Abstract
Longstanding theories and models classify mineral-associated organic matter as the large ( ~ 60%) but slow-cycling and persistent portion of soil organic matter. Strong physico-chemical interactions and diffusion limitations restrict the turnover of mineral-associated organic matter, allowing carbon and nitrogen bound therein to persist in soil for as long as centuries to millennia. However, mineral-associated organic matter is a chemically and functionally diverse pool with a substantial portion cycling at relatively fast (i.e., minutes to years) timescales. Despite a growing body of evidence for the heterogenous and multi-pool nature of mineral-associated organic matter, we lack consensus on how to conceptualize and directly quantify fast-cycling mineral-associated organic matter and its ecological significance. We demonstrate that the dynamic qualities of fast-cycling mineral-associated organic matter vary based on 1) the chemistry of the mineral particles and organic matter, 2) the complex set of interactions between organic matter and the mineral matrix, and 3) the presence and strength of destabilizing forces that lead to decomposition or loss of mineral-associated organic matter (i.e., plant-microbe interactions, agricultural intensification, and climate change). Finally, we discuss potential implications and research opportunities for how we measure, manage, and model the dynamic subfraction of this otherwise persistent pool of soil organic matter.
Similar content being viewed by others
Introduction
Soil organic matter (SOM) plays a central role in terrestrial ecosystem functioning, providing nitrogen (N) and other nutrients to plants, and holding the largest pool of organic carbon (C) on land. A portion of the SOM pool cycles slowly – with turnover time ranging from decades to millennia. Our understanding of SOM persistence has evolved from an emphasis on chemical complexity and recalcitrance towards a growing appreciation for the interactions between relatively simple plant and microbial inputs and reactive mineral surfaces as key controls on SOM persistence1. As such, there is considerable focus on characterizing and measuring the mineral-associated organic matter (MAOM; see Box 1) pool, the fraction of SOM which holds the majority of organic C and N in the terrestrial biosphere (60–65% of C and 75% of N)2,3. Strong physico-chemical interactions and diffusion limitations restrict the turnover of some MAOM, allowing C within this pool to persist for centuries to millennia4.
Particulate organic matter (POM) is considered a more bioavailable and faster cycling pool of soil C and N, relative to MAOM. POM is dominated by larger fragments of plant and microbial residues, which are at early stages of decomposition. The POM pool responds rapidly to changes in tillage or plant inputs and often correlates strongly with whole soil measures of decomposition5, contributing to the view of POM as an SOM pool that is particularly sensitive to disturbances and physically accessible to decomposers. However, the decomposition of POM, in addition to litter decomposition, is also needed to build MAOM6. The leaching and depolymerization of POM and litter releases OM compounds into solution, which can then adsorb to a reactive mineral surface before or after cycling through microbial biomass7,8,9. While microaggregates provide further protection to MAOM10, aggregate turnover and disturbance can promote MAOM formation as well11. MAOM formation is also more efficient within the rhizosphere relative to the bulk soil, which suggests MAOM forms under highly dynamic conditions12. MAOM formation is fast: transfer of 15N or 13C-labeled plant residues into MAOM fractions can occur within days to months13,14,15,16.
The abundance of fine mineral particles and high mineral specific surface area are strong predictors of soil C storage17,18; emerging evidence suggests that so too are existing MAOM stocks and the overall MAOM saturation deficit19,20. As such, MAOM is often examined for its capacity to store and sequester C and nutrients, with less attention to the heterogeneity underlying the MAOM fraction and its implications for potential destabilization following disturbance21,22. Although the majority of MAOM is likely persistent and long-lived, there is increasing evidence for a dynamic, fast-cycling MAOM pool.
Given the vast quantity of C and N stored in MAOM, the presence of even a small active MAOM pool can substantially influence ecosystem processes. For example, a hypothetical and very conservative annual MAOM turnover rate of 1% in a grassland with 3000 kg MAOM N ha−1 in the top 10 cm of soil23 would supply 30 kg N ha−1 per year, which is approximately a third of the N uptake by grassland species24. The fast-cycling MAOM pool may function as a distinct SOM pool with essential roles in ecosystems, including retaining nutrients prone to loss and supplying nutrients to plants and microbes. At the same time, this fast-cycling MAOM pool may become a larger source of greenhouse gas emissions over time, especially under accelerated land use and climate change, as we discuss below.
The idea of a fast-cycling MAOM pool is not new (e.g., Kleber et al. and Torn et al.)25,26, but the concept is rarely acknowledged in research and policies related to the management of MAOM (e.g., natural climate solutions-oriented work) or in Earth system models. In this review, we seek to clarify the existence and ecological importance of fast-cycling MAOM with a targeted summary of research on this crucial SOM pool. Others have synthesized relevant geochemical and plant-mediated mechanisms of MAOM cycling27,28, and the land use and climate change implications of POM versus MAOM29 but have not focused explicitly on the fast-cycling MAOM pool. Here, we synthesize recent studies that confirm the existence of a fast-cycling MAOM pool across various ecosystem types and provide insight into its size, stability, and mechanisms of turnover. We describe its key role in ecosystem functions such as nutrient provisioning, soil C cycling, and plant-microbe interactions, and suggest implications for how we measure, manage, and model this dynamic subfraction of MAOM.
MAOM is chemically heterogeneous
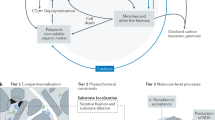
MAOM is a large and heterogenous pool of SOM, containing mineral particles and organic molecules that vary widely in age, size, and reactivity. Variations in MAOM binding mechanisms and chemical characteristics determine how this pool of OM cycles through terrestrial ecosystems, by influencing its availability to microbes, turnover rate, and potential to serve as a plant nutrient source. At the scale of mineral surface-OM associations, the behavior of MAOM and the chemical bonds therein will depend largely on characteristics of the interacting organic compounds, mineral particles, and soil solution27,30,31,32,33 (Fig. 1). Minerals can have permanent and/or variable charges of varying strength, distribution, and density and such differences will impact the type and number of binding sites, adsorption-desorption behavior, and hence, the bioavailability of associated molecules34,35. For example, minerals with a relatively high point-of-zero-charge that are positively charged under neutral and acidic conditions, such as goethite, may be able to bind SOM more strongly via ligand exchange reactions compared to negatively charged clay minerals that bind to OM via cation bridging36. Mineral types that bind compounds more weakly may harbor more vulnerable and accessible forms of MAOM.
The distribution of MAOM into persistent or fast-cycling forms will depend largely on the properties of the minerals and associated organic matter. Key ecological drivers of MAOM destabilization include plant-microbial forces, climate change, land use change, and agricultural management. Original artwork by Elena Harley (www.elabarts.com).
Characteristics of organic substrates expected to influence the mechanism and strength of association include molecular size, hydrophobicity, acidity, and abundance of carboxylic, phenolic and aromatic constituents, which interact with mineral surface chemistry to influence the mechanism and reversibility of organo-mineral associations37,38,39. For example, stronger covalent bonds tend to form between organic substrates enriched in aromatic acid and phenolic groups and mineral fractions with a high abundance of reactive Fe and Al (oxy)hydroxide phases, especially under acidic soil solution conditions38,40. Stoichiometry (e.g., the C:N ratio) of organic matter will also influence its potential for adsorption or desorption41. Empirical work and direct imaging of organo-mineral interfaces at sub-micron scales indicate that N-rich organic compounds (e.g., N-containing groups in proteins) bind preferentially to mineral surfaces42,43,44,45,46,47, and it is often suggested that MAOM-N is more stable than MAOM-C as a result.
The stability and turnover dynamics of MAOM are also shaped by its fine-scale spatial and compositional heterogeneity45,48,49,50,51,52. Several lines of evidence support the existence of spatially or chemically distinct portions of MAOM that are more vulnerable to mineralization. Kleber et al. proposed a model suggesting that OM binds to mineral surfaces in a zonal structure25. In this multi-layer model, the outermost zone is referred to as the kinetic zone and hosts organic molecules that are weakly bound via van der Waals forces, hydrogen bonding, and cation bridging interactions. Secondary interactions among organic molecules (i.e., organo-organic interactions45) are consequently an important mechanism within this kinetic zone, and provide a mechanism for loading of new organic substrates to pre-existing MAOM33,52. In the zonal model, the more weakly-associated kinetic material is hypothesized to exchange readily with the soil solution. Similarly, Kaiser and Guggenberger argued that the potential bioavailability of MAOM depends on the degree of OM loading on mineral surfaces53,54. As more organic molecules occupy binding sites, a proportion of MAOM may become more susceptible to degradation due to fewer functional groups being involved in sorption. Further, the enrichment of N-rich OM at the mineral surface is consistent with a zonal model driven by preferential retention of N-containing functional groups (e.g., through hydrogen bonding, cation bridges, or ligand exchange)30. However, in batch adsorption experiments using dissolved organic matter (DOM) and goethite, N-containing compounds were the last to adsorb, populating the kinetic zone, which is hypothesized to constitute the most dynamic, exchangeable fraction55. Variations in mineral-organic interactions, including those regulated by variation in chemical bonds, organic matter composition and fine-scale spatial heterogeneity, warrants deeper engagement with the idea of MAOM containing a fast-cycling and bioavailable subfraction.
