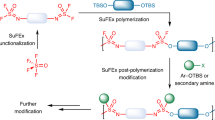
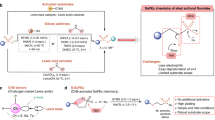
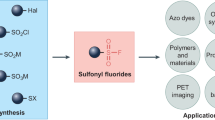
Abstract
Click chemistry is a conceptual strategy to rapidly synthesize and discover functional molecules. Sulfur fluoride exchange (SuFEx) is a click reaction that has revolutionized multiple research fields. In this Primer, we delve into the essential elements of SuFEx operation, catalysis and SuFExable connective hubs. We also explore the cutting-edge applications of SuFEx in drug development, polymer science and biochemistry. Additionally, we examine the potential limitations and promising prospects for this versatile click reaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kolb, H. C., Finn, M. G. & Sharpless, K. B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 40, 2004–2021 (2001). This is the original click chemistry manifesto presented by K. Barry Sharpless, outlining the synthesis philosophy and requirements for a reaction to achieve ‘click’ status.
Rostovtsev, V. V., Green, L. G., Fokin, V. V. & Sharpless, K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective ‘ligation’ of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002).
Tornøe, C. W., Christensen, C. & Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 67, 3057–3064 (2002).
Agard, N. J., Prescher, J. A. & Bertozzi, C. R. A strain-promoted [3 + 2] azide−alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Dong, J., Krasnova, L., Finn, M. G. & Sharpless, K. B. Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed. 53, 9430–9448 (2014). This publication represents the re-emergence of SuFEx as a click reaction and describes in detail many of the unique properties of S–F-containing substrates.
Giel, M., Smedley, C. J. & Moses, J. E. in Science of Synthesis: Click Chemistry Vol. 1 Ch. 5 (ed. Rutjes, F. P. J. T.) 435 (Georg Thieme, 2022). This book chapter provides a comprehensive overview of the SuFEx reaction, including recent advancements in the field and current limitations.
Davies, W. & Dick, J. H. Benzenesulphonyl fluoride derivatives. J. Chem. Soc. https://doi.org/10.1039/JR9320002042 (1932).
Steinkopf, W. Über aromatische sulfofluoride. J. Prakt. Chem. 117, 1–82 (1927).
Mortenson, D. E. et al. ‘Inverse drug discovery’ strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates. J. Am. Chem. Soc. 140, 200–210 (2018).
Zheng, Q. et al. ‘Sleeping beauty’ phenomenon: SuFEx-enabled discovery of selective covalent inhibitors of human neutrophil elastase. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv.7842020.v1 (2019).
Barrow, A. S. et al. The growing applications of SuFEx click chemistry. Chem. Soc. Rev. 48, 4731–4758 (2019).
Chinthakindi, P. K. & Arvidsson, P. I. Sulfonyl fluorides (SFs): more than click reagents? Eur. J. Org. Chem. 2018, 3648–3666 (2018).
Davies, A. T., Curto, J. M., Bagley, S. W. & Willis, M. C. One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides. Chem. Sci. 8, 1233–1237 (2017).
Tribby, A. L., Rodríguez, I., Shariffudin, S. & Ball, N. D. Pd-catalyzed conversion of aryl iodides to sulfonyl fluorides using SO2 surrogate DABSO and Selectfluor. J. Org. Chem. 82, 2294–2299 (2017).
Lee, C., Ball, N. D. & Sammis, G. M. One-pot fluorosulfurylation of Grignard reagents using sulfuryl fluoride. Chem. Commun. 55, 14753–14756 (2019).
Gilbert, E. E. Recent developments in preparative sulfonation and sulfation. Synthesis 1969, 3–10 (1969).
Lou, T. S.-B. & Willis, M. C. Sulfonyl fluorides as targets and substrates in the development of new synthetic methods. Nat. Rev. Chem. 6, 146–162 (2022).
