Abstract
Ecosystem structure and biophysical processes mediate biological responses to climate changes, but few studies have examined impacts of this dynamic among upper trophic levels. We investigated ecosystem differences in how diverse seabird populations across the northern hemisphere have responded to changes in regional mixed layer temperature and water column stratification. Using 138 time series of breeding productivity over the past half-century, we show that seabird reproductive productivity has declined in the Arctic and North Atlantic but not in the Pacific during a period of ubiquitous mixed layer warming and regionally-variable stratification trends. Models of breeding productivity and ocean drivers show that seabird responses to climate change vary by ecosystem. Additionally, ecosystems in which seabirds exhibit detectibly declining productivity tend to have lower overall diet diversity across seabird species. These findings emphasize the importance of ecosystem processes and structure in determining the vulnerability of marine predators to climate change.
Similar content being viewed by others
Introduction
In marine systems, surface and subsurface waters have warmed substantially since the 1950s1, and the rate of warming is expected to increase over the coming decades1,2. Warming can generate thermal stratification of the water column, reinforced by surface freshening from enhanced sea-ice melt at high latitudes, resulting in stronger vertical density gradients and deepening of the mixed layer3,4. At macro- or ecosystem-scales (i.e., >1000 km) physical drivers of climate change have the potential to alter productivity-generating processes, which, via bottom-up controls, influence the productivity of upper trophic levels5,6. Extensive work has shown that warming and stratification affect primary productivity2 as well as the timing of peak phytoplankton blooming7 at ecosystem-scales. However, fewer studies have examined whether ecosystem-scale responses to climate change are detectable at top trophic levels. Quantifying responses of upper tropic levels over large spatial and temporal scale is considerably more difficult than for lower trophic levels (e.g., remote sensing of primary productivity). Upper trophic level responses also tend to be more closely linked to meso- or ecoregional scales (i.e., ~100–500 km)5,8, show more spatial variation in trends9, and diverge from patterns observed at low trophic levels10. However, if biotic responses to physical drivers of climate change are detectable among upper trophic levels at ecosystem-scales, particularly if occurring via bottom-up mechanisms, then this may indicate where and how climate change is effecting synoptic ecosystem change. Therefore, ecosystem-scale investigations of upper trophic level responses to climate change are necessary for understanding how environmental variability propagates up to predators. Such studies also help identify similarities and differences among ecosystems in biological responses to accelerating climate change, supporting resource prioritization for ecological conservation.
Seabirds, cetaceans, and pinnipeds, have been used as ‘elucidating sentinels’11 of ecosystem responses to climate change because they are wide-ranging and potentially integrate bottom-up signals at large spatial scales12,13. Seabirds are particularly useful sentinels in that they are easily observed while on colonies, and long-term time series are available across a wide range of ecosystems11,14,15,16. Consequently, several studies have successfully used seabirds to document global-scale phenological17 and demographic16 responses to environmental variability as well as prey availability18. Seabirds are long-lived, near-apex predators that rely on marine systems for at least part of their life cycle. Species vary markedly in their foraging strategies. Some pursue benthic or pelagic prey by diving after fish, macro-invertebrates, or plankton; while others scan the surface for prey, steal from other seabirds, or scavenge19. Maximum diving depths vary by species, and are related to body size, but diving seabirds typically forage within the top 200 m of the ocean20. Seabird foraging strategies tend to be either specialized, focusing on few prey species, or generalist, switching among alternate prey species based on factors like availability21.
Most seabirds reproduce in colonies, where they lay and incubate eggs and raise chicks in nests and burrows on coastlines and offshore islands. During the breeding period, seabirds tend to exhibit constricted foraging ranges because they must return to the colony to incubate eggs and provision their chicks. During this sensitive life stage, they are central-place foragers22, generally relying on prey resources within a 3–300 km radius around their colonies, though foraging ranges vary with body size, diet, and foraging strategies23. Reproductive performance, measured by breeding productivity (number of chicks fledged per female per year), may be directly tied to prey abundance and accessibility near colonies18,24 and is thus controlled by bottom-up processes. Breeding productivity is also indirectly tied to meso-scale (ecoregional) ocean conditions at annual to sub-annual time scales (seasonal, in mid to high latitude systems) to the extent that these conditions determine localized prey fields5,8. For instance, warming near colonies during and prior to breeding may alter the timing of localized prey availability, resulting in a mismatch between predators and prey, potentially limiting seabird breeding capacity17. Stratification of the water column near breeding colonies may also play an important role in determining reproductive output of resident seabirds. Density gradients between surface and sub-surface waters inhibit the vertical transport of nutrients and alter the vertical and horizontal distributions of seabird prey25. Strengthened density gradients thus have potential to impact foraging success for seabirds because prey density controls foraging behavior and efficiency26. Warming and stratification, where they co-occur, can also have compounding impacts on seabird breeding productivity. For example, reduced breeding performance among kittiwakes in Northern Europe has been linked to regional increases of both ocean temperature and stratification, likely due to changes in the spatiotemporal availability of prey27.