MAOM contains young and mineralizable compounds
MAOM is generally characterized as old and slow-cycling due to a consistent increase in SOC mean residence time (aka mean system age)56 with decreasing particle size. Radiocarbon analyses demonstrate that silt- and clay-associated OM has a longer estimated turnover time than sand-associated OM57. However, a small but fast-cycling portion of MAOM (i.e., days to years) may be obscured in these estimates by much slower-cycling compounds (i.e., centuries to millennia) that substantially shift the mean estimate of residence time in these observational studies58,59. Radiocarbon tracer studies can more accurately track the rate of incorporation of new inputs to MAOM, though they are rarely employed. However, one site in an oak forest with accidental radiocarbon release found that up to 37% of MAOM is replaced on an annual basis and has a mean residence time of four years60. In the same soils, the steady-state mean residence time estimated from natural abundance radiocarbon measurements was 108 years, which suggests that natural abundance-based averages may not be a reliable measure of typical MAOM residence times in topsoil60.
Recent work suggests that a primary driver of skewness in radiocarbon-based SOM and MAOM age data may be organic fossil C in sedimentary soil parent materials, including kerogen, lignite, and coal, which could contribute substantially to MAOM C pools. The presence of fossil C may skew radiocarbon ages in MAOM and other SOM fractions61,62,63. The use of ramped thermal analysis has proved an effective means of illustrating how the average radiocarbon value of a MAOM fraction may obscure the strongly contrasting ages of its constituent compounds, which may range from post-bomb (modern) to nearly radiocarbon dead (~ 60,000 years old or older)64. Thus, the reliance on radiocarbon dating alone to infer the mean age or persistence of MAOM may lead to spurious conclusions regarding MAOM durability in the context of climate change and its potential to store new C additions over long timescales.
Mineral surfaces provide MAOM with some protection from decomposition, but they are also host to active microbial communities. Mineral surfaces support high microbial biomass and accelerated rates of microbial turnover and enzyme production65,66,67. The abundance of low-molecular weight and low C:N compounds support a high mineralization potential within MAOM68. The intrinsic bioavailability of MAOM is illustrated by several incubation-based experiments. In a study of 156 diverse soils from across the United States, both MAOM and POM were strong predictors of whole soil C decomposition dynamics69. They suggest that the large pool size of MAOM may compensate for its lower decomposition rates in some soils creating similar overall contributions to CO2 flux as POM. When SOM fractions are incubated under ideal temperature and moisture conditions, MAOM can even exhibit higher N and C mineralization potential compared to POM or light fractions70,71,72,73,74,75. This was recently observed for soils collected from forest, grassland, and cropland land uses76.
Two main methodological approaches provide direct estimates of the potentially bioavailable and fast-cycling portion of the MAOM pool: (1) incubation experiments to trace the mineralization of clay or MAOM-bound organic compounds (Table 1) and (2) batch adsorption-desorption experiments (Table 2). In the former, DOM (either an isotopically labelled substrate or DOM extracted from soil or plant litter) is added to pristine minerals or to isolated MAOM fractions, and the decomposition of the newly formed MAOM is monitored over the course of a days or weeks-long incubation. Many of these studies demonstrate an intrinsic capacity for MAOM to supply C (Table 1). Over half of adsorbed organic compounds in such experiments were bioavailable and mineralizable, with the magnitude of decomposition often mediated by mineral type.
Batch adsorption-desorption studies highlight how rapidly compounds may desorb from mineral surfaces (Table 2). Such experiments quantify the equilibrium partitioning of a sorbate between solid and solution phases, in a liquid suspension over 24-48 hours77 and using a wide range in soil:solution ratios (1:4 to 1:10,000). Results demonstrate the rapid and largely irreversible adsorption of organic compounds. However, approximately half of adsorbed OC can be removed with water within a few hours, which highlights the high potential bioavailability of MAOM. Reported rates of desorption and mineralization vary widely due to the variety of experimental conditions including mineral type, desorption agents, background electrolytes, and pH. Likewise, the short duration, highly disturbed, and often artificial context limit our ability to interpret and translate results to natural soil systems. Another major limitation of the experimental approaches highlighted in Tables 1, 2 is that the newly formed MAOM may not behave like MAOM formed under months or years of incubation, which would better capture the suite of biotic and abiotic pathways of MAOM stabilization. Nonetheless, the selected laboratory experiments tracing the desorption and mineralization of OC from clays highlight the potential for microbes and plant roots to access and decompose MAOM. As discussed below, there are certain ecological contexts and processes that may allow for this mineralization potential to be realized at the ecosystem scale.
Ecological drivers of MAOM destabilization
While there has been much emphasis on the controls of SOM formation and persistence, a growing body of research has focused on the drivers of SOM destabilization21. Forces of destabilization have been categorized into those that disrupt soil physical structure (such as soil aggregates), those that disrupt mineral-organic associations, and those that enhance C metabolism21. Below, we focus on three proximal drivers of MAOM destabilization: plant-microbial interactions, climate change, and agricultural intensification. The transfer of OM from mineral-associated to more bioavailable pools will depend on the presence and strength of particular environmental conditions that favor MAOM destabilization.
Plant-microbial forces
Rhizosphere environments provide conditions that can facilitate the destabilization and turnover of MAOM. MAOM in the rhizosphere is more likely to be released from mineral surfaces than MAOM in bulk soil due to a higher concentration of plant and microbial exudates in the soil near active roots78,79. These exudates may stimulate specific extracellular enzymes or more generally stimulate decomposition (i.e. glucose, organic acids). Additionally, plant and microbial exudates can form associations with soil minerals directly, contributing to the MAOM pool80. In fact, root-derived organic matter, particularly root exudates, is suggested to be a dominant source of mineral-associated C and N12,81,82,83,84. As a result, rhizosphere MAOM acts as both a source and sink for organic compounds and is likely more dynamic compared to MAOM in the bulk soil.
MAOM may be particularly vulnerable to destabilization by plant exudates in ecosystems with rapidly growing vegetation and high demand for soil nutrients. These conditions are common in early successional forests, agricultural systems, and ecosystems recovering from disturbance or responding to global change85,86,87. Some evidence for these patterns comes from analyses of MAOM radiocarbon content where in the eastern U.S., MAOM turns over more quickly in ecosystems with higher root mass88. This suggests that ecosystems with high plant nutrient demand may have a higher proportion of fast-cycling MAOM compared to other ecosystems, particularly in the rhizosphere.
The rhizosphere is also characterized by the presence and enrichment of mineral-weathering soil bacteria. Rhizosphere activity can accelerate the formation of metal oxides89 or facilitate rock weathering and clay transformation processes90,91,92. Phosphate-dissolving bacteria have been reported in the rhizosphere of agricultural soils93 and around the roots of mangrove trees94. Similarly, the dissolution of clay particles was highest in rhizosphere of Norway spruce and oak compared to bulk soil95. Although mineral weathering is generally seen as a slow pedogenic process, it can occur over short time scales, especially in the microbially-active zone around roots. Processes occurring at the root-soil interface can significantly accelerate pedogenesis such that mineral dissolution or alteration can occur within 20 years95,96 or even within a growing season97. It is unclear how these localized, accelerated alterations to clay particles in the rhizosphere alter the formation and cycling of MAOM specifically.
Mycorrhizal fungi, which receive energy-rich C compounds from plant hosts, are equipped to destabilize and mobilize MAOM. The capacity for ectomycorrhizal fungi (ECM) to liberate MAOM was recently reviewed by Tunlid et al.98. Many ECM fungi secrete low molecular weight compounds like oxalic acid that can disrupt mineral-organic associations99, dissolve minerals100, release nutrients98 and facilitate the direct assimilation of N from MAOM101. Some ECM species also mediate the reductive dissolution of iron-bearing minerals and the subsequent generation of reactive oxygen species102. MAOM was shown to be more sensitive to hydroxyl radicals than POM, which was attributed to the enrichment in MAOM of low molecular weight compounds with lower activation energies103. Thus, ECM-facilitated dissolution of minerals may accelerate the decomposition of MAOM. Arbuscular mycorrhizal fungi (AMF) also generate many of the same compounds with known weathering capacities104,105 but may have more limited capacity to directly mobilize nutrients from MAOM compared to ECM106. AMF depend on other soil biota to mobilize and mine nutrients directly from SOM107,108. AMF-generated organic acids were shown to mobilize phosphorus bound to iron oxides109, but this area of research remains highly understudied. Overall, evidence suggests that the activities of roots, mycorrhizal fungi and free-living microbes contribute substantially to MAOM mobilization, likely making the rhizosphere a hotspot for dynamic and fast-cycling MAOM.
Climate-related forces
Climate is a major driver of plant growth, microbial activity, and abiotic properties of soils. Therefore, it is also likely to alter the formation and destabilization of MAOM, with direct implications for the fast-cycling MAOM pool. Shifts in climate may disrupt and alter MAOM formation and destabilization processes, potentially limiting MAOM accrual and/or accelerating the loss of MAOM. Multiple studies report how changes in precipitation, temperature, and atmospheric CO2 may destabilize MAOM across varied ecosystems.