Liu, Z. et al. Sufex click chemistry enabled late-stage drug functionalization. J. Am. Chem. Soc. 140, 2919–2925 (2018). This article presents a prime example of how the high-throughput SuFEx derivatization of drug molecules can lead to pronounced increases in potency.
Padma, D. K., Subrahmanya Bhat, V. & Vasudeva Murthy, A. R. Reactions of sulphuryl fluoride, sulphuryl chlorofluoride and sulphuryl chloride with amines. J. Fluor. Chem. 20, 425–437 (1982).
Liang, D.-D. et al. Silicon-free SuFEx reactions of sulfonimidoyl fluorides: scope, enantioselectivity, and mechanism. Angew. Chem. Int. Ed. 59, 7494–7500 (2020). This publication demonstrates the first asymmetric SuFEx reaction and presents a thorough investigation into some key aspects of the underlying SuFEx mechanism.
Liang, D.-D., Pujari, S. P., Subramaniam, M., Besten, M. & Zuilhof, H. Configurationally chiral sufex-based polymers. Angew. Chem. Int. Ed. 61, e202116158 (2022).
Krutak, J. J., Burpitt, R. D., Moore, W. H. & Hyatt, J. A. Chemistry of ethenesulfonyl fluoride. Fluorosulfonylethylation of organic compounds. J. Org. Chem. 44, 3847–3858 (1979).
Chen, Q., Mayer, P. & Mayr, H. Ethenesulfonyl fluoride: the most perfect Michael acceptor ever found? Angew. Chem. Int. Ed. 55, 12664–12667 (2016).
Meng, Y.-P. et al. Ethenesulfonyl fluoride (ESF) and its derivatives in SuFEx click chemistry and more. Synthesis 52, 673–687 (2019).
Leng, J. & Qin, H.-L. 1-Bromoethene-1-sulfonyl fluoride (1-Br-ESF), a new SuFEx clickable reagent, and its application for regioselective construction of 5-sulfonylfluoro isoxazoles. Chem. Commun. 54, 4477–4480 (2018).
Smedley, C. J. et al. Diversity oriented clicking (DOC): divergent synthesis of SuFExable pharmacophores from 2-substituted-alkynyl-1-sulfonyl fluoride (SASF) hubs. Angew. Chem. Int. Ed. 59, 12460–12469 (2020). This article presents the concept of DOC, an innovative drug discovery technique that generates function by rapidly accessing structural diversity through sequential click reactions.
Zhang, X., Fang, W.-Y. & Qin, H.-L. Regio- and stereoselective installation of bromide onto vinyl sulfonyl fluorides: construction of a class of versatile sulfur fluoride exchange hubs. Org. Lett. 24, 4046–4051 (2022).
Nie, X. et al. Introducing a new class of sulfonyl fluoride hubs via radical chloro-fluorosulfonylation of alkynes. Angew. Chem. Int. Ed. 60, 22035–22042 (2021).
Kaljurand, I. et al. Extension of the self-consistent spectrophotometric basicity scale in acetonitrile to a full span of 28 pKa units: unification of different basicity scales. J. Org. Chem. 70, 1019–1028 (2005).
Hyde, A. M., Calabria, R., Arvary, R., Wang, X. & Klapars, A. Investigating the underappreciated hydrolytic instability of 1,8-diazabicyclo[5.4.0]undec-7-ene and related unsaturated nitrogenous bases. Org. Process. Res. Dev. 23, 1860–1871 (2019).
Li, S., Wu, P., Moses, J. E. & Sharpless, K. B. Multidimensional SuFEx click chemistry: sequential sulfur(vi) fluoride exchange connections of diverse modules launched from an SOF4 hub. Angew. Chem. Int. Ed. 56, 2903–2908 (2017). This publication reports the first use of SOF4 as a multidimensional SuFEx hub.
Perea-Buceta, J. E. et al. Diverting hydrogenations with Wilkinson’s catalyst towards highly reactive rhodium(I) species. Angew. Chem. Int. Ed. 54, 14321–14325 (2015).