Studies investigating how climate change influences seabird breeding productivity have, so far, tended to focus on individual species or regions. In this study, we synthesize seabird responses to warming of the surface mixed layer (where both diving and surface-feeding seabirds concentrate foraging effort) and increasing water column stratification across ecosystems and species, providing an integrative perspective on how physical drivers of climate change impact predator demographics at ecosystem-scales. We use a hemispheric subset of the Global Seabird Breeding Success Dataset (see Data Availability section), including 138 time series of seabird reproductive performance. These data include 39 species with diverse life history strategies collected over the past half-century by researchers around the world (Supplementary Table 1). Time series are situated in seven northern hemisphere coastal ecosystems with distinct biotas, hydrographic features, and productivity-generating processes, ranging from mid- to high-latitude spring bloom systems to Eastern Boundary Upwelling and tropical oligotrophic systems (Fig. 1). These systems include the Arctic, Northwest Atlantic, Hawaii, Warm Northeast Pacific, Cold Northeast Pacific, Northwest Pacific, and Northern European. While long-term breeding productivity time series are also available in the southern hemisphere, they are fewer in number and more sparsely distributed, hampering among-ecosystem comparison. We predicted that relationships between northern hemisphere breeding productivity and ocean climate factors will vary among systems due to underlying ecosystem-specific differences in biophysical processes and available prey diversity. To test these predictions, we (i) characterized multidecadal temperature, stratification, and seabird standardized breeding productivity (SBP) trends across all seven ecosystems, (ii) examined relationships between standardized breeding productivity and interannual climate variation, and (iii) quantified diet diversity across ecosystems to assess whether prey diversity mediate seabird responses to climate change. This study contributes to the goals of the UN Decade of Ocean Science for Sustainable Development project Global Integration of Seabird Time Series (No. 24.7) and is, to our knowledge, the first study to investigate marine predator breeding productivity responses to climate change at ecosystem-scales.
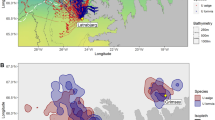
Point coloration and shading show ‘ecosystem’ classification of each time series. Polygons edged with thin white lines show ‘ecoregion’ structure. Arctic (teal); Northwest Atlantic (purple); Hawaii (green); Warm Northeast Pacific (brown); Cold Northeast Pacific (orange); Northwest Pacific (pink); Northern European Seas (yellow).
Results & Discussion
Long term environmental trends
We modeled linear trends in environmental conditions for each ecosystem during the pre-breeding season (adult arrival at breeding colonies till egg laying) and breeding season (egg laying till chick fledging) from 1993 to 2020 (Fig. 2, Supplementary Table 2), the period of time for which GLORYS (Copernicus Marine Service) model output is available. Linear models mask climate cycles discernable in all seven ecosystems (Supplementary Figs. 1 and 2), but allowed us to quantify multi-decadal climate trends over a period corresponding with the bulk of data in the Global Breeding Success Dataset. Models included spatially and temporally averaged data from all ecoregions where seabird colonies are located (Fig. 1, see Methods). Mixed layer temperature has increased (within 90% confidence interval [CI]) during seabird breeding seasons across all seven marine ecosystems (Supplementary Tables 9 and 10). Similarly, pre-breeding season temperature of the mixed layer (Supplementary Tables 5 and 6) has increased significantly in five of the seven ecosystems: the Arctic, Northwest Atlantic, Hawaii, Northwest Pacific, and Northern European Seas. Trend estimates were positive but included zero in the 90% CI for the Warm and Cold Northeast Pacific. Water column stratification trends varied widely by ecosystem. Pre-breeding season stratification (Supplementary Tables 7 and 8) declined in both the Arctic and Northwest Atlantic; all other trends were non-significant. Breeding season stratification (Supplementary Tables 11 and 12) declined in the Arctic and trends were non-significant elsewhere (though increasing in all but the Northeast Pacific ecosystems). Global analyses on stratification trends have found that the upper ocean has become increasingly stratified over recent decades4. Our analysis did not confirm this pattern, but that may be because our analysis was geographically limited to coastal and continental shelf seas (<200 m depth). In coastal areas the formation and maintenance of density gradients are influenced by coastal topography, runoff, upwelling, eddies, and other processes. Greater hydrodynamic complexity in coastal regions, compared to pelagic regions, may explain the lack of discernable stratification trends in recent decades as shown in Fig. 2.
Modeled trends in mixed layer temperature (red) and water column stratification (blue, higher scaled values indicate greater stratification) in seven northern hemisphere ecosystems (facets), where seabird colonies are located. Values are averaged over the pre-breeding (dashed) and breeding seasons (solid). Linear trend and standard error for each ecoregion is available in Supplementary Figs. 1 and 2.
Long term seabird breeding productivity trends
We found that seabird SBP is declining in recent decades in the Arctic, Northern European Seas, and Northwest Atlantic (Fig. 3, Supplementary Tables 13 and 14). In contrast, we found no trend in seabird SBP across four Pacific Ocean ecosystems: the Cold Northeast Pacific, Northwest Pacific, Warm Northeast Pacific, and Hawaii, though our ability to detect trends in the latter two systems was likely hampered by the existence of substantially fewer time series (Supplementary Table 1). Notably, breeding productivity showed a near-significant pattern of decline in the Northwest Pacific.
Modeled trends with standard error of standardized seabird breeding productivity among seven northern hemisphere ecosystems. Rug plot in plot margins show the density of raw data values on each axis. Dashed lines show standardized breeding productivity trends for individual time series, raw data and standard error for each time series is available in Supplementary Fig. 3.
All three ecosystems where we detected statistically significant declines in SBP also exhibited increasing pre-breeding and breeding season mixed layer temperature. In two of these systems (Arctic and Northern European Seas), and the Warm Northeast Pacific, linear mixed models of seabird SBP and seasonally stratified mixed layer temperature showed that breeding productivity decreases with increasing temperature (Supplementary Tables 15–18). Standardized breeding productivity was not linked to stratification (90% CI) in any of the seven ecosystems, except the Warm Northeast Pacific during the pre-breeding season where increasing stratification was associated with reduced breeding productivity (Supplementary Tables 19–22). In the Arctic and Northern European Seas, the negative association between warming and breeding productivity was likely mediated by reduced prey availability. Warm conditions during and preceding the pre-breeding season have been linked to early sea ice retreat and reduced plankton abundance in the Arctic28, and mismatched occurrence of key prey species with seabird chick-rearing in European Seas29,30,31. Our results show that warming has impacted seabird breeding productivity at ecosystem-scales in several systems, but it is unclear why similar warming trends have not precipitated breeding productivity declines in other systems. Two potential explanations for this observation are (1) that ecosystems differ in how they respond to incremental temperature change due to fundamental differences in biophysical processes, and (2) that seabirds may be insulated from bottom-up climate impacts in some systems by food web structure. We explore both of these possibilities in the sections below.