Given projected shifts in climate toward intensification of hydrologic cycles, it is important to understand how shifting moisture regimes may alter fast-cycling MAOM. In arid systems where moisture limitation is a physiological control on microbial mineralization, changing precipitation patterns (and increased DOC fluxes to subsoil) may facilitate MAOM losses88. Moisture availability indices (e.g., MAP/PET) and correlated moisture-driven mineralogy gradients (e.g., reactive metal vs. base cation-dominated mineralogy) have therefore been applied as organizing concepts to predict differences in MAOM formation mechanisms110,111,112,113 and susceptibility to destabilization under changing moisture availability across broad-scale climatic regions112.
In addition to the role of moisture regime at the ecosystem scale, MAOM destabilization processes are influenced by moisture-driven changes in soil redox status at the pore scale. Most notably, the reduction of iron-bearing minerals under saturated conditions can release mineral-bound C into soluble forms, leading to potential net losses of C following a return to oxic conditions114,115,116,117,118. However, saturation may also cause more dynamic sorption-desorption events in soils less dominated by iron-bearing minerals119. In environments experiencing frequent wet-dry cycles, shifting aerobic to anaerobic conditions has been shown to increase the bioavailability of MAOM, possibly due to a loss of Fe-MAOM associations45. Wet-dry cycling was also shown to enhance the decomposition of MAOM and its vulnerability to simulated exudates120. This suggests that increases in moisture variability due to climate intensification may increase the proportion of the MAOM pool that cycles quickly.
Temperature is an important control on SOM decomposition rates121, but the sensitivity of faster-cycling MAOM components to changing temperature is less well described. In general, MAOM is expected to be less temperature-sensitive overall compared to POM122,123. However, there have been few direct studies of the responses of different components of MAOM to changes in temperature. Available evidence suggests larger and lighter components of MAOM are more responsive to warming124,125. One possibility, which warrants further investigation, is that larger and lighter portions of MAOM are held more loosely to mineral surfaces, making them more susceptible to mineralization with increased microbial activity. In addition, OM protected by reactive metal associations were found to be more vulnerable to warming temperatures than OM protected by base cations, potentially due to the temperature sensitivity of biological processes involved in overcoming stable organo-metal interaction mechanisms112.
As the CO2 fertilization effect promotes higher rates of photosynthesis, plants require more nutrients from soil, particularly N126. As a result, rising atmospheric CO2 concentrations may indirectly promote MAOM desorption and turnover as a consequence of increasing plant nutrient demand. In ecosystems without a large standing stock of bioavailable N, plants may rely on MAOM as a source of limiting nutrients to fuel higher rates of productivity. This may occur through greater root exudation87, which could cause desorption of N-rich MAOM that would subsequently be available for mineralization. However, rhizosphere-induced priming may act on the POM pool rather than the MAOM pool127,128. Indeed, in support of a minimal MAOM response to CO2 enrichment, a meta-analysis of global change manipulations found no significant effect of elevated CO2 (eCO2) on MAOM-C concentrations123. However, in studies conducted for more than five years, MAOM-C concentrations tended to decrease with eCO2123. This pattern suggests that the effects of eCO2 may not be immediately apparent, but could become more evident over time as the diminishing soil nutrient pool prompts plants and microbes to begin mining resources from MAOM. Moreover, the biotic demand by plants and their associated fungi may also mediate how elevated CO2 affects MAOM pools. In a synthesis of 19 CO2 enrichment experiments, MAOM pools decreased in plots where the dominant plants associated with ECM fungi but increased in plots where the dominant plants associated with AM fungi129. This suggests that MAOM vulnerability may depend, in part, on the nutrient requirements and nutrient acquisition capabilities of the plants and their associated microbes.
Land use change and agricultural management
In general, land management that alters the input and microbial processing of OM will influence MAOM cycling130. Agricultural management systems may shift disturbance regimes, external inputs, and plant diversity, as well as the abiotic environment (e.g. temperature and moisture), with both biotic and abiotic drivers influencing MAOM120,131,132. For example, the physical disruption of tillage and changes in irrigation can disproportionately impact the MAOM protected within macro-aggregates133. MAOM turnover also depends on the C:N ratio and chemical composition of organic inputs. For example, a clover-rye mixed cover crop facilitated transfer of POM-C to MAOM-C more than a rye or clover crop alone134. Manure additions have been linked to faster MAOM turnover times than synthetic inputs135, perhaps because inorganic fertilizer applications may suppress the biological mechanisms that mobilize MAOM136.
MAOM response to management depends on both MAOM formation and loss; management that increases soil microbial activity can also enhance MAOM mineralization. The balance between stabilization and destabilization processes is heavily context-dependent and may result in either increases or decreases in MAOM21. For example, in a study on intensively managed Mollisols, incorporating legume cover crops and adding manure did not result in increased MAOM, as was hypothesized137. The researchers attributed this finding to nutrient mining of MAOM by the maize crop, which offset potential gains in MAOM due to enhanced microbial efficiency and biomass production. Similarly, long-term organic fertilization increased MAOM-C turnover, but not accrual135. Organic cropping with manure on an Alfisol also did not increase MAOM138, but adding a legume cover crop did increase MAOM in an arid wheat cropping systems on silt loam soils139. These studies highlight how measuring net changes overlooks active-cycling MAOM, which may be a large flux in systems that experience high rates of both MAOM loss and accrual. This fast-cycling MAOM may prove to be important in predicting N available to crops, as N mineralization remains an important, and poorly predicted, element of crop yield136,140,141,142.
In some cases, management may not alter the quantity of POM or MAOM but rather cause shifts in the chemical composition of one or both fractions. For example, MAOM chemical composition shifted in response to contrasting cover crop functional types143 and in organic cropping systems with cover crops and manure138, even though MAOM-C concentration was not affected. Similarly, in a system shifting from tropical savanna to pasture, losses of savanna-derived MAOM-C were offset by gains from pasture-derived C144. These shifts in MAOM chemical composition or quantity may correspond with faster-cycling MAOM, but it is uncertain how to measure or manage plant access to this nutrient supply.
Implications of the multi-pool and dynamic nature of MAOM
The implications of a fast-cycling MAOM pool for both agricultural management and representation within Earth system models have largely gone unexplored. Below, we discuss the relevance of fast-cycling MAOM for these applications and highlight key research opportunities to improve our understanding and measurement of this MAOM pool’s importance.
Managing fast-cycling MAOM in managed landscapes
While some management practices may destabilize MAOM, there are other practices that may be harnessed to promote fast-cycling MAOM for multiple benefits such as soil nutrient availability and accrual of soil C145. This perspective aligns with recent calls for more holistic stewardship of SOM that supports all forms —not only the stable forms—and accounts for the continuous flow of nutrients into and out of SOM146. In this context, Daly et al. outlined several key hypotheses about the potential role of the fast-cycling MAOM pool that require further testing136. First, fast-cycling MAOM can provide key nutrients for crops, particularly in low input systems that promote plant investment in root production and mycorrhizal symbioses136. Furthermore, fast-cycling MAOM may play a key role in the accumulation of more persistent SOC. Movement of compounds into and out of the fast-cycling MAOM fraction could help equilibrate the concurrent, though seemingly opposed, processes of SOM accrual and SOM mobilization in soils. Fast-cycling MAOM fraction could briefly stabilize soluble C and N compounds during periods when their concentrations are high, thereby acting as a temporary buffer against losses of C and N via leaching, microbial respiration, and transformations like denitrification. For example, in agroecosystems, the rapid, early season movement of inorganic N fertilizers into microbial biomass and MAOM may enhance its availability later, during periods of peak crop growth. When soluble SOM pools are low, desorption and decomposition of fast-cycling MAOM could supply plants and microbes with energy and nutrients and thus may regulate short-term nutrient availability. Over time, some fast-cycling MAOM may form stronger bonds with mineral surfaces or be incorporated into larger SOM complexes like aggregates, thereby transforming it to slower-cycling MAOM. This pipeline of loss-prone dissolved compounds moving through fast-cycling MAOM into persistent SOM may be key to increasing retention of C, N, and other nutrients in the soil system.
Ultimately, the ecosystem and management implications of fast-cycling MAOM depend on the fate(s) of MAOM following desorption. Fast-cycling MAOM may be functionally similar to POM, but the lower C:N ratio of MAOM suggests that it should be utilized relatively efficiently by microbes, resulting in less CO2 losses and greater C and N incorporation into microbial biomass and metabolites compared to POM. This cycle of SOM temporarily sorbing to mineral surfaces, undergoing microbial consumption and transformation, and re-release into soil solution as DOM, has been suggested to result in the cascade theory of SOM downward translocation147. This theory is supported by the observation of relatively older, more microbially-processed SOM in deeper soil horizons, as well as by empirical observations of DOM chemical composition changes during downward transport through grassland soil profiles using ultrahigh-resolution mass spectrometry148. Specific surface soil management strategies promoting SOC transport to, and accumulation within, deeper soils remain a critical knowledge gap149, and fast cycling MAOM may be key to this process. Research focusing specifically on MAOM dynamics across soil depths could help clarify our understanding of the fate and importance of fast-cycling MAOM in long-term C storage.
Integrating fast-cycling MAOM into models of soil C and N dynamics
Earth system models typically conceptualize soil C as multiple pools that decompose via first-order decay kinetics and differ in their turnover times150. In the last decade, models that explicitly include nonlinear microbial-mineral interactions are becoming more prevalent but vary widely in their process representations and parameterizations151,152,153,154. Mechanisms of stabilization vary from DOC sorption only155,156 to accumulation of microbial necromass154,157 or chemically recalcitrant compounds128 to a combination of the two158,159,160,161. As a result of these different model formulations, the predicted temporal dynamics of SOM, and the underlying pools conceptualized as MAOM can vary widely in response to disturbance151,162.