Smedley, C. J. et al. Accelerated SuFEx click chemistry for modular synthesis. Angew. Chem. Int. Ed. 61, e202112375 (2022). The ‘accelerated’ method presented in this paper represents a enhancement of the traditional SuFEx reaction, allowing direct coupling of S–F hubs with unprotected alcohols in minutes.
Liu, C. et al. A general approach to o-sulfation by a sulfur(VI) fluoride exchange reaction. Angew. Chem. Int. Ed. 59, 18435–18441 (2020).
Kotsuki, H. H. in Superbases for Organic Synthesis. Guanidines, Amidines, Phosphazenes and Related Organocatalysts (ed. Ishikawa, T.) 187 (Wiley, 2009).
Dong, J., Sharpless, K. B., Kwisnek, L., Oakdale, J. S. & Fokin, V. V. SuFEx-based synthesis of polysulfates. Angew. Chem. Int. Ed. 53, 9466–9470 (2014).
Mukherjee, P. et al. Sulfonamide synthesis via calcium triflimide activation of sulfonyl fluorides. Org. Lett. 20, 3943–3947 (2018).
Han, B. et al. Calcium bistriflimide-mediated sulfur(VI)–fluoride exchange (SuFEx): mechanistic insights toward instigating catalysis. Inorg. Chem. 61, 9746–9755 (2022).
Gao, B. et al. Bifluoride-catalysed sulfur(VI) fluoride exchange reaction for the synthesis of polysulfates and polysulfonates. Nat. Chem. 9, 1083–1088 (2017).
Lee, C. et al. The emerging applications of sulfur(VI) fluorides in catalysis. ACS Catal. 11, 6578–6589 (2021).
Gembus, V., Marsais, F. & Levacher, V. An efficient organocatalyzed interconversion of silyl ethers to tosylates using DBU and p-toluenesulfonyl fluoride. Synlett 10, 1463–1466 (2008).
Chao, Y. et al. Sulfur–phenolate exchange: SuFEx-derived dynamic covalent reactions and degradation of sufex polymers. Angew. Chem. Int. Ed. 61, e202207456 (2022).
Zheng, Q. et al. Sulfur [18F]fluoride exchange click chemistry enabled ultrafast late-stage radiosynthesis. J. Am. Chem. Soc. 143, 3753–3763 (2021).
van den Boom, A. F. J., Subramaniam, M. & Zuilhof, H. Sulfur-phenolate exchange as a fluorine-free approach to S(VI) exchange chemistry on sulfonyl moieties. Org. Lett. 24, 8621–8626 (2022).
van den Boom, A. F. J. & Zuilhof, H. Sulfur-phenolate exchange as a mild, fast, and high-yielding method toward the synthesis of sulfonamides. Org. Lett. 25, 788–793 (2023).
Dean, J. A. & Lange, N. A. Lange’s Handbook of Chemistry (McGraw-Hill, 1999).
Mahapatra, S. et al. SuFEx activation with Ca(NTF2)2: a unified strategy to access sulfamides, sulfamates, and sulfonamides from S(VI) fluorides. Org. Lett. 22, 4389–4394 (2020).
Chanda, A. & Fokin, V. V. Organic synthesis ‘on water’. Chem. Rev. 109, 725–748 (2009).
Cortes-Clerget, M. et al. Water as the reaction medium in organic chemistry: from our worst enemy to our best friend. Chem. Sci. 12, 4237–4266 (2021).
Jung, Y. & Marcus, R. A. On the theory of organic catalysis ‘on water’. J. Am. Chem. Soc. 129, 5492–5502 (2007).
Narayan, S. et al. ‘On water’: unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 44, 3275–3279 (2005).
Zheng, Q., Dong, J. & Sharpless, K. B. Ethenesulfonyl fluoride (ESF): an on-water procedure for the kilogram-scale preparation. J. Org. Chem. 81, 11360–11362 (2016).
Meng, G. et al. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 574, 86–89 (2019).