Ecosystem-specific breeding productivity responses to environmental change
To explore ecosystem-specific patterns in how climate factors influence breeding productivity, we modeled mixed layer temperature and stratification effects on SBP using values from which the long-term trend has been removed (i.e., detrended values) for each of the seven ecosystems (Table 1, Supplementary Tables 23–50). This approach focuses on mechanistic linkages at the interannual scale, and allows comparison of climate-productivity mechanisms, rather than long-term trends, among ecosystems. We found that interannual increases in temperature and stratification occurring during the pre-breeding season tended to have a detrimental impact on SBP, and that detrended stratification played a greater role than systematic stratification variation for determining SBP in some systems.
In five of the seven ecosystems, pre-breeding season conditions were linked to interannual variation in SBP (within the 90% CI). Specifically, interannual increases in pre-breeding mixed layer temperature were associated with SBP negatively in the Arctic (Supplementary Table 23), Cold Northeast Pacific (Supplementary Table 35) and Warm Northeast Pacific (Supplementary Table 39); and positively in Hawaii (Supplementary Table 47). Pre-breeding season stratification was also negatively associated with SBP in the Northwest Atlantic (Supplementary Table 32). The importance of pre-breeding season conditions across many northern hemisphere ecosystems suggests that this period plays an outsized role in determining annual seabird reproductive productivity. Changes in pre-breeding season conditions may be generally linked to shifts in ecosystem-specific productivity-generating processes in mid- to high-latitude systems. For example, in the California Current Eastern Boundary Upwelling System, where the Warm and Cold Northeast Pacific ecosystems are located, winter and spring upwelling pre-conditions habitats of the North American West Coast, generating strong primary, secondary, and predator productivity32. Farther north, springtime warming in the Arctic has been linked to reduced seabird breeding productivity in some species33 and decreasing population sizes34 via reduced sea-ice concentrations, which, like upwelling, influences primary productivity and food web dynamics28,35. By altering seasonal productivity-generating processes, variation in pre-breeding season conditions thus has potential to impact numerous seabird species, including species that rely on different prey types, occupy different trophic levels, and exhibit different foraging strategies and behaviors. While pre-breeding warming has already been linked to declining breeding productivity in the Arctic and Northern European Seas (Supplementary Tables 15 and 16), the importance of pre-breeding conditions to interannual breeding productivity variation in other systems suggests that continued change in pre-breeding conditions may be a key driver and indicator of future demographic shifts among predators in mid- to high-latitude systems.
In our detrended analysis, we also found evidence that stratification is particularly influential for seabird breeding productivity in two ecosystems: Hawaii and the Northwest Atlantic. In the Northwest Atlantic, SBP was significantly negatively associated with both pre-breeding and breeding season stratification (Supplementary Tables 32 and 34). This finding corroborates prior work relating low ecosystem productivity (during both warm and cold conditions) to increased stratification, which precipitated low copepod (Calanus finmarchicus) abundance and low predator reproductive success36. Interestingly, detrended stratification had a stronger influence on SBP than detrended temperature in the Northwest Atlantic, a hotspot for ocean warming and marine heatwave occurrence16. Nowhere else was breeding productivity as impacted by stratification as in this region, suggesting that changes in stratification have the potential to influence ecosystem-scale predator productivity, even eclipsing the effects of temperature, but that impacts depend strongly on ecosystem characteristics and are difficult to generalize.
The influence of stratification on breeding productivity was more difficult to ascertain in Hawaii, where within-season mixed layer temperature and water column stratification are strongly correlated (~80%, Table 1). Pre-breeding season temperature and stratification both had strongly positive associations with SBP (Supplementary Tables 47 and 48). Additionally complicating is the fact that available Hawaiian time series were predominantly comprised of surface/near-surface-foraging albatrosses and shearwaters (Procellariformes), and thus included less diverse life history strategies than exist in other northern hemisphere ecosystems. Evidence from other regions suggests that surface-foraging omnivores may benefit from both vertical (Southwest Atlantic37) and horizontal (Celtic Sea38) heterogeneity associated with strongly stratified waters, and that stratification onset may influence breeding phenology29. These mechanisms may be important for Hawaiian albatross and shearwaters, as species with relatively low flight costs and broad foraging ranges, may be able to offset reductions in prey availability near colonies by traveling farther from the nest39, where they could benefit from stratification-associated prey concentrations in more remote, pelagic habitats. It is also possible that warm conditions near the Hawaiian Islands are a proxy for the position or strength of the subtropical transition zone, known to be an important foraging ground for Hawaiian albatross39, but testing this was outside the scope of this study. Our focus on coastal ecosystems (Fig. 1) as the spatial scale for analysis was appropriate for many species of breeding seabirds, but did not completely reflect the scale at which long-distance foraging species, such as albatross and shearwaters, forage. In the southern hemisphere, long-distance foraging species represent a much larger proportion of seabirds. Moreover, physical features relevant for foraging may extend over much larger spatial scales in the southern hemisphere, linking seabird breeding productivity to very large pelagic areas40. Future studies investigating ecosystem-scale seabird responses in the southern hemisphere will need to adopt more expansive and potentially dynamic concepts of what defines an ecosystem.