The vast majority of SOM models, including all models used at global scales, assume that modeled pools are homogenous and that any particle within a pool has an equal probability of entering or leaving as its neighbor150. Within this framework, turnover time and age is estimated as a single value for each pool, such that representing MAOM of varying ages would require increasing the number of pools and defining specific stabilization mechanisms or a combination of mechanisms for each pool. However, when MAOM is explicitly represented in SOM models, it is most often defined as a single pool155,156,158,160. Moreover, while clay content or soil texture may partly represent organo-mineral interactions159, this oversimplifies the spectrum of reactivity across clay types as well as secondary rock-derived minerals such as iron and aluminum oxides163. Multiple SOM models were found to overestimate MAOM turnover154. Further dividing the MAOM pool may better represent components with different turnover times154.
Select models do include a second MAOM pool that is considered faster cycling – for example, the exchangeable MAOM pool in the MEMS v2 model164, the Q pool in the MEND model161, and the Q_DOM pool in the COMISSION model159, which all represent DOC sorbed to minerals that can readily exchange with DOC in solution. Moreover, fast-cycling MAOM is expected to interact with minerals in ways that differ from more persistent MAOM, which suggests potential benefits of separately representing these pools. However, parameterizing sorption/desorption rates and specific mineral relationships for these distinct MAOM pools, and benchmarking their relative sizes, remains a challenge due to data limitations especially at larger scales. Alternatively, other modeling frameworks exist that focus on a continuum of individual particles traveling through model pools, with the ability to estimate the distribution of ages and transit times within a single pool56,165,166. Models can also represent the fast-cycling MAOM pool by allowing for MAOM desorption at relatively high rates. For instance, a study using the SOMic model, which represents microbial dynamics and allows for rapid MAOM desorption, found that this modeling framework generated predictions of global SOC distribution and long-term SOC dynamics that were closely aligned with empirical data167. Enhanced representation of mineral composition may improve predictions of fast-cycling MAOM. Although data on soil minerology exists at global scales, understanding of how specific mineral interactions affect MAOM cycling rates is still emerging168,169. Regardless of the modeling framework, the existence of fast-cycling MAOM suggests that modeling MAOM as a single homogenous, passively cycling SOM pool is likely insufficient for accurately capturing temporal dynamics.
Measurement of fast-cycling MAOM
Although SOM fractionation methods drive our operational and conceptual understanding of POM and MAOM pools, we lack a standard method for directly quantifying the fast-cycling MAOM pool. MAOM can be further separated along physical (density, particle size) and chemical (solubility in acid or base, oxidation, thermal lability) gradients, which may, in theory, be able to isolate a more bioavailable subfraction. However, these methods also often require further dispersion and disruption that may facilitate the inter-fraction transfer of C and N. Common procedures for isolating SOM fractions may remove or obscure the fast-cycling portion of MAOM. Fractionation procedures require a degree of mixing and dispersion in liquid that will release and redistribute DOM between POM and MAOM. The quantity of DOM leached during fractionation can be significant170 and it is currently not possible to distinguish between POM or MAOM in leached compounds. MAOM that desorbs and enters dissolved pools during fractionation may be an important component of fast-cycling MAOM. More studies are needed to identify the extent of leaching under contrasting fractionation and dispersion conditions as it is possible that certain methods favor the transfer of POM C or N into the MAOM pool, potentially inflating the stabilization capacity of this fraction.
Sequential chemical extractions can isolate phases of MAOM that differ in solubility and potentially bioavailability. Selective dissolutions with Na-pyrophosphate, hydroxylamine, and dithionite-HCl can be used to target organo-metal complexes, short-range order Al and Fe hydroxides, and crystalline Fe hydroxides, respectively171. These well-stabilized forms of MAOM are still vulnerable to loss, such as the iron-associated C that is destabilized and released by low molecular weight organic acids or under saturated conditions116,172. Physical and chemical fractionation methods can also be combined with thermal and/or chemometric approaches to estimate the chemical and biological stability of particle size fractions of SOM173. Otherwise, a combined chemical and biological assay is a potentially promising method for simulating destabilizing agents and the conditions that accelerate MAOM cycling. For example, by incubating isolated MAOM fractions with organic acids and glucose to simulate root exudates, one can estimate the destabilization potential of MAOM172,174. However, this has not been examined comprehensively across soil types and experimental conditions to generate a standard method.
Radiocarbon and 13C-based experiments can provide estimates of pool turnover times. However, these methods provide an average turnover time, which may obscure the smaller, faster cycling subfraction, unless paired with ramped thermal analysis techniques that can quantify an underlying distribution of turnover times. MAOM generally has a wide distribution of radiocarbon mean ages, with considerable overlap with the distribution for POM9,113. Measurements of CO2 respiration in long-term incubations can be used to calculate the size and turnover rate of active and slow pools of C175 although calculated residence times may vary based on experiment duration176 and these incubations are unable to determine the origin of the CO2. Isotope tracer-based experiments in which POM and MAOM fractions are incubated separately or differentially labelled would allow for the tracing of fast-cycling MAOM and provide critical, mechanistic insights into the potential size of and controls on this pool. If using stable isotope labelling to target MAOM subfractions, researchers should ensure that newly-formed MAOM reflects both abiotic and biotic pathways of stabilization (i.e., not relying exclusively on short-term, adsorption-based approaches to labelling MAOM, which favor abiotic pathways). In general, future work should identify a consistent method and guidance for how to pair fractionation methods with isotope tracer and/or spectroscopic/imaging methods to isolate the various MAOM subfractions.
Conclusion
For decades, the soil science community has acknowledged the existence of fast-cycling MAOM. Although the majority of MAOM is highly persistent and cycles on decadal and millennial time-scales, a portion is bioavailable, exchangeable, and an active contributor to C and N fluxes. As we have summarized, the size of this pool will likely depend on the intrinsic properties of the MAOM – namely, the availability and physicochemical properties of minerals as well as the composition, quantity, and structure of the associated OM. In addition, fast-cycling MAOM will likely respond readily to drivers of destabilization, such as plant-microbe interactions, climate change, agricultural intensification, and land use change. Therefore, clarifying its size and responses in varying ecological scenarios is important to improve model predictions and make better recommendations for land managers. While counterintuitive to the goals of soil C sequestration, within certain contexts, especially agroecosystems that could benefit from MAOM's potential to supply N, it may be beneficial to promote the existence of a fast-cycling MAOM pool146. Accurate quantification of this pool and its role in ecological processes will enhance our understanding of these contexts and could inform agricultural management approaches. Likewise, our previous understanding of MAOM as a persistent, slowly-cycling pool is clearly oversimplified, and including more realistic representations of fast-cycling MAOM in Earth system models is needed to accurately quantify both the current and future size of this SOM pool. These applications are currently hindered by methodological limitations that have made it challenging to characterize this dynamic pool of MAOM. Continued development of methods to isolate and trace fast-cycling MAOM will enable better quantification of this pool and subsequent integration of these insights into modeling and soil management practices.
References
Grandy, A. S. & Neff, J. C. Molecular C dynamics downstream: The biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci. Total Environ. 404, 297–307 (2008).
Heckman, K. et al. Beyond bulk: Density fractions explain heterogeneity in global soil carbon abundance and persistence. Glob. Change Biol. 28, 1178–1196 (2022).
Sokol, N. W. et al. Global distribution, formation and fate of mineral-associated soil organic matter under a changing climate: A trait-based perspective. Funct. Ecol. 36, 1411–1429 (2022).
Torn, M. S., Trumbore, S. E., Chadwick, O. A., Vitousek, P. M. & Hendricks, D. M. Mineral control of soil organic carbon storage and turnover. Nature 389, 170–173 (1997).
Bu, R. et al. Particulate organic matter affects soil nitrogen mineralization under two crop rotation systems. PLoS ONE 10, e0143835 (2015).
Angst, G. et al. Unlocking complex soil systems as carbon sinks: multi-pool management as the key. Nat. Commun. 14, 2967 (2023).
Chenu, C. & Plante, A. F. Clay-sized organo-mineral complexes in a cultivation chronosequence: revisiting the concept of the ‘primary organo-mineral complex’. Eur. J. Soil Sci. 57, 596–607 (2006).
Cotrufo, F. M. et al. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. https://doi.org/10.1038/NGEO2520 (2015).
Cotrufo, M. F. & Lavallee, J. M. Chapter One - Soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration. in Advances in Agronomy (ed. Sparks, D. L.) 172, 1–66 (Academic Press, 2022).
Totsche, K. U. et al. Microaggregates in soils. J. Plant Nutr. Soil Sci. 181, 104–136 (2018).
Even, R. J. & Francesca Cotrufo, M. The ability of soils to aggregate, more than the state of aggregation, promotes protected soil organic matter formation. Geoderma 442, 116760 (2024).
Sokol, N. W., Sanderman, J. & Bradford, M. A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Change Biol. 25, 12–24 (2019).
Poirier, V. et al. Organo-Mineral Interactions Are More Important for Organic Matter Retention in Subsoil Than Topsoil. Soil Syst 4, 4 (2020).