Veryser, C., Demaerel, J., Bieliūnas, V., Gilles, P. & De Borggraeve, W. M. Ex situ generation of sulfuryl fluoride for the synthesis of aryl fluorosulfates. Org. Lett. 19, 5244–5247 (2017).
Guo, T. et al. A new portal to SuFEx click chemistry: a stable fluorosulfuryl imidazolium salt emerging as an ‘F−SO2+’ donor of unprecedented reactivity, selectivity, and scope. Angew. Chem. 130, 2635–2640 (2018). This publication presents the development and deployment of a fluorosulfuryl imidazolium salt as a potent, bench-stable surrogate for sulfuryl fluoride gas.
Zhou, H. et al. Introduction of a crystalline, shelf-stable reagent for the synthesis of sulfur(VI) fluorides. Org. Lett. 20, 812–815 (2018).
Abbasov, M. E. et al. A proteome-wide atlas of lysine-reactive chemistry. Nat. Chem. 13, 1081–1092 (2021).
Zhang, W. et al. A practical fluorosulfonylating platform via photocatalytic imidazolium-based SO2F radical reagent. Nat. Commun. 13, 3515 (2022).
Abdul Fattah, T., Saeed, A. & Albericio, F. Recent advances towards sulfur (VI) fluoride exchange (SuFEx) click chemistry. J. Fluor. Chem. 213, 87–112 (2018).
Carneiro, S. N. et al. Sulfur(VI) fluorides as tools in biomolecular and medicinal chemistry. Org. Biomol. Chem. 21, 1356–1372 (2023).
Kolb, H. C. & Sharpless, K. B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 8, 1128–1137 (2003).
Wilson Lucas, S., Zijian Qin, R., Rakesh, K. P., Sharath Kumar, K. S. & Qin, H.-L. Chemical and biology of sulfur fluoride exchange (SuFEx) click chemistry for drug discovery. Bioinorg. Chem. 130, 106227 (2023).
Glassford, I. et al. Ribosome-templated azide–alkyne cycloadditions: synthesis of potent macrolide antibiotics by in situ click chemistry. J. Am. Chem. Soc. 138, 3136–3144 (2016).
Rodrik-Outmezguine, V. S. et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature 534, 272 (2016).
Scott, S. K. in DNA-Encoded Chemical Libraries: Methods and Protocols (eds Israel, D. & Ding, Y.) 39–43 (Springer, 2022).
Jemielity, J., Chrominski, M., Ziemkiewicz, K. & Kowalska, J. Introducing SuFNucs: sulfamoyl-fluoride-functionalized nucleosides that undergo sulfur fluoride exchange reaction. Org. Lett. 24, 4977–4981 (2022).
You, Y. et al. Sulfur(VI) fluoride exchange as a key reaction for synthesizing biaryl sulfate core derivatives as potent hepatitis C virus NS5A inhibitors and their structure–activity relationship studies. RSC Adv. 8, 31803–31821 (2018).
Zhao, W. J. et al. Synthesis of novel pesticidal N,N’-disubstituted sulfamide derivatives using sulfur(VI) fluorine exchange click reaction. J. Argric. Food Chem. 69, 5798–5803 (2021).
Liu, F. et al. Biocompatible sufex click chemistry: thionyl tetrafluoride (SOF4)-derived connective hubs for bioconjugation to DNA and proteins. Angew. Chem. Int. Ed. 58, 8029–8033 (2019).
Kitamura, S. et al. Sulfur(VI) fluoride exchange (SuFEx)-enabled high-throughput medicinal chemistry. J. Am. Chem. Soc. 142, 10899–10904 (2020).
Garnar-Wortzel, L. et al. Chemical inhibition of ENL/AF9 YEATS domains in acute leukemia. ACS Cent. Sci. 7, 815–830 (2021).
Singh, J., Petter, R. C., Baillie, T. A. & Whitty, A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 10, 307–317 (2011).
De Vita, E. 10 years into the resurgence of covalent drugs. Future Med. Chem. 13, 193–210 (2021).