Besides the importance of pre-breeding environmental conditions for breeding productivity across most mid- to high-latitude systems, we found few commonalities across the seven ecosystems. Our inability to generalize across ecosystems emphasizes the importance of ecosystem-specific characteristics and processes in mediating how climate drivers influence upper trophic level productivity. Food web structure is one such characteristic that varies by ecosystem and may be important for determining predator responses to environmental change.
Prey diversity mitigates climate impacts on marine predators
Ecological theory suggests that high prey diversity helps stabilize marine food web dynamics via so-called “portfolio effects”, which spread the risk of predator nutritional stress across many potential prey species41,42. Thus, greater diversity of prey species may alleviate bottom-up impacts of climate change on predators. To examine evidence for climate-mediating portfolio effects among seabirds, we identified up to the top three prey items historically utilized by each species in our dataset based on published literature and expert input (Supplementary Table 3, see Methods). We then compared diet diversity patterns across five ecosystems, excluding Hawaii and the Warm Northeast Pacific, both of which had fewer seabird species and time series, using the Shannon-Wiener diversity index (Fig. 4, Supplementary Fig. 8 for counts). Finally, we used simple linear models to explore heuristic relationships between time series trends, biophysical response coefficients, and diet diversity across ecosystems (Fig. 5). We found that low diet diversity was associated with declining seabird breeding productivity, and that low diet diversity ecosystems tended to exhibit more negative breeding productivity responses to incremental increases in temperature and stratification (Supplementary Table 51).
Portfolios are ordered left to right by increasing diversity score (Shannon-Wiener Index) shown as H’ values along with the number of seabird species at top. Bars show the proportion of seabirds using each prey item across ecosystems. Color-coding displays coarse prey groups, white outlines indicate particular prey taxa within the group (see Methods and Supplementary Table 3).
Trend estimates for ecosystem and seasonally stratified linear mixed effects models of seabird standardized breeding productivity plotted against ecosystem prey diversity H’ index. Left plot displays time series trends (Fig. 3) per ecosystem (squares); center (right) plot displays temperature (stratification) response (Table 1). Linear regression and standard error are plotted for each subplot (Supplementary Table 51).
The diet portfolios we assembled through our review (Supplementary Table 3) reflect a combination of prey availability and foraging behaviors of seabirds. Consequently, increases in prey diversity (e.g., through range shifts) may not translate into increased foraging opportunities as seabird communities have evolved to exploit particularly, locally-abundant prey species, often becoming highly specialized. Specialized predators, sensitive to reductions in preferred prey items and unable to exploit alternative prey even if they are locally available, may thus be more vulnerable to prey fluctuations driven by climate change43,44. We found that obligate piscivore seabirds were more commonly represented in ecosystems with low diet diversity and declining reproductive success (Fig. 6, Supplementary Fig. 7). In the Northern European Seas, for example, seabirds tend to concentrate foraging effort on few fish species, particularly Ammodytes spp (sandeel; Figs. 4 and 6). Widespread dependence on Ammodytes spp abundance among Northern European Seas seabirds means that changes in the Ammodytes spp availability alone can induce fluctuations in the productivity of a whole predator community. These findings are consistent with those presented by Sydeman and colleagues (2021)16, specifically, that piscivorous species are likely more vulnerable to global environmental change than omnivorous or planktivorous species via changes in prey availability.
Summary figure depicting seabird prey-use diversity by ecosystem with key seabird species. Colors show representative seabird species and key prey items for the Arctic (teal), Northwest Atlantic (purple), Hawaii (green), Warm Northeast Pacific (brown), Cold Northeast Pacific (orange), Northwest Pacific (pink), Northern European Seas (yellow). Percentages for each prey item indicate the percentage of seabird colonies that exploit that prey item in each ecosystem. Design of image follows the format of Cury et al. 2011.
Ecosystems where seabirds exploit diverse prey species were more likely to exhibit resilience to the bottom-up impacts of climate change. Within highly productive habitats, such as upwelling ecosystems, moderate diversity among just a few dominant forage fish species can sustain food supplies for seabirds when prey exhibit strong asynchronous fluctuations in distribution and abundance45, or, when low frequency variability occurs across functionally similar species of small forage fish46. Such mechanisms likely play a role in insulating Northeast Pacific seabirds, broadly, from climate-related pressures. However, when environmental conditions induce synchronous reductions in forage fish biodiversity, as occurred in the Northeast Pacific during the coupled 2014–2016 marine heatwave and El Niño event, portfolio effects dissipate, and even generalist breeding seabirds may experience reproductive failures and in some cases mass mortality events47,48. Recent research has found common murres (Uria aalge) in the Northeast Pacific have failed to rebound following mass mortality induced by the 2014–2016 heatwave49, suggesting that the heatwave may have precipitated a fundamental shift in the carrying capacity of an ecosystem with historically stable seabird productivity (Fig. 3). Consequently, past trends and climate-predator associations may not be indicative of future dynamics.
As long as seabird populations remain within physiological limits, behavioral shifts in general may occur before demographic changes. Our analysis suggests that foraging flexibility may be insulating seabirds in some high prey-biodiversity ecosystems from climate effects. However, future studies should quantify prey-switching more explicitly using diet studies to confirm our findings. Another aspect of behavior flexibility relevant to seabirds, and marine predators generally, is in the timing (phenology) of breeding, which may allow species to maintain spatiotemporal overlap with prey during the reproductive season. Shifts to earlier breeding among seabirds has been documented in some regions of the Arctic50. However, Keogan and colleagues (2018)17 found in their global meta-analysis that seabird breeding phenology is generally insensitive to changes in sea surface temperature, limiting seabirds’ abilities to offset environmentally-induced prey reductions. With limited flexibility to alter the timing of breeding, maintenance of diverse and abundant prey resources may be among the most effective ways to prevent or slow demographic decline of seabird species.
