain the stability of crop mycobiomes and ecosystem functions. Environ. Microbiol. 23, 1907–1924 (2021).
Hou, J. et al. Biogeography and diversity patterns of abundant and rare bacterial communities in rice paddy soils across China. Sci. Total Environ. 730, 139116 (2020).
Geisen, S. et al. Soil protists: a fertile frontier in soil biology research. FEMS Microbiol. Rev. 42, 293–323 (2018).
Xiong, W. et al. Rhizosphere protists are key determinants of plant health. Microbiome 8, 1–9 (2020).
Adl, M. S. & Gupta, V. V. S. R. Protists in soil ecology and forest nutrient cycling. Can. J. Res. 36, 1805–1817 (2006).
Matson, P. A., Parton, W. J., Power, A. G. & Swift, M. J. Agricultural intensification and ecosystem properties. Science 277, 504–509 (1997).
de Vries, F. T. et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 110, 14296–14301 (2013).
Rouphael, Y., Kyriacou, M. C., Petropoulos, S. A., De Pascale, S. & Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 234, 275–289 (2018).
Shen, Y. et al. Pharmaceutical exposure changed antibiotic resistance genes and bacterial communities in soil-surface- and overhead-irrigated greenhouse lettuce. Environ. Int. 131, 105031 (2019).
Shen, W. et al. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 463, 1–18 (2021).
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R. & Polasky, S. Agricultural sustainability and intensive production practices. Nature 418, 671–677 (2002).
Lou, Y., Xu, M., Wang, W., Sun, X. & Liang, C. Soil organic carbon fractions and management index after 20 yr of manure and fertilizer application for greenhouse vegetables. Soil Use Manag. 27, 163–169 (2011).
Hickman, G. W. A review of current data on international production of vegetables in greenhouses. Statistics Cuesta Roble Consulting, Mariposa, CA. www.cuestaroble.com (2011).
Garland, G. et al. Crop cover is more important than rotational diversity for soil multifunctionality and cereal yields in European cropping systems. Nat. Food 2, 28–37 (2021).
de Carvalho, T. S. et al. Land use intensification in the humid tropics increased both alpha and beta diversity of soil bacteria. Ecology 97, 2760–2771 (2016).
Hartmann, M., Frey, B., Mayer, J., Maeder, P. & Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 9, 1177–1194 (2015).
Nemergut, D. R. et al. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 77, 342–356 (2013).
Jiang, F. et al. Global patterns and drivers of plant-soil microbe interactions. Ecol. Lett. 27, e14364 (2024).
Lynch, M. D. J. & Neufeld, J. D. Ecology and exploration of the rare biosphere. Nat. Rev. Microbiol. 13, 217–229 (2015).
de Graaff, M.-A., Hornslein, N., Throop, H. L., Kardol, P. & van Diepen, L. T. A. Effects of agricultural intensification on soil biodiversity and implications for ecosystem functioning: A meta-analysis. Adv. Agron. 155, 1–44 (2019).
Wan, W. et al. Environmental adaptation is stronger for abundant rather than rare microorganisms in wetland soils from the Qinghai-Tibet Plateau. Mol. Ecol. 30, 2390–2403 (2021).
Dai, T. et al. Nutrient supply controls the linkage between species abundance and ecological interactions in marine bacterial communities. Nat. Commun. 13, 175 (2022).
Romdhane, S. et al. Land-use intensification differentially affects bacterial, fungal and protist communities and decreases microbiome network complexity. Environ. Microbiome 17, 1–15 (2022).
Binzer, A. et al. The susceptibility of species to extinctions in model communities. Basic Appl. Ecol. 12, 590–599 (2011).
Sexton, J. P., Montiel, J., Shay, J. E., Stephens, M. R. & Slatyer, R. A. Evolution of ecological niche breadth. Annu. Rev. Ecol. Evol. Syst. 48, 183–206 (2017).
Feng, K. et al. Niche width of above- and below-ground organisms varied in predicting biodiversity profiling along a latitudinal gradient. Mol. Ecol. 29, 1890–1902 (2020).
Oliverio, A. M. et al. The global-scale distributions of soil protists and their contributions to belowground systems. Sci. Adv. 6, eaax8787 (2020).
Jiao, S., Yang, Y., Xu, Y., Zhang, J. & Lu, Y. Balance between community assembly processes mediates species coexistence in agricultural soil microbiomes across eastern China. ISME J. 14, 202–216 (2020).
Zhou, X., Rahman, M. K. U., Liu, J. & Wu, F. Soil acidification mediates changes in soil bacterial community assembly processes in response to agricultural intensification. Environ. Microbiol. 23, 4741–4755 (2021).
Wang, X.-B. et al. Habitat-specific patterns and drivers of bacterial β-diversity in China’s drylands. ISME J. 11, 1345–1358 (2017).
Peng, Z. et al. Land conversion to agriculture induces taxonomic homogenization of soil microbial communities globally. Nat. Commun. 15, 3624–3624 (2024).
Delgado-Baquerizo, M. et al. Global homogenization of the structure and function in the soil microbiome of urban greenspaces. Sci. Adv. 7, eabg5809 (2021).
Venter, P. C., Nitsche, F. & Arndt, H. The hidden diversity of flagellated protists in soil. Protist 169, 432–449 (2018).
Guo, S. et al. Protists as main indicators and determinants of plant performance. Microbiome 9, 64 (2021).
Buhk, C. et al. Homogenizing and diversifying effects of intensive agricultural land-use on plant species beta diversity in Central Europe - A call to adapt our conservation measures. Sci. Total Environ. 576, 225–233 (2017).