Gehringer, M. & Laufer, S. A. Emerging and re-emerging warheads for targeted covalent inhibitors: applications in medicinal chemistry and chemical biology. J. Med. Chem. 62, 5673–5724 (2019).
Jones, L. H. in Design of Covalent-Based Inhibitors Vol. 56 (eds Ward, R. A. & Grimster, N. P.) 95–134 (Elsevier, 2021).
Myers, D. K. & Kemp, A. Inhibition of esterases by the fluorides of organic acids. Nature 173, 33–34 (1954).
Fahrney, D. E. & Gold, A. M. Sulfonyl fluorides as inhibitors of esterases. I. Rates of reaction with acetylcholinesterase, α-chymotrypsin, and trypsin. J. Am. Chem. Soc. 85, 997–1000 (1963).
Gold, A. M. & Fahrney, D. Sulfonyl fluorides as inhibitors of esterases. II. Formation and reaction of phenylmethanesulfonyl alpha-chymotrypsin. Biochemistry 3, 783–791 (1964).
Gold, A. M. Sulfonyl fluorides as inhibitors of esterases. III. Identification of serine as the site of sulfonylation in phenylmethanesulfonyl α-chymotrypsin. Biochemistry 4, 897–901 (1965).
Baker, B. R. & Hurlbut, J. A. Irreversible enzyme inhibitors. CXIV. Proteolytic enzymes. 4. Additional active-site-directed irreversible inhibitors of α-chymotrypsin derived from phenoxyacetamides bearing a terminal sulfonyl fluoride. J. Med. Chem. 11, 241–245 (1968).
Baker, B. R. & Hurlbut, J. A. Irreversible enzyme inhibitors. CXIII. Proteolytic enzymes. 3. Active-site-directed irreversible inhibitors of α-chymotrypsin derived from phenoxyacetamides with an N-fluoro-sulfonylphenyl substituent. J. Med. Chem. 11, 233–241 (1968).
Baker, B. R. Specific irreversible enzyme inhibitors. Annu. Rev. Pharmacol. 10, 35–50 (1970).
Laura, R., Robison, D. J. & Bing, D. H. (p-Amidinophenyl)methanesulfonyl fluoride, an irreversible inhibitor of serine proteases. Biochem 19, 4859–4864 (1980).
Lively, M. O. & Powers, J. C. Specificity and reactivity of human granulocyte elastase and cathepsin G, porcine pancreatic elastase, bovine chymotrypsin and trypsin toward inhibition with sulfonyl flourides. Biochim. Biophys. Acta Enzymol. 525, 171–179 (1978).
Yoshimura, T., Barker, L. N. & Powers, J. C. Specificity and reactivity of human-leukocyte elastase, porcine pancreatic elastase, human granulocyte cathepsin-G, and bovine pancreatic chymotrypsin with arylsulfonyl fluorides. Discovery of a new series of potent and specific irreversible elastase inhibitors. J. Biol. Chem. 257, 5077–5084 (1982).
Shannon, D. A. et al. Sulfonyl fluoride analogues as activity-based probes for serine proteases. ChemBioChem 13, 2327–2330 (2012).
Zheng, Q. et al. SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase. Proc. Natl Acad. Sci. USA 116, 18808–18814 (2019).
Keeley, A., Petri, L., Abranyi-Balogh, P. & Keseru, G. M. Covalent fragment libraries in drug discovery. Drug Discov. Today 25, 983–996 (2020).
Zhang, J. et al. Identification of simple arylfluorosulfates as potent agents against resistant bacteria. Proc. Natl Acad. Sci. USA 118, e2103513118 (2021).
Tolmachova, K. A. et al. (Chlorosulfonyl)benzenesulfonyl fluorides — versatile building blocks for combinatorial chemistry: design, synthesis and evaluation of a covalent inhibitor library. ACS Comb. Sci. 20, 672–680 (2018).
Cheng, Y. et al. Diversity oriented clicking delivers β-substituted alkenyl sulfonyl fluorides as covalent human neutrophil elastase inhibitors. Proc. Natl Acad. Sci. USA 119, e2208540119 (2022).