Conclusion
We investigated predator responses to environmental change across and among ecosystems by compiling and analyzing a hemispheric dataset of seabird reproductive success over the past fifty years. Our results demonstrate that breeding productivity of North Atlantic (collectively the Northern European Seas and Northwest Atlantic ecosystems) and Arctic seabirds is in decline, and that productivity of North and Central Pacific ecosystems has remained comparatively stable. These patterns have arisen in a period of persistent warming of the surface mixed layer, but changes in pre-breeding season conditions tended to be most strongly associated with decreases in productivity. We also found that seabird diet diversity was lowest in the North Atlantic and Arctic, lending support to the suggestion that seabirds, and perhaps other higher-order consumers, may be buffered from rapid environmental change by diverse prey portfolios. As marine ecosystems continue to be exposed to the pressures of climate change, maintenance or recovery of diverse prey communities, through spatial protections, targeted fisheries regulations, and ecosystem-based management, may be among the most effective tools we have to conserve predator communities. Finally, it is essential to maintain long-term seabird (and other predator) monitoring programs to track biological responses to ongoing climate change and provide indicators of changes in ecosystem structure and function. Expansion of monitoring programs in under-represented regions of the globe, including much of the southern hemisphere, tropical, and equatorial regions is also critical to monitoring and conserving predator populations and ecosystems around the world.
Methods
Seabird breeding productivity
The northern hemisphere subset of the Global Breeding Success Dataset contains over 4000 observations in 138 unique time series, representing 39 species across 48 sites throughout the northern hemisphere. This unique dataset enables investigation of seabird-climate dynamics at ecosystem scales across seabird families and functional traits (i.e., foraging strategies, trophic level, etc.; Supplementary Figs. 6 and 7).
Annual seabird breeding productivity data were compiled primarily from direct contributions by seabird researchers (Supplementary Table 1) and some data were collected from published sources. Generally, we excluded time series shorter than eleven years, for which climate-driven trends were unlikely to be distinguished from interannual variability51. In some cases, individual years (n = 15) were excluded from the time series when it was known that exogenous factors (e.g., predation, DDT) strongly influenced breeding productivity. Data providers specified these determinations and we did not question these recommendations. We also excluded whole time series (n = 4) in cases where exogenous factors were persistently influential or multiple time series reported on the same population.
Regionalization
For each time series (seabird species by site combination) we assigned two nested regional geographic classifications using the Marine Ecoregions of the World (MEOW) approach outlined by Spalding and colleagues (2007)52. MEOW builds on other regionalization approaches (Large Marine Ecosystems, Longhurst Regions, and others) to spatially parse marine spaces into a hierarchical, nested structure based largely on biogeographic and environmental characteristics. A unique characteristic of MEOW is that they are constrained to coastal shelf areas (out to the 200 m isobath). Each time series was first assigned an ‘ecoregion’ (the smallest-scale MEOW unit), likely corresponding to the area with the strongest influence over breeding productivity, i.e., where parents are likely to concentrate foraging effort while incubating and provisioning chicks. Each ecoregion was then nested within an ‘ecosystem’ (analogous to ‘province’ in the MEOW approach52). This larger regionalization corresponds to areas with distinct biotas and consistent oceanography and hydrography. Seven ecosystems are represented in the Dataset: the Arctic, Cold Temperate Northeast Pacific (Cold Northeast Pacific), Cold Temperate Northwest Pacific (Northwest Pacific), Warm Temperate Northeast Pacific (Warm Northeast Pacific), Hawaii, Northern European Seas (Northern European Seas), and Cold Temperate Northwest Atlantic (Northwest Atlantic). As we have defined them, these seven ecosystems are thus roughly equivalent to coastal Large Marine Ecosystems. Ecoregion and ecosystem classifications for each time series are available in the supplementary information (Supplementary Table 1). One time series (Ebro Delta, Audouin’s gull) was omitted from our analysis because it was the only time series located within the Mediterranean ecosystem. Lack of broader representation from this ecosystem limited our ability to make meaningful among-ecosystem comparisons.
Environmental conditions
We used the GLORYS12V1 global ocean reanalysis to describe the marine environment of the ecoregions and ecosystems of this study53. This reanalysis resolves the ocean at 1/12-degree, daily resolution at 50 vertical depth levels. The model has been validated against independent observations and represents the state-of-the-art in hydrodynamic modeling54,55. GLORYS12V1 is developed by Mercator Ocean and is an operational service from the Copernicus Marine Service Center (marine.copernicus.eu). The GLORYS12V1 reanalysis assimilates available historical data (e.g., satellite, CTD, XBT, buoys), and the period used for this study covers 1993–2019.
For each ecoregion (Fig. 1), we extracted temperature, mixed layer depth, and salinity for the upper 200 m of the water column to calculate the coastal stratification. We used the Potential Energy Anomaly (PEA) as a metric for seasonal stratification of the water column. PEA offers a valuable metric for seasonal stratification, as it is an inherent property that does not rely on specific thresholds. More specifically, the PEA is calculated as an integral property of the density differences of the water column and the energy required to thoroughly mix the water column56. The seasonal nature of the PEA makes it particularly useful for coastal regions and more shallow waters where a permanent stratification may be lacking entirely due to strong mixing from tides and wind. It is important to note that seasonal stratification derived from PEA differs from stratification derived from temperature thresholds.