Bates, S. T. et al. Global biogeography of highly diverse protistan communities in soil. ISME J. 7, 652–659 (2013).
Stefana, G., Cornelia, B., Joerg, R. & Michael, B. Soil water availability strongly alters the community composition of soil protists. Pedobiologia 57, 205–213 (2014).
Seppey, C. V. W. et al. Distribution patterns of soil microbial eukaryotes suggests widespread algivory by phagotrophic protists as an alternative pathway for nutrient cycling. Soil Biol. Biochem. 112, 68–76 (2017).
Petters, S. et al. The soil microbial food web revisited: Predatory myxobacteria as keystone taxa?. ISME J. 15, 2665–2675 (2021).
Zhong, Y. et al. Arbuscular mycorrhizal trees influence the latitudinal beta-diversity gradient of tree communities in forests worldwide. Nat. Commun. 12, 3137 (2021).
Liang, Y. et al. Long-term soil transplant simulating climate change with latitude significantly alters microbial temporal turnover. ISME J. 9, 2561–2572 (2015).
Zhang, J., Van der Heijden, M. G. A., Zhang, F. & Bender, S. F. Soil biodiversity and crop diversification are vital components of healthy soils and agricultural sustainability. Front. Agric. Sci. Eng. 7, 236–242 (2020).
Delgado-Baquerizo, M. et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 4, 210–220 (2020).
Leifheit, E. F., Veresoglou, S. D., Lehmann, A., Morris, E. K. & Rillig, M. C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation-a meta-analysis. Plant Soil 374, 523–537 (2014).
Gao, Z., Karlsson, I., Geisen, S., Kowalchuk, G. & Jousset, A. Protists: Puppet Masters of the Rhizosphere Microbiome. Trends Plant Sci. 24, 165–176 (2019).
Geisen, S., Hu, S. R., dela Cruz, T. E. E. & Veen, G. F. Protists as catalyzers of microbial litter breakdown and carbon cycling at different temperature regimes. ISME J. 15, 618–621 (2021).
Hu, W. et al. Aridity-driven shift in biodiversity-soil multifunctionality relationships. Nat. Commun. 12, 5350 (2021).
Zhou, Z., Wang, C. & Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 11, 3072 (2020).
Luo, J. et al. Long-term organic fertilization promotes the resilience of soil multifunctionality driven by bacterial communities. Soil Biol. Biochem. 177, 108922 (2023).
Dupont, A. O. C., Griffiths, R. I., Bell, T. & Bass, D. Differences in soil micro-eukaryotic communities over soil pH gradients are strongly driven by parasites and saprotrophs. Environ. Microbiol. 18, 2010–2024 (2016).
Wang, Y.-F. et al. Biological interactions mediate soil functions by altering rare microbial communities. Environ. Sci. Technol. 58, 5866–5877 (2024).
Wang, C., Guo, L. & Shen, R. F. Rare microbial communities drive ecosystem multifunctionality in acidic soils of southern China. Appl. Soil Ecol. 189, 104895 (2023).
Hautier, Y. et al. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 348, 336–340 (2015).
Tang, L. et al. Plant community associates with rare rather than abundant fungal taxa in alpine grassland soils. Appl. Environ. Microbiol. 89, e01862–e01922 (2023).
van Elsas, J. D. et al. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc. Natl. Acad. Sci. USA 109, 1159–1164 (2012).
He, X. G. et al. Soil rare microorganisms mediated the plant cadmium uptake: the central role of protists. Sci. Total Environ. 908, 168505 (2024).
Jiang, M. et al. Home-based microbial solution to boost crop growth in low-fertility soil. N. Phytol. 239, 752–765 (2023).
Thakur, M. P. & Geisen, S. Trophic regulations of the soil microbiome. Trends Microbiol. 27, 771–780 (2019).
Guillou, L. et al. The protist ribosomal reference database (PR2): a catalog of unicellular eukaryote small sub-unit rRNA sequences with curated taxonomy. Nucleic Acids Res. 41, D597–D604 (2013).
Guo, S. et al. Predatory protists reduce bacteria wilt disease incidence in tomato plants. Nat. Commun. 15, 829 (2024).
Zhang, J. et al. High-throughput cultivation and identification of bacteria from the plant root microbiota. Nat. Protoc. 16, 988–1012 (2021).
Hol, W. H. G. et al. Reduction of rare soil microbes modifies plant-herbivore interactions. Ecol. Lett. 13, 292–301 (2010).
Acknowledgements
This research was supported by the National Key Research and Development Program of China (2023YFD1902000), the National Natural Science Foundation of China (U21A20226), Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) CX(23)1038, and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_1825).
Author information
Authors and Affiliations
Contributions
Yuanyuan Yan performed research, contributed data and analysis, wrote original draft. Xing Zhou performed research and contributed analysis. Liangliang Liu performed research. Ruimin Li performed research. Jun Zhao contributed data and analysis. Jinbo Zhang designed research. Zucong Cai designed research and revised the paper. Francisco Dini-Andreote revised the paper. Josep Penuelas revised the paper. Xinqi Huang designed research, contributed data and analysis, and revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks Alejandro Berlinches De Gea and Sara Sánchez Moreno for their contribution to the peer review of this work. Primary Handling Editors: Somaparna Ghosh [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yan, Y., Zhou, X., Liu, L. et al. Transitions within agroecosystems impact protists diversity and soil multifunctionality. Commun Earth Environ 6, 634 (2025). https://doi.org/10.1038/s43247-025-02647-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02647-w