Bum-Erdene, K. et al. Small-molecule covalent bond formation at tyrosine creates a binding site and inhibits activation of Ral GTPases. Proc. Natl Acad. Sci. USA 117, 7131–7139 (2020).
Grimster, N. P. et al. Aromatic sulfonyl fluorides covalently kinetically stabilize transthyretin to prevent amyloidogenesis while affording a fluorescent conjugate. J. Am. Chem. Soc. 135, 5656–5668 (2013).
Baranczak, A. et al. A fluorogenic aryl fluorosulfate for intraorganellar transthyretin imaging in living cells and in Caenorhabditis elegans. J. Am. Chem. Soc. 137, 7404–7414 (2015).
Hett, E. C. et al. Rational targeting of active-site tyrosine residues using sulfonyl fluoride probes. ACS Chem. Biol. 10, 1094–1098 (2015).
Fadeyi, O. O. et al. Covalent enzyme inhibition through fluorosulfate modification of a noncatalytic serine residue. ACS Chem. Biol. 12, 2015–2020 (2017).
Gambini, L. et al. Covalent inhibitors of protein–protein interactions targeting lysine, tyrosine, or histidine residues. J. Med. Chem. 62, 5616–5627 (2019).
Teng, M. X. et al. Rationally designed covalent BCL6 inhibitor that targets a tyrosine residue in the homodimer interface. ACS Med. Chem. Lett. 11, 1269–1273 (2020).
Ippolito, J. A. et al. Covalent inhibition of wild-type HIV-1 reverse transcriptase using a fluorosulfate warhead. ACS Med. Chem. Lett. 12, 249–255 (2021).
Bolding, J. E. et al. Aryl fluorosulfate based inhibitors that covalently target the SIRT5 lysine deacylase. Angew. Chem. Int. Ed. 61, e2022045 (2022).
Baggio, C. et al. Aryl-fluorosulfate-based Lysine covalent pan-inhibitors of apoptosis protein (IAP) antagonists with cellular efficacy. J. Med. Chem. 62, 9188–9200 (2019).
Udompholkul, P., Baggio, C., Gambini, L., Alboreggia, G. & Pellecchia, M. Lysine covalent antagonists of melanoma inhibitors of apoptosis protein. J. Med. Chem. 64, 16147–16158 (2021).
Beerkens, B. L. H. et al. Development of subtype-selective covalent ligands for the adenosine A(2B) receptor by tuning the reactive group. RSC Med. Chem. 13, 850–856 (2022).
Cruite, J. T. et al. Cereblon covalent modulation through structure-based design of histidine targeting chemical probes. RSC Chem. Biol. 3, 1105–1110 (2022).
Gushwa, N. N., Kang, S., Chen, J. & Taunton, J. Selective targeting of distinct active site nucleophiles by irreversible Src-family kinase inhibitors. J. Am. Chem. Soc. 134, 20214–20217 (2012).
Zhao, Q. et al. Broad-spectrum kinase profiling in live cells with lysine-targeted sulfonyl fluoride probes. J. Am. Chem. Soc. 139, 680–685 (2017).
Chen, W. et al. Arylfluorosulfates inactivate intracellular lipid binding protein(s) through chemoselective sufex reaction with a binding site Tyr residue. J. Am. Chem. Soc. 138, 7353–7364 (2016).
Brighty, G. J. et al. Using sulfuramidimidoyl fluorides that undergo sulfur(VI) fluoride exchange for inverse drug discovery. Nat. Chem. 12, 906–913 (2020).
Kline, G. M., Nugroho, K. & Kelly, J. W. Inverse drug discovery identifies weak electrophiles affording protein conjugates. Curr. Opin. Chem. Biol. 67, 102113 (2022).
Dong, J., Krasnova, L. & Sharpless, K. B. Fluorosulfonyl sEH inhibitors. Patent WO2015188060A1 (2015).