Prey use
We searched published and gray literature for the preferred historical breeding season diet of each individual seabird species and site combination (e.g., prey important for Atlantic Puffins at Røst). Dietary preferences were established using various methods, depending on the source, and included direct observations of prey delivered to chicks, regurgitated prey, or molecular techniques. We also included adult dietary information as adult diets influence breeding productivity. When published reports of diet prey were unavailable for a particular seabird time series, we consulted data providers (Supplementary Table 1) and/or further identified prey based on expert knowledge. We recorded up to the top three prey for each seabird time series (some had less than three prey identified) in order to capture the diversity of the most important dietary components, and examine overlapping preferences across seabird species within an ecosystem. Prey species were sorted into 34 taxonomic categories based on taxonomic relationships and global importance, and were assigned to one of eight coarse prey types (Supplementary Table 3). To characterize ecosystem-scale differences in seabird diet diversity, we calculated a Shannon-Wiener diversity index score57 within R for the prey categories within each ecosystem using formula 1, where pi is the proportional abundance of each species i.
Statistical analyses
We constructed four families of hierarchical linear mixed models (LMMs) to evaluate whether seabird demographic trends are associated with key indicators linked to climate change (see Supplementary Note 3). Specifically, these models estimate within and among ecosystem differences in (1) ML temperature and water column stratification, (2) seabird breeding productivity, and (3) the relationship between environmental and demographic variables. A fourth family of models utilized quadratic detrended measures to estimate the response of seabirds to interannual variability in climate drivers. To examine how seasonal timing plays a role in the seabird responses to climate measures, all models (except family 2) were fitted to physical data averaged over both the pre-breeding season, when birds are arriving to breeding colonies and nesting, and the breeding season, when birds are incubating eggs and provisioning chicks (see also Supplementary Table 2). All trend models (families one through three) quantified ecosystem variation by incorporating ecosystem as a fixed term. Detrended biophysical models (family four) were fitted for each of the seven northern hemisphere ecosystems independently because the degree of collinearity among temperature and stratification varied regionally. Before fitting each of these models we checked for correlation among terms (Table 1). All linear models were evaluated for linearity, normality, and homoscedasticity graphically using standard diagnostic plots (Supplementary Figs. 3.1–4 and 5).
All models were constructed using the nlme package58 using R version 4.3.159. Figures and tables were made using the packages ggplot260, kableExtra61, and performance62.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The northern hemisphere subset of the Global Breeding Success Dataset is available at https://doi.org/10.17605/OSF.IO/9DBUE and via a GitHub repository at https://github.com/hjkilleen/seabirds-northern-hemisphere. Temperature, salinity, and mixed layer depth data are publicly available via the Copernicus Marine Service at https://data.marine.copernicus.eu/product/GLOBAL_MULTIYEAR_PHY_001_030/description.
Code availability
The code used generate the results presented in this study is available at https://doi.org/10.17605/OSF.IO/9DBUE and via a GitHub repository at https://github.com/hjkilleen/seabirds-northern-hemisphere.
References
Cheng, L. et al. Past and future ocean warming. Nat. Rev. Earth Environ. 3, 776–794 (2022).
Bindoff, N. L. et al. Chapter 5: Changing Ocean, Marine Ecosystems, and Dependent Communities — Special Report on the Ocean and Cryosphere in a Changing Climate. In (Cambridge University Press, Cambridge, UK and New York, NY, USA, 2019).
Li, G. et al. Increasing ocean stratification over the past half-century. Nat. Clim. Change 10, 1116–1123 (2020).
Sallée, J.-B. et al. Summertime increases in upper-ocean stratification and mixed-layer depth. Nature 591, 592–598 (2021).
Hunt, G. L. & Schneider, D. C. Chapter 2: Scale dependent processes in the physical and biological environment of marine birds. In Seabird Feeding Ecology (Cambridge University Press, 1987).
Doney, S. C. et al. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37 (2012).
Yamaguchi, R. et al. Trophic level decoupling drives future changes in phytoplankton bloom phenology. Nat. Clim. Change 12, 469–476 (2022).
Haury, L. R., McGowan, J. A. & Wiebe, P. H. Patterns and Processes in the Time-Space Scales of Plankton Distributions. In Spatial Pattern in Plankton Communities (ed. Steele, J. H.) 277–327. https://doi.org/10.1007/978-1-4899-2195-6_12 (Springer US, Boston, MA, 1978).
Heim, K. C., Thorne, L. H., Warren, J. D., Link, J. S. & Nye, J. A. Marine ecosystem indicators are sensitive to ecosystem boundaries and spatial scale. Ecol. Indic. 125, 107522 (2021).
Kirby, R. R. & Beaugrand, G. Trophic amplification of climate warming. Proc. R. Soc. B Biol. Sci. 276, 4095–4103 (2009).
Hazen, E. L. et al. Ecosystem Sentinels as Early-Warning Indicators in the Anthropocene. Annu. Rev. Environ. Resour. 49, 573–598 (2024).
Aebischer, N. J., Coulson, J. C. & Colebrookl, J. M. Parallel long-term trends across four marine trophic levels and weather. Nature 347, 753–755 (1990).
Hazen, E. L. et al. Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 17, 565–574 (2019).
Cairns, D. K. Seabirds as Indicators of Marine Food Supplies. Biol. Oceanogr. 5, 261–271 (1988).
Piatt, J. F., Sydeman, W. J. & Wiese, F. Introduction: a modern role for seabirds as indicators. Mar. Ecol. Prog. Ser. 352, 199–204 (2007).
Sydeman, W. J. et al. Hemispheric asymmetry in ocean change and the productivity of ecosystem sentinels. Science 372, 980–983 (2021).
Keogan, K. et al. Global phenological insensitivity to shifting ocean temperatures among seabirds. Nat. Clim. Change 8, 313–318 (2018).
Cury, P. M. et al. Global Seabird Response to Forage Fish Depletion—One-Third for the Birds. Science 334, 1703–1706 (2011).
Oro, D. Seabirds and climate: knowledge, pitfalls, and opportunities. Front. Ecol. Evol. 2, 79 (2014).
Piatt, J. F. & Nettleship, D. N. Diving Depths of Four Alcids. The Auk 102, 293–297 (1985).