Haddix, M. L., Paul, E. A. & Cotrufo, M. F. Dual, differential isotope labeling shows the preferential movement of labile plant constituents into mineral-bonded soil organic matter. Glob. Change Biol. 22, 2301–2312 (2016).
Fulton-Smith, S., Even, R. & Cotrufo, M. F. Depth impacts on the aggregate-mediated mechanisms of root carbon stabilization in soil: Trade-off between MAOM and POM pathways. Geoderma 452, 117078 (2024).
Shabtai, I. A. et al. Calcium promotes persistent soil organic matter by altering microbial transformation of plant litter. Nat. Commun. 14, 6609 (2023).
Angst, G. et al. Soil organic carbon stocks in topsoil and subsoil controlled by parent material, carbon input in the rhizosphere, and microbial-derived compounds. Soil Biol. Biochem. 122, 19–30 (2018).
Barré, P., Fernandez-Ugalde, O., Virto, I., Velde, B. & Chenu, C. Impact of phyllosilicate mineralogy on organic carbon stabilization in soils: incomplete knowledge and exciting prospects. Geoderma 235–236, 382–395 (2014).
King, A. E. & Sokol, N. W. Soil carbon formation is promoted by saturation deficit and existing mineral-associated carbon, not by microbial carbon-use efficiency. Sci. Adv. 11, eadv9482 (2025).
Georgiou, K. et al. Global stocks and capacity of mineral-associated soil organic carbon. Nat. Commun. 13, 3797 (2022).
Bailey, V. L., Pries, C. H. & Lajtha, K. What do we know about soil carbon destabilization?. Environ. Res. Lett. 14, 083004 (2019).
Dynarski, K. A., Bossio, D. A. & Scow, K. M. Dynamic Stability of Soil Carbon: Reassessing the “Permanence” of Soil Carbon Sequestration. Front. Environ. Sci. 8, (2020).
Piñeiro, G., Paruelo, J. M., Jobbágy, E., Jackson, R. B. & Oesterheld, M. Grazing effects on belowground C and N stocks along a network of cattle exclosures in temperate and subtropical grasslands of South America. Glob. Biogeochem. Cycles 23, (2009).
Woodmansee, R. G. & Duncan, D. A. Nitrogen and phosphorus dynamics and budgets in annual grasslands. Ecology 61, 893–904 (1980).
Kleber, M., Sollins, P. & Sutton, R. A conceptual model of organo-mineral interactions in soils: self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 85, 9–24 (2007).
Torn, M. S. et al. A dual isotope approach to isolate soil carbon pools of different turnover times. Biogeosciences 10, 8067–8081 (2013).
Kleber, M. et al. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2, 402–421 (2021).
Bölscher, T. et al. Vulnerability of mineral-organic associations in the rhizosphere. Nat. Commun. 16, 5527 (2025).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. https://doi.org/10.1111/gcb.14859 (2019).
Kleber, M. et al. Mineral-organic associations: formation, properties, and relevance in soil environments. Adv. Agron. 130, 1–140 (2015).
Mikutta, R. et al. Biogeochemistry of mineral-organic associations across a long-term mineralogical soil gradient (0.3-4100 kyr), Hawaiian Islands. Geochim. Cosmochim. Acta 73, 2034–2060 (2009).
Sanderman, J., Maddern, T. & Baldock, J. Similar composition but differential stability of mineral retained organic matter across four classes of clay minerals. Biogeochemistry 121, 409–424 (2014).
Underwood, T. R., Bourg, I. C. & Rosso, K. M. Mineral-associated organic matter is heterogeneous and structured by hydrophobic, charged, and polar interactions. Proc. Natl. Acad. Sci. 121, e2413216121 (2024).
Kögel-Knabner, I. et al. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 171, 61–82 (2008).
Scheidegger, A. M. & Sparks, D. L. A critical assessment of sorption-desorption mechanisms at the soil mineral/water interface. Soil Sci 161, 813 (1996).
Bramble, D. S. E. et al. Formation of mineral-associated organic matter in temperate soils is primarily controlled by mineral type and modified by land use and management intensity. Glob. Change Biol. 30, e17024 (2024).
Deng, Y. & Dixon, J. B. Soil Organic Matter and Organic-Mineral Interactions. in Soil Mineralogy with Environmental Applications 69–107 (John Wiley & Sons, Ltd, 2002). https://doi.org/10.2136/sssabookser7.c3.
Lv, J. et al. Molecular-Scale Investigation with ESI-FT-ICR-MS on Fractionation of Dissolved Organic Matter Induced by Adsorption on Iron Oxyhydroxides. Environ. Sci. Technol. 50, 2328–2336 (2016).
Oren, A. & Chefetz, B. Sorptive and Desorptive Fractionation of Dissolved Organic Matter by Mineral Soil Matrices. J. Environ. Qual. 41, 526–533 (2012).
Kramer, M. C., Sanderman, J., Chadwick, O. A., Chorover, J. & Vitousek, P. M. Long - term carbon storage through retention of dissolved aromatic acids by reactive particles in soil. Glob. Change Biol. 18, 2594–2605 (2012).
Rocci, K. S. et al. Impacts of nutrient addition on soil carbon and nitrogen stoichiometry and stability in globally-distributed grasslands. Biogeochemistry 159, 353–370 (2022).
Heckman, K. et al. Sorptive fractionation of organic matter and formation of organo-hydroxy-aluminum complexes during litter biodegradation in the presence of gibbsite. Geochim. Cosmochim. Acta 121, 667–683 (2013).
Inagaki, T. M. et al. Subsoil organo-mineral associations under contrasting climate conditions. Geochim. Cosmochim. Acta 270, 244–263 (2020).
Keiluweit, M. et al. Nano-scale investigation of the association of microbial nitrogen residues with iron (hydr)oxides in a forest soil O-horizon. Geochim. Cosmochim. Acta 95, 213–226 (2012).
Possinger, A. R. et al. Organo-mineral interactions and soil carbon mineralizability with variable saturation cycle frequency. Geoderma 375, 114483 (2020).
Rillig, M. C. & Caldwell, B. A. Role of proteins in soil carbon and nitrogen storage: Controls on persistence. Biogeochemistry 85, 25–44 (2007).
Spohn, M. Preferential adsorption of nitrogen- and phosphorus-containing organic compounds to minerals in soils: A review. Soil Biol. Biochem. 194, 109428 (2024).
Lehmann, J., Kinyangi, J. & Solomon, D. Organic matter stabilization in soil microaggregates: implications from spatial heterogeneity of organic carbon contents and carbon forms. Biogeochemistry 85, 45–57 (2007).
Kinyangi, J. et al. Nanoscale Biogeocomplexity of the Organomineral Assemblage in Soil. Soil Sci. Soc. Am. J. 70, 1708–1718 (2006).
Lehmann, J. et al. Spatial complexity of soil organic matter forms at nanometre scales. Nat. Geosci 1, 238–242 (2008).
Steffens, M. et al. Identification of Distinct Functional Microstructural Domains Controlling C Storage in Soil. Environ. Sci. Technol. 51, 12182–12189 (2017).
Vogel, C. et al. Submicron structures provide preferential spots for carbon and nitrogen sequestration in soils. Nat. Commun. 5, 2947 (2014).
Kaiser, K. & Guggenberger, G. Mineral surfaces and soil organic matter. Eur J Soil Sci 54, 219–236 (2003).
Kaiser, K. & Guggenberger, G. Sorptive stabilization of organic matter by microporous goethite: sorption into small pores vs. surface complexation. Eur. J. Soil Sci. 58, 45–59 (2007).
Coward, E. K., Ohno, T. & Sparks, D. L. Direct Evidence for Temporal Molecular Fractionation of Dissolved Organic Matter at the Iron Oxyhydroxide Interface. Environ. Sci. Technol. 53, 642–650 (2019).
Sierra, C. A., Hoyt, A. M., He, Y. & Trumbore, S. E. Soil Organic Matter Persistence as a Stochastic Process: Age and Transit Time Distributions of Carbon in Soils. Glob. Biogeochem. Cycles 32, 1574–1588 (2018).
Feng, W. et al. Methodological uncertainty in estimating carbon turnover times of soil fractions. Soil Biol. Biochem. 100, 118–124 (2016).
Hall, S. J., McNicol, G., Natake, T. & Silver, W. L. Large fluxes and rapid turnover of mineral-associated carbon across topographic gradients in a humid tropical forest: insights from paired 14C analysis. Biogeosciences 12, 2471–2487 (2015).
Schrumpf, M. & Kaiser, K. Large differences in estimates of soil organic carbon turnover in density fractions by using single and repeated radiocarbon inventories. Geoderma 239–240, 168–178 (2015).
Heckman, K. A. et al. Soil organic matter is principally root derived in an Ultisol under oak forest. Geoderma 403, 115385 (2021).
Fox, P. M. et al. Shale as a Source of Organic Carbon in Floodplain Sediments of a Mountainous Watershed. J. Geophys. Res. Biogeosciences 125, e2019JG005419 (2020).
Grant, K. E. et al. Diverse organic carbon dynamics captured by radiocarbon analysis of distinct compound classes in a grassland soil. Biogeosciences 21, 4395–4411 (2024).
Schrumpf, M. et al. Storage and stability of organic carbon in soils as related to depth, occlusion within aggregates, and attachment to minerals. Biogeosciences 10, 1675–1691 (2013).