Liu, Z., Meng, G., Guo, T., Dong, J. & Wu, P. Novel approaches to access arylfluorosulfates and sulfamoyl fluorides based on sulfur (VI) fluoride exchange. Curr. Protoc. Chem. Biol. 11, e64 (2019).
Gambhir, S. S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2, 683–693 (2002).
Phelps, M. E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl Acad. Sci. USA 97, 9226–9233 (2000).
Inkster, J. A. et al. Sulfonyl fluoride-based prosthetic compounds as potential 18F labelling agents. Eur. J. Chem. 18, 11079–11087 (2012).
Zhang, B. et al. Synthesis, bioconjugation and stability studies of F-18 ethenesulfonyl fluoride. J. Label. Comp. Radiopharm. 61, 847–856 (2018).
Zhang, W. et al. Synthesis and F-18 labeling of alkenyl sulfonyl fluorides via an unconventional elimination pathway. Org. Lett. 24, 4992–4997 (2022).
Kwon, Y.-D. et al. Synthesis of 18F-labeled aryl fluorosulfates via nucleophilic radiofluorination. Org. Lett. 22, 5511–5516 (2020).
Jeon, M. H. et al. Late-stage F-18/F-19 isotopic exchange for the synthesis of F-18-labeled sulfamoyl fluorides. Org. Lett. 23, 2766–2771 (2021).
Li, H. et al. High-performing polysulfate dielectrics for electrostatic energy storage under harsh conditions. Joule 7, 95–111 (2023).
Li, S. et al. SuFExable polymers with helical structures derived from thionyl tetrafluoride. Nat. Chem. 13, 858–867 (2021). This work prepares unprecedented helical SuFEx-based polymers from SOF4 and showcases the SuFEx-based post-polymerization modifications that are possible.
Liang, D.-D. et al. Silicon-free SuFEx reactions of sulfonimidoyl fluorides: scope, enantioselectivity, and mechanism. Angew. Chem. Int. Ed. 59, 7494–7500 (2020).
Kim, H. et al. Chain-growth sulfur(VI) fluoride exchange polycondensation: molecular weight control and synthesis of degradable polysulfates. ACS Cent. Sci. 7, 1919–1928 (2021).
Subramaniam, M., Ruggeri, F. S. & Zuilhof, H. Degradable click-reaction-based polymers as highly functional materials. Matter 5, 2490–2492 (2022).
Choi, E. J., Jung, D., Kim, J.-S., Lee, Y. & Kim, B. M. Chemoselective tyrosine bioconjugation through sulfate click reaction. Chem. Eur. J. 24, 10948–10952 (2018).
Chen, W. T. et al. Synthesis of sulfotyrosine-containing peptides by incorporating fluorosulfated tyrosine using an Fmoc-based solid-phase strategy. Angew. Chem. Int. Ed. 55, 1835–1838 (2016).
McCann, H. M. et al. Covalent immune proximity-induction strategy using SuFEx engineered bifunctional viral peptides. ACS Chem. Biol. 17, 1269–1281 (2022).
Faucher, F. F. et al. Solid phase synthesis of fluorosulfate containing macrocycles for chemoproteomic workflows. Isr. J. Chem. 63, e202300020 (2023).
Hoppmann, C. & Wang, L. Proximity-enabled bioreactivity to generate covalent peptide inhibitors of p53-Mdm4. Chem. Commun. 52, 5140–5143 (2016).
Tabuchi, Y., Watanabe, T., Katsuki, R., Ito, Y. & Taki, M. Direct screening of a target-specific covalent binder: stringent regulation of warhead reactivity in a matchmaking environment. Chem. Commun. 57, 5378–5381 (2021).
Tabuchi, Y., Yang, J. & Taki, M. Inhibition of thrombin activity by a covalent-binding aptamer and reversal by the complementary strand antidote. Chem. Commun. 57, 2483–2486 (2021).
Tabuchi, Y., Yang, J. & Taki, M. Relative nuclease resistance of a DNA aptamer covalently conjugated to a target protein. Int. J. Mol. Sci. 23, 7778 (2022).