Watanuki, Y., Sato, K., Shiomi, K., Wanless, S. & Daunt, F. Foraging habitat and site selection do not affect feeding rates in European shags. J. Exp. Biol. 226, jeb244461 (2023).
MacArthur, R. H. & Pianka, E. R. On Optimal Use of a Patchy Environment. Am. Nat. 100, 603–609 (1966).
Furness, R. W. & Tasker, M. L. Seabird-fishery interactions: quantifying the sensitivity of seabirds to reductions in sandeel abundance, and identification of key areas for sensitive seabirds in the North Sea. Mar. Ecol. Prog. Ser. 202, 253–264 (2000).
Durant, J., Ottersen, G. & Stenseth, N. C. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283 (2007).
McInnes, A. M. et al. Small pelagic fish responses to fine-scale oceanographic conditions: implications for the endangered African penguin. Mar. Ecol. Prog. Ser. 569, 187–203 (2017).
Cox, S. L., Embling, C. B., Hosegood, P. J., Votier, S. C. & Ingram, S. N. Oceanographic drivers of marine mammal and seabird habitat-use across shelf-seas: A guide to key features and recommendations for future research and conservation management. Estuar. Coast. Shelf Sci. 212, 294–310 (2018).
Carroll, M. J. et al. Effects of sea temperature and stratification changes on seabird breeding success. Clim. Res. 66, 75–89 (2015).
Ramírez, F. et al. Sea ice phenology and primary productivity pulses shape breeding success in Arctic seabirds. Sci. Rep. 7, 4500 (2017).
Ramírez, F. et al. Oceanographic drivers and mistiming processes shape breeding success in a seabird. Proc. R. Soc. B Biol. Sci. 283, 20152287 (2016).
Slotte, A. et al. Earlier hatching and slower growth: a key to survival in the early life history of Norwegian spring spawning herring. Mar. Ecol. Prog. Ser. 617–618, 25–39 (2019).
Tiedemann, M. et al. Environmental influences on Norwegian spring-spawning herring (Clupea harengus L.) larvae reveal recent constraints in recruitment success. ICES J. Mar. Sci. 78, 640–652 (2021).
Schroeder, I. D. et al. Winter pre-conditioning of seabird phenology in the California Current. Mar. Ecol. Prog. Ser. 393, 211–223 (2009).
Sauser, C. et al. Demographic responses of Arctic seabirds to spring sea-ice variations. Front. Ecol. Evol. 11, 1107992 (2023).
Descamps, S. & Ramírez, F. Species and spatial variation in the effects of sea ice on Arctic seabird populations. Divers. Distrib. 27, 2204–2217 (2021).
Cusset, F. et al. Arctic seabirds and shrinking sea ice: egg analyses reveal the importance of ice-derived resources. Sci. Rep. 9, 15405 (2019).
Pershing, A. J. & Kemberling, A. Decadal comparisons identify the drivers of persistent changes in the zooplankton community structure in the northwest Atlantic. ICES J. Mar. Sci. 81, 564–574 (2024).
Costa, P. L., Bugoni, L., Kinas, P. & Madureira, L. Seabirds, environmental features and the Argentine anchovy (Engraulis anchoita) in the southwestern Atlantic Ocean. Mar. Ecol. Prog. Ser. 651 (2020).
Waggitt, J. J. et al. Combined measurements of prey availability explain habitat selection in foraging seabirds. Biol. Lett. 14, 20180348 (2018).
Antolos, M., Shaffer, S. A., Weimerskirch, H., Tremblay, Y. & Costa, D. P. Foraging behavior and energetics of albatrosses in contrasting breeding environments. Front. Mar. Sci. 4, 414 (2017).
Hunt, G. L. et al. Physical processes, prey abundance, and the foraging ecology of seabirds. In (eds. Adams, N. J. & Slotow, R. H.) 2040–2056 (Johannesburg: BirdLife South Africa, 1999).
Schindler, D. E. et al. Population diversity and the portfolio effect in an exploited species. Nature 465, 609–612 (2010).
Schindler, D. E., Armstrong, J. B. & Reed, T. E. The portfolio concept in ecology and evolution. Front. Ecol. Environ. 13, 257–263 (2015).
Blondin, H. E. et al. Land-dependent marine species face climate-driven impacts on land and at sea. Mar. Ecol. Prog. Ser. 699, 181–198 (2022).
Carroll, G., Abrahms, B., Brodie, S. & Cimino, M. A. Spatial match–mismatch between predators and prey under climate change. Nat. Ecol. Evol. 8, 1593–1601 (2024).
Cury, P. et al. Small pelagics in upwelling systems: patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 57, 603–618 (2000).
Lindegren, M., Checkley, D. M., Rouyer, T., MacCall, A. D. & Stenseth, N. Chr. Climate, fishing, and fluctuations of sardine and anchovy in the California Current. Proc. Natl. Acad. Sci. 110, 13672–13677 (2013).
Piatt, J. F. et al. Extreme mortality and reproductive failure of common murres resulting from the northeast Pacific marine heatwave of 2014-2016. Plos One 15, e0226087 (2020).
Arimitsu, M. L. et al. Heatwave-induced synchrony within forage fish portfolio disrupts energy flow to top pelagic predators. Glob. Change Biol. 27, 1859–1878 (2021).
Renner, H. M. et al. Catastrophic and persistent loss of common murres after a marine heatwave. Science 386, 1272–1276 (2024).
Descamps, S. et al. Diverging phenological responses of Arctic seabirds to an earlier spring. Glob. Change Biol. 25, 4081–4091 (2019).
Orgeret, F. et al. Climate change impacts on seabirds and marine mammals: The importance of study duration, thermal tolerance and generation time. Ecol. Lett. 25, 218–239 (2021).