Stoner, S. et al. Relating mineral–organic matter stabilization mechanisms to carbon quality and age distributions using ramped thermal analysis. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 381, 20230139 (2023).
Allison, S. D. & Jastrow, J. D. Activities of extracellular enzymes in physically isolated fractions of restored grassland soils. Soil Biol. Biochem. 38, 3245–3256 (2006).
Breulmann, M. et al. Short-term bioavailability of carbon in soil organic matter fractions of different particle sizes and densities in grassland ecosystems. Sci. Total Environ. 497–498, 29–37 (2014).
Kandeler, E. et al. The mineralo-sphere – Succession and physiology of bacteria and fungi colonising pristine minerals in grassland soils under different land-use intensities. Soil Biol. Biochem. 136, 107534 (2019).
Jilling, A. et al. Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry https://doi.org/10.1007/s10533-018-0459-5 (2018).
Yu, W., Huang, W., Weintraub-Leff, S. R. & Hall, S. J. Where and why do particulate organic matter (POM) and mineral-associated organic matter (MAOM) differ among diverse soils?. Soil Biol. Biochem. 172, 108756 (2022).
Bailey, T. et al. Opposing patterns of carbon and nitrogen stability in soil organic matter fractions compared to whole soil. Eur. J. Soil Sci. 75, e13495 (2024).
Bimüller, C. et al. Decoupled carbon and nitrogen mineralization in soil particle size fractions of a forest topsoil. Soil Biol. Biochem. 78, 263–273 (2014).
Mueller, C. W. et al. Bioavailability and isotopic composition of CO2 released from incubated soil organic matter fractions. Soil Biol. Biochem. 69, 168–178 (2014).
Parfitt, R. L., Salt, G. J. Carbon and nitrogen mineralisation in sand, silt, and clay fractions of soils under maize and pasture. Aust. J. Soil Res. Aust. J. Soil Res. 39, 361–371 (2001).
Sollins, P., Spycher, G. & Glassman, C. A. Net nitrogen mineralization from light- and heavy-fraction forest soil organic matter. Soil Biol. Biochem. 16, 31–37 (1984).
Whalen, J. K., Bottomley, P. J. & Myrold, D. D. Carbon and nitrogen mineralization from light- and heavy-fraction additions to soil. Soil Biol. Biochem. 32, 1345–1352 (2000).
Villarino, S. H. et al. A large nitrogen supply from the stable mineral-associated soil organic matter fraction. Biol. Fertil. Soils 59, 833–841 (2023).
Abramoff, R. Z. et al. How much carbon can be added to soil by sorption?. Biogeochemistry 152, 127–142 (2021).
Neurath, R. A. et al. Root Carbon Interaction with Soil Minerals Is Dynamic, Leaving a Legacy of Microbially Derived Residues. Environ. Sci. Technol. 55, 13345–13355 (2021).
Shabtai, I. A. et al. Root exudates simultaneously form and disrupt soil organo-mineral associations. Commun. Earth Environ. 5, 1–12 (2024).
Chari, N. R. & Taylor, B. N. Soil organic matter formation and loss are mediated by root exudates in a temperate forest. Nat. Geosci. 15, 1011–1016 (2022).
Kaštovská, E. et al. Root but not shoot litter fostered the formation of mineral-associated organic matter in eroded arable soils. Soil Tillage Res 235, 105871 (2024).
Sokol, N. W. et al. The path from root input to mineral-associated soil carbon is dictated by habitat-specific microbial traits and soil moisture. Soil Biol. Biochem. 193, 109367 (2024).
Sokol, N. W. & Bradford, M. A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 12, 46–53 (2019).
Villarino, S. H., Pinto, P., Jackson, R. B. & Piñeiro, G. Plant rhizodeposition: A key factor for soil organic matter formation in stable fractions. Sci. Adv. 7, eabd3176 (2021).
Bélanger, N., C^oté, B., Fyles, J. W., Courchesne, F. & Hendershot, W. H. Forest regrowth as the controlling factor of soil nutrient availability 75 years after fire in a deciduous forest of Southern Quebec. Plant Soil 262, 363–272 (2004).
Lovett, G. M. et al. Nutrient retention during ecosystem succession: a revised conceptual model. Front. Ecol. Environ. 16, 532–538 (2018).
Phillips, R. P., Bernhardt, E. S. & Schlesinger, W. H. Elevated CO2 increases root exudation from loblolly pine (Pinus taeda) seedlings as an N-mediated response. Tree Physiol 29, 1513–1523 (2009).
Heckman, K. A. et al. Moisture-driven divergence in mineral-associated soil carbon persistence. Proc. Natl. Acad. Sci. 120, e2210044120 (2023).
Seguin, V. et al. Mineral weathering in the rhizosphere of forested soils. in Biogeochemistry of Trace Elements in the Rhizpshere 22–55 (2005).
Hinsinger, P. & Jaillard, B. Root-induced release of interlayer potassium and vermiculitization of phlogopite as related to potassium depletion in the rhizosphere of ryegrass. J. Soil Sci. 44, 525–534 (1993).
Uroz, S. et al. Bacterial weathering and its contribution to nutrient cycling in temperate forest ecosystems. Res. Microbiol. 162, 821–831 (2011).
Zhang, Z., Huang, J., He, L. & Sheng, X. Distinct Weathering Ability and Populations of Culturable Mineral-Weathering Bacteria in the Rhizosphere and Bulk Soils of Morus Alba. Geomicrobiol. J. 33, 39–45 (2016).
Chabot, R., Antoun, H. & Cescas, M. P. Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar. phaseoli. Plant Soil 39, 311–321 (1996).
Vazquez, P., Holguin, G., Puente, M. E., Lopez-Cortes, A. & Bashan, Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertil. Soils 30, 460–468 (2000).
Calvaruso, C., Mareschal, L., Turpault, M.-P. & Leclerc, E. Rapid clay weathering in the rhizosphere of norway spruce and oak in an acid forest ecosystem. Soil Sci. Soc. Am. J. 73, 331 (2009).
Mareschal, L., Turpault, M.-P., Bonnaud, P. & Ranger, J. Relationship between the weathering of clay minerals and the nitrification rate: a rapid tree species effect. Biogeochemistry 112, 293–309 (2013).
Paola, A., Pierre, B., Vincenza, C., Vincenzo, D. M. & Bruce, V. Short term clay mineral release and re-capture of potassium in a Zea mays field experiment. Geoderma 264, 54–60 (2016).
Tunlid, A., Floudas, D., Op De Beeck, M., Wang, T. & Persson, P. Decomposition of soil organic matter by ectomycorrhizal fungi: Mechanisms and consequences for organic nitrogen uptake and soil carbon stabilization. Front. For. Glob. Change 5, (2022).
Keiluweit, M. et al. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 5, 588–595 (2015).
Bonneville, S. et al. Tree-mycorrhiza symbiosis accelerate mineral weathering: Evidences from nanometer-scale elemental fluxes at the hypha–mineral interface. Geochim. Cosmochim. Acta 75, 6988–7005 (2011).
Wang, T., Persson, P. & Tunlid, A. A widespread mechanism in ectomycorrhizal fungi to access nitrogen from mineral-associated proteins. Environ. Microbiol. 23, 5837–5849 (2021).
Krumina, L., Op De Beeck, M., Meklesh, V., Tunlid, A. & Persson, P. Ectomycorrhizal Fungal Transformation of Dissolved Organic Matter: Consequences for Reductive Iron Oxide Dissolution and Fenton-Based Oxidation of Mineral-Associated Organic Matter. Front. Earth Sci. 10, (2022).
Liu, F. et al. Production of reactive oxygen species and its role in mediating the abiotic transformation of organic carbon in sandy soil under vegetation restoration. Carbon Res 2, 35 (2023).
Verbruggen, E., Struyf, E. & Vicca, S. Can arbuscular mycorrhizal fungi speed up carbon sequestration by enhanced weathering?. PLANTS PEOPLE PLANET 3, 445–453 (2021).
Wu, S. et al. Soil organic matter dynamics mediated by arbuscular mycorrhizal fungi – an updated conceptual framework. New Phytol 242, 1417–1425 (2024).
Tisserant, E. et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. 110, 20117–20122 (2013).
Cao, T. et al. Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 410, 115662 (2022).
Rozmoš, M. et al. Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. ISME J 16, 676–685 (2022).
Andrino, A. et al. Production of Organic Acids by Arbuscular Mycorrhizal Fungi and Their Contribution in the Mobilization of Phosphorus Bound to Iron Oxides. Front. Plant Sci. 12, (2021).
King, A. E. et al. A soil matrix capacity index to predict mineral-associated but not particulate organic carbon across a range of climate and soil pH. Biogeochemistry 165, 1–14 (2023).
Kramer, M. G. & Chadwick, O. A. Climate-driven thresholds in reactive mineral retention of soil carbon at the global scale. Nat. Clim. Change 8, 1104–1108 (2018).
Possinger, A. R. et al. Climate Effects on Subsoil Carbon Loss Mediated by Soil Chemistry. Environ. Sci. Technol. 55, 16224–16235 (2021).
Rasmussen, C. et al. Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 137, 297–306 (2018).
Adhikari, D. et al. Dynamics of ferrihydrite-bound organic carbon during microbial Fe reduction. Geochim. Cosmochim. Acta 212, 221–233 (2017).