Spalding, M. D. et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 57, 573–583 (2007).
Jean-Michel, L. et al. The copernicus global 1/12° oceanic and sea ice GLORYS12 reanalysis. Front. Earth Sci. 9, 698876 (2021).
Global Ocean Physics Reanalysis. E.U. Copernicus Marine Service Information (CMEMS). Marine Data Store (MDS). https://doi.org/10.48670/moi-00021.
Verezemskaya, P. et al. Assessing eddying (1/12°) ocean reanalysis GLORYS12 using the 14-yr instrumental record from 59.5°N section in the atlantic. J. Geophys. Res. Oceans 126, e2020JC016317 (2021).
Holt, J., Harle, J., Wakelin, S., Jardine, J. & Hopkins, J. Why is seasonal density stratification in shelf seas expected to increase under future climate change? Geophys. Res. Lett. 49, e2022GL100448 (2022).
Shannon, C. E. & Wiener, W. The Mathematical Theory of Communication. (University of Illinois Press, Urbana, Illinois, USA, 1963).
Pinheiro, J., Bates, D. & R Core Team. Linear and nonlinear mixed effects models. (2024).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing (2024).
Wickham, H. ggplot2: Elegant graphics for data analysis. (2016).
Zhu, H. kableExtra: Construct complex table with ‘kable’ and pipe syntax. (2024).
Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P. & Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 6, 3139 (2021).
Acknowledgements
This study results from the dedication and commitment to long-term seabird population studies by many governmental, non-governmental, and self-funded individuals across the globe. We sincerely thank and acknowledge all these researchers, technicians, and data managers who contributed to seabird monitoring at the 48 sites in this study. Analysis and writing presented in this study were supported by the U.S. National Science Foundation (Award No. 2142918). We are additionally grateful to D.S. Schoeman, whose code developed for prior studies using the Global Seabird Breeding Success Database was consulted in this study, and to the three anonymous reviewers, who provided thoughtful feedback on an earlier draft. Additional acknowledgements for data collection efforts and funding at each site are provided in Supplementary Note 1.
Author information
Authors and Affiliations
Contributions
Helen Killeen: Conceptualization, Methodology, Formal Analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration William J. Sydeman: Conceptualization, Methodology, Formal Analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration, Funding acquisition Brian Hoover: Conceptualization, Methodology, Formal Analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization Sarah Ann Thompson: Data curation, Writing - original draft, Writing - review & editing, Project administration, Funding acquisition Trond Kristiansen: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Funding acquisition Marisol García-Reyes: Writing - review & editing, Funding acquisition Gammon Koval: Data curation, Writing - review & editing Erendira Ceballos: Writing - review & editing, Visualization Molly Heal: Investigation, Data curation Tycho Anker-Nilssen: Investigation, Data curation, Writing - review & editing Robert Barrett: Investigation, Data curation Peter Becker: Investigation, Data curation Per-Arvid Berglund: Investigation, Data curation Tim Birkhead: Investigation, Data curation Thierry Boulinier: Investigation, Data curation Sandra Bouwhuis: Investigation, Data curation, Writing - review & editing Francis Daunt: Investigation, Data curation, Writing - review & editing Nina Dehnhard: Investigation, Data curation, Writing - review & editing Antony Diamond: Investigation, Data curation, Writing - review & editing Kjell Einar Erikstad: Investigation, Data curation Kyle Elliott: Investigation, Data curation, Writing - review & editing Annette L. Fayet: Investigation, Data curation, Writing - review & editing Elizabeth Flint: Investigation, Data curation Robert Furness: Investigation, Data curation, Writing - review & editing Elena Golubova: Investigation, Data curation, Writing - review & editing Erpur Snær Hansen: Investigation, Data curation, Writing - review & editing Mike Harris: Investigation, Data curation Scott Hatch: Investigation, Data curation, Writing - review & editing April Hedd: Investigation, Data curation Jonas Hentati-Sundberg: Investigation, Data curation, Writing - review & editing Jaime Jahncke: Investigation, Data curation Alexander Kitaysky: Investigation, Data curation Svein-Håkon Lorentsen: Investigation, Data curation Don Lyons: Investigation, Data curation Heather L. Major: Investigation, Data curation, Writing - review & editing David Mazurkiewicz: Investigation, Data curation Will Miles: Investigation, Data curation Mark Newell: Investigation, Data curation Rachael A. Orben: Investigation, Data curation, Writing - review & editing Daniel Oro: Investigation, Data curation, Writing - review & editing Michael Parker: Investigation, Data curation Jonathan Plissner: Investigation, Data curation Jean-François Rail: Investigation, Data curation Tone Kristin Reiertsen: Investigation, Data curation Heather Renner: Investigation, Data curation, Writing - review & editing Jennifer C. Rock: Investigation, Data curation Hallvard Strøm: Investigation, Data curation Robert M. Suryan: Investigation, Data curation, Writing - review & editing Julie Thayer: Investigation, Data curation, Writing - review & editing Jannelle Trowbridge: Investigation, Data curation Enriqueta Velarde: Investigation, Data curation, Writing - review & editing Sarah Wanless: Investigation, Data curation, Writing - review & editing Pete Warzybok: Investigation, Data curation Yutaka Watanuki: Investigation, Data curation, Writing - review & editing Shannon Whelan: Investigation, Data curation, Writing - review & editing Lindsay Young: Investigation, Data curation, Writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth and Environment thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Alice Drinkwater, Heike Langenberg, and Mengjie Wang. [A peer review file is available.]
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Killeen, H., Sydeman, W.J., Hoover, B. et al. Ecosystems mediate climate impacts on northern hemisphere seabirds. Commun Earth Environ 6, 804 (2025). https://doi.org/10.1038/s43247-025-02717-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-025-02717-z