Huang, W. & Hall, S. J. Elevated moisture stimulates carbon loss from mineral soils by releasing protected organic matter. Nat. Commun. 8, 1774 (2017).
Patzner, M. S. et al. Iron mineral dissolution releases iron and associated organic carbon during permafrost thaw. Nat. Commun. 11, 6329 (2020).
Zhao, Q. et al. Coupled dynamics of iron and iron-bound organic carbon in forest soils during anaerobic reduction. Chem. Geol. 464, 118–126 (2017).
Zhao, Q. et al. Oxidation of soil organic carbon during an anoxic-oxic transition. Geoderma 377, 114584 (2020).
Lieberman, H. P., Rothman, M., von Sperber, C. & Kallenbach, C. M. Experimental flooding shifts carbon, nitrogen, and phosphorus pool distribution and microbial activity. Biogeochemistry 165, 75–90 (2023).
Jilling, A., Sokol, N. W., Morán-Rivera, K. & Stuart Grandy, A. Wet-dry cycling influences the formation of mineral-associated organic matter and its sensitivity to simulated root exudates. Geoderma 445, 116869 (2024).
von, Lützow, M. & Kögel-Knabner, I. Temperature sensitivity of soil organic matter decomposition—what do we know?. Biol. Fertil. Soils 46, 1–15 (2009).
Georgiou, K. et al. Emergent temperature sensitivity of soil organic carbon driven by mineral associations. Nat. Geosci. 17, 205–212 (2024).
Rocci, K. S., Lavallee, J. M., Stewart, C. E. & Cotrufo, M. F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 793, 148569 (2021).
He, N., Chen, Q., Han, X., Yu, G. & Li, L. Warming and increased precipitation individually influence soil carbon sequestration of Inner Mongolian grasslands. China. Agric. Ecosyst. Environ. 158, 184–191 (2012).
Phillips, C. L., Murphey, V., Lajtha, K. & Gregg, J. W. Asymmetric and symmetric warming increases turnover of litter and unprotected soil C in grassland mesocosms. Biogeochemistry 128, 217–231 (2016).
Luo, Y. et al. Progressive Nitrogen Limitation of Ecosystem Responses to Rising Atmospheric Carbon Dioxide. BioScience 54, 731–739 (2004).
Rocci, K. S. et al. Bridging 20 Years of Soil Organic Matter Frameworks: Empirical Support, Model Representation, and Next Steps. J. Geophys. Res. Biogeosciences 129, e2023JG007964 (2024).
Sulman, B. N., Phillips, R. P., Oishi, A. C., Shevliakova, E. & Pacala, S. W. Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Change 4, 1099–1102 (2014).
Terrer, C. et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 591, 599–603 (2021).
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K. & Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter?. Glob. Change Biol. 19, 988–995 (2013).
Cates, A. M., Jilling, A., Tfaily, M. M. & Jackson, R. D. Temperature and moisture alter organic matter composition across soil fractions. Geoderma 409, 115628 (2022).
Leitner, Z. R., Daigh, A. L. M. & DeJong-Hughes, J. Temporal fluctuations of microbial communities within the crop growing season. Geoderma 391, 114951 (2021).
Fu, Z., Hu, W., Beare, M. & Baird, D. Soil macroaggregate-occluded mineral-associated organic carbon drives the response of soil organic carbon to land use change. Soil Tillage Res 244, 106271 (2024).
Connell, R. K., James, T. Y. & Blesh, J. A legume-grass cover crop builds mineral-associated organic matter across variable agricultural soils. Soil Biol. Biochem. 203, 109726 (2025).
Mayer, M. et al. Dynamic stability of mineral-associated organic matter: enhanced stability and turnover through organic fertilization in a temperate agricultural topsoil. Soil Biol. Biochem. 184, 109095 (2023).
Daly, A. B. et al. A holistic framework integrating plant-microbe-mineral regulation of soil bioavailable nitrogen. Biogeochemistry 154, 211–229 (2021).
Rui, Y. et al. Persistent soil carbon enhanced in Mollisols by well-managed grasslands but not annual grain or dairy forage cropping systems. Proc. Natl. Acad. Sci. 119, e2118931119 (2022).
Kauer, K., Pärnpuu, S., Talgre, L., Eremeev, V. & Luik, A. Soil Particulate and Mineral-Associated Organic Matter Increases in Organic Farming under Cover Cropping and Manure Addition. Agriculture 11, 903 (2021).
van der Pol, L. K. et al. Addressing the soil carbon dilemma: Legumes in intensified rotations regenerate soil carbon while maintaining yields in semi-arid dryland wheat farms. Agric. Ecosyst. Environ. 330, 107906 (2022).
Osterholz, W. R. et al. Predicting Gross Nitrogen Mineralization and Potentially Mineralizable Nitrogen using Soil Organic Matter Properties. Soil Sci. Soc. Am. J. 81, 1115–1126 (2017).
Spackman, J. A., Fernández, F. G., Paiao, G. D., Venterea, R. T. & Coulter, J. A. Corn 15N uptake and partitioning in response to fertilizer application rate and timing. Agron. J. 116, 1991–2006 (2024).
White, C. M., Finney, D. M., Kemanian, A. R. & Kaye, J. P. Modeling the contributions of nitrogen mineralization to yield of corn. Agron. J. 113, 490–503 (2021).
Zhang, Z., Kaye, J. P., Bradley, B. A., Amsili, J. P. & Suseela, V. Cover crop functional types differentially alter the content and composition of soil organic carbon in particulate and mineral-associated fractions. Glob. Change Biol. 28, 5831–5848 (2022).
Teixeira, R. et al. Land-use change with pasture and short rotation eucalypts impacts the soil C emissions and organic C stocks in the Cerrado biome. Land Degrad. Dev. 31, 909–923 (2020).
Grandy, A. S. et al. A microbial framework for nitrogen cycling solutions in agroecosystems. One Earth 7, 2103–2107 (2024).
Janzen, H. H. RUSSELL REVIEW Soil carbon stewardship: Thinking in circles. Eur. J. Soil Sci. 75, e13536 (2024).
Kaiser, K. & Kalbitz, K. Cycling downwards – dissolved organic matter in soils. Soil Biol. Biochem. 52, 29–32 (2012).
Roth, V.-N. et al. Persistence of dissolved organic matter explained by molecular changes during its passage through soil. Nat. Geosci. 12, 755–761 (2019).
Cagnarini, C. et al. Zones of influence for soil organic matter dynamics: A conceptual framework for data and models. Glob. Change Biol. 25, 3996–4007 (2019).
Todd-Brown, K. E. O. et al. Causes of variation in soil carbon simulations from CMIP5 Earth system models and comparison with observations. Biogeosciences 10, 1717–1736 (2013).
Sulman, B. N. et al. Multiple models and experiments underscore large uncertainty in soil carbon dynamics. Biogeochemistry 141, 109–123 (2018).
Todd-Brown, K. E. O., Hopkins, F. M., Kivlin, S. N., Talbot, J. M. & Allison, S. D. A framework for representing microbial decomposition in coupled climate models. Biogeochemistry 109, 19–33 (2012).
Treseder, K. K. et al. Integrating microbial ecology into ecosystem models: challenges and priorities. Biogeochemistry 109, 7–18 (2012).
Wieder, W. R., Grandy, A. S., Kallenbach, C. M. & Bonan, G. B. Integrating microbial physiology and physio-chemical principles in soils with the MIcrobial-MIneral Carbon Stabilization (MIMICS) model. Biogeosciences 11, 3899–3917 (2014).
Ahrens, B., Braakhekke, M. C., Guggenberger, G., Schrumpf, M. & Reichstein, M. Contribution of sorption, DOC transport and microbial interactions to the 14C age of a soil organic carbon profile: Insights from a calibrated process model. Soil Biol. Biochem. 88, 390–402 (2015).
Tang, J. & Riley, W. J. Weaker soil carbon–climate feedbacks resulting from microbial and abiotic interactions. Nat. Clim. Change 5, 56–60 (2015).
Wieder, W. R., Grandy, A. S., Kallenbach, C. M., Taylor, P. G. & Bonan, G. B. Representing life in the Earth system with soil microbial functional traits in the MIMICS model. Geosci. Model Dev. 8, 1789–1808 (2015).
Abramoff, R. et al. The Millennial model: in search of measurable pools and transformations for modeling soil carbon in the new century. Biogeochemistry 137, 51–71 (2018).
Ahrens, B. et al. Combination of energy limitation and sorption capacity explains 14C depth gradients. Soil Biol. Biochem. 148, 107912 (2020).
Robertson, A. D. et al. Unifying soil organic matter formation and persistence frameworks: the MEMS model. Biogeosciences 16, 1225–1248 (2019).
Wang, G., Post, W. M. & Mayes, M. A. Development of microbial-enzyme-mediated decomposition model parameters through steady-state and dynamic analyses. Ecol. Appl. 23, 255–272 (2013).
Wieder, W. R. et al. Simulating Global Terrestrial Carbon and Nitrogen Biogeochemical Cycles With Implicit and Explicit Representations of Soil Microbial Activity. J. Adv. Model. Earth Syst. 16, e2023MS004156 (2024).
Kirsten, M. et al. Iron oxides and aluminous clays selectively control soil carbon storage and stability in the humid tropics. Sci. Rep. 2021 111 11, 1–12 (2021).
Zhang, Y. et al. Simulating measurable ecosystem carbon and nitrogen dynamics with the mechanistically defined MEMS 2.0 model. Biogeosciences 18, 3147–3171 (2021).
Agren, G. I. & Bosatta, N. Theoretical Analysis of the Long-Term Dynamics of Carbon and Nitrogen in Soils. Ecology 68, 1181–1189 (1987).
Waring, B. G. et al. From pools to flow: The PROMISE framework for new insights on soil carbon cycling in a changing world. Glob. Change Biol. https://doi.org/10.1111/gcb.15365 (2020).
Woolf, D. & Lehmann, J. Microbial models with minimal mineral protection can explain long-term soil organic carbon persistence. Sci. Rep. 9, 6522 (2019).
Ito, A. & Wagai, R. Global distribution of clay-size minerals on land surface for biogeochemical and climatological studies. Sci. Data 4, 170103 (2017).
von Fromm, S. F. et al. Moisture and soil depth govern relationships between soil organic carbon and oxalate-extractable metals at the global scale. Biogeochemistry 168, 20 (2025).
Plaza, C., Giannetta, B., Benavente, I., Vischetti, C. & Zaccone, C. Density-based fractionation of soil organic matter: effects of heavy liquid and heavy fraction washing. Sci. Rep. 9, 10146 (2019).
Heckman, K., Lawrence, C. R. & Harden, J. W. A sequential selective dissolution method to quantify storage and stability of organic carbon associated with Al and Fe hydroxide phases. Geoderma 312, 24–35 (2018).
Li, H. et al. Simple Plant and Microbial Exudates Destabilize Mineral-Associated Organic Matter via Multiple Pathways. Environ. Sci. Technol. 55, 3389–3398 (2021).
Schiedung, M., Barré, P. & Peoplau, C. Separating fast from slow cycling soil organic carbon – A multi-method comparison on land use change sites. Geoderma 453, 117154 (2025).
Jilling, A., Keiluweit, M., Gutknecht, J. L. M. & Grandy, A. S. Priming mechanisms providing plants and microbes access to mineral-associated organic matter. Soil Biol. Biochem. 158, 108265 (2021).
Paul, E. A., Morris, S. J. & Böhm, S. The determination of soil C pool sizes and turnover rates: biophysical fractionation and tracers. in Assessment Methods for Soil Carbon 193–206 (CRC Press, 2000).
Paul, E. A., Morris, S. J., Conant, R. & Plante, A. F. Does the Acid Hydrolysis–Incubation Method Measure Meaningful Soil Organic Carbon Pools?. Soil Sci. Soc. Am. J. 70, 1023–1035 (2006).
Konrad, A. et al. Microbial carbon use efficiency of mineral-associated organic matter is related to its desorbability. Soil Biol. Biochem. 203, 109740 (2025).
Jagadamma, S., Steinweg, J. M., Mayes, M., Wang, G. & Post, W. Decomposition of added and native organic carbon from physically separated fractions of diverse soils. Biol. Fertil. Soils 1–9 https://doi.org/10.1007/s00374-013-0879-2 (2013).
Jones, D. L. & Edwards, A. C. Influence of sorption on the biological utilization of two simple carbon substrates. Soil Biol. Biochem. 30, 1895–1902 (1998).
McGhee, I., Sannino, F., Gianfreda, L. & Burns, R. G. Bioavailability of 2,4-D sorbed to a chlorite-like complex. Chemosphere 39, 285–291 (1999).
Mikutta, R. et al. Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochim. Cosmochim. Acta 71, 2569–2590 (2007).
Saidy, A. R., Smernik, R. J., Baldock, J. A., Kaiser, K. & Sanderman, J. Microbial degradation of organic carbon sorbed to phyllosilicate clays with and without hydrous iron oxide coating. Eur. J. Soil Sci. 66, 83–94 (2015).
Singh, N. et al. Bioavailability of an Organophosphorus Pesticide, Fenamiphos, Sorbed on an Organo Clay. J. Agric. Food Chem. 51, 2653–2658 (2003).
Gu, B., Schmitt, J., Chen, Z., Llang, L. & Mccarthyt, J. F. Adsorption and Desorption of Natural Organic Matter on Iron Oxide: Mechanisms and Models. Env. Sci Technol 28, 38–48 (1994).
Gu, B., Schmitt, J., Chen, Z., Liang, L. & McCarthy, J. F. Adsorption and desorption of different organic matter fractions on iron oxide. Geochim. Cosmochim. Acta 59, 219–229 (1995).
Joo, J. C., Shackelford, C. D. & Reardon, K. F. Association of humic acid with metal (hydr)oxide-coated sands at solid–water interfaces. J. Colloid Interface Sci. 317, 424–433 (2008).
Kahle, M., Kleber, M. & Jahn, R. Retention of dissolved organic matter by phyllosilicate and soil clay fractions in relation to mineral properties. Org. Geochem. 35, 269–276 (2004).
Kaiser, K. & Zech, W. Release of Natural Organic Matter Sorbed to Oxides and a Subsoil. Soil Sci. Soc. Am. J. 63, 1157–1166 (1999).
Navon, R., Hernandez-Ruiz, S., Chorover, J. & Chefetz, B. Interactions of Carbamazepine in Soil: Effects of Dissolved Organic Matter. J. Environ. Qual. 40, 942–948 (2011).
Saidy, A. R., Smernik, R. J., Baldock, J. A., Kaiser, K. & Sanderman, J. The sorption of organic carbon onto differing clay minerals in the presence and absence of hydrous iron oxide. Geoderma 209–210, 15–21 (2013).
Singh, M., Sarkar, B., Biswas, B., Churchman, J. & Bolan, N. S. Adsorption-desorption behavior of dissolved organic carbon by soil clay fractions of varying mineralogy. Geoderma 280, 47–56 (2016).
Singh, M. et al. Influence of physico-chemical properties of soil clay fractions on the retention of dissolved organic carbon. Environ. Geochem. Health 39, 1335–1350 (2017).
Yeasmin, S. et al. Influence of mineral characteristics on the retention of low molecular weight organic compounds: A batch sorption-desorption and ATR-FTIR study. J. Colloid Interface Sci. 432, 246–257 (2014).
Leuthold, S., Lavallee, J. M., Haddix, M. L. & Cotrufo, M. F. Contrasting properties of soil organic matter fractions isolated by different physical separation methodologies. Geoderma 445, 116870 (2024).
Acknowledgements
Work by A.J. was funded by the National Science Foundation award #2103187. The research at the University of New Hampshire was supported by NSF award #2103114. Work by R.H. was funded by the National Science Foundation Award #2103076. ARP was supported in part by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture. Work at LLNL was supported by LLNL-LDRD Program Project No. 24-LW-053 (KG) and by the U.S. Department of Energy, Office of Biological and Environmental Research, Genomic Science Program ‘Microbes Persist’ Scientific Focus Area, award #SCW1632 (KG, NS) and was performed under the auspices of DOE Contract DE-AC52-07NA27344. MK was supported through an NSF CAREER (EAR-2046284) and SNF Project Funding (No. 200021_213101). K.S.R. was supported by the U.S. National Science Foundation’s Macrosystem Biology and NEON-Enabled Science program grants DEB-1926482 and DEB-1926413 and the Biosciences Initiative at the University of Michigan. Work by RZA was supported in part by the Schmidt Futures Virtual Earth System Research Institute (VESRI) CALIPSO (Carbon Loss in Plants, Soils, and Oceans) Project. I.A.S. was supported by the Foundation for Food and Agriculture Research New Innovator Award (#23-000598). The findings and conclusions in this publication are those of the author(s) and should not be construed to represent any official USDA or U.S. Government determination or policy. R.P.P. was supported by the National Science Foundation’s Division of Environmental Biology (Award #2106096) – a highly-efficient division that’s used its modest budget to support visionary, transformative, interdisciplinary research for decades. We thank science illustrator Elena Hartley for the design of the figure (www.elabarts.com).
Author information
Authors and Affiliations
Contributions
Andrea Jilling: conceptualization, investigation, writing, and revision. A. Stuart Grandy: conceptualization, investigation, writing, and revision. Amanda B. Daly: conceptualization, investigation, writing, and revision. Rachel Hestrin: conceptualization, investigation, writing, and revision. Angela Possinger: conceptualization, investigation, writing, and revision. Rose Abramoff: investigation, writing, and revision. Madison Annis: investigation, writing. Anna M. Cates: investigation, writing. Katherine Dynarski: investigation, writing, and revision. Katerina Georgiou: investigation, writing, and revision. Katherine Heckman: investigation, writing, and revision. Marco Keiluweit: conceptualization, investigation, and revision. Ashley K. Lang: investigation, writing, and revision. Richard P. Phillips: investigation, writing, and revision. Katherine Rocci: investigation, writing, and revision. Itamar A. Shabtai: investigation, writing, and revision. Noah W. Sokol: investigation, writing, and revision. Em Whalen: writing and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth & Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Somaparna Ghosh. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jilling, A., Grandy, A.S., Daly, A.B. et al. Evidence for the existence and ecological relevance of fast-cycling mineral-associated organic matter. Commun Earth Environ 6, 690 (2025). https://doi.org/10.1038/s43247-025-02681-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43247-025-02681-8