Wang, N. et al. Genetically encoding fluorosulfate-l-tyrosine to react with lysine, histidine, and tyrosine via SuFEx in proteins in vivo. J. Am. Chem. Soc. 140, 4995–4999 (2018).
Liu, J. et al. A genetically encoded fluorosulfonyloxybenzoyl-l-lysine for expansive covalent bonding of proteins via SuFEx chemistry. J. Am. Chem. Soc. 143, 10341–10351 (2021).
Yu, B. et al. Accelerating PERx reaction enables covalent nanobodies for potent neutralization of SARS-CoV-2 and variants. Chem 8, 2766–2783 (2022).
Han, Y. et al. Covalently engineered protein minibinders with enhanced neutralization efficacy against escaping SARS-CoV-2 variants. J. Am. Chem. Soc. 144, 5702–5707 (2022).
Li, Q. et al. Developing covalent protein drugs via proximity-enabled reactive therapeutics. Cell 182, 85–97.e16 (2020).
Sun, W. et al. Genetically encoded chemical crosslinking of RNA in vivo. Nat. Chem. 15, 21–32 (2023).
Li, S., Wang, N., Yu, B., Sun, W. & Wang, L. Genetically encoded chemical crosslinking of carbohydrate. Nat. Chem. 15, 33–42 (2023).
Yang, B. et al. Genetically introducing biochemically reactive amino acids dehydroalanine and dehydrobutyrine in proteins. J. Am. Chem. Soc. 141, 7698–7703 (2019).
Narayanan, A. & Jones, L. H. Sulfonyl fluorides as privileged warheads in chemical biology. Chem. Sci. 6, 2650–2659 (2015).
Mukherjee, H. et al. A study of the reactivity of S(VI)–F containing warheads with nucleophilic amino-acid side chains under physiological conditions. Org. Biomol. Chem. 15, 9685–9695 (2017).
Fadeyi, O. et al. Chemoselective preparation of clickable aryl sulfonyl fluoride monomers: a toolbox of highly functionalized intermediates for chemical biology probe synthesis. ChemBioChem 17, 1925–1930 (2016).
Mallia, C. J. & Baxendale, I. R. The use of gases in flow synthesis. Org. Process Res. Dev. 20, 327–360 (2016).
Zhang, Z.-X. & Willis, M. C. Sulfondiimidamides as new functional groups for synthetic and medicinal chemistry. Chem 8, 1137–1146 (2022).
Greed, S. et al. Synthesis of highly enantioenriched sulfonimidoyl fluorides and sulfonimidamides by stereospecific sulfur–fluorine exchange (SuFEx) reaction. Eur. J. Chem. 26, 12533–12538 (2020).
Zhao, Z. et al. Organoids. Nat. Rev. Methods Primers 2, 94 (2022).
Sun, S. et al. Phosphorus fluoride exchange: multidimensional catalytic click chemistry from phosphorus connective hubs. Chem 9, 1–16 (2023).
Baum, Z. J. et al. Artificial intelligence in chemistry: current trends and future directions. J. Chem. Inf. Model. 61, 3197–3212 (2021).
No authors listed. New horizons in chemical space. Nat. Rev. Drug Discov. 3, 375–375 (2004).
Moorhouse, A. D., Homer, J. A. & Moses, J. E. The certainty of a few good reactions. Chem 9, 1–15 (2023).
Acknowledgements
J.E.M. is financially supported by the NCI Cancer Center Support Grant 5P30CA045508, The F. M. Kirby Foundation, Sunshine Foundation, S. J. Edwards, The Starr Foundation and The Wasily Family Foundation. J.D. is thankful for the financial support from Ministry of Science and Technology of China, Major State Basic Research Development Program of China (2021YFF0701704) and Shanghai Pilot Program for Basic Research, Shanghai Jiao Tong University. H.Z. is supported by the National Science Foundation of China (Grants 21871208 and 22011530163, both to H.Z.), Tianjin University and Wageningen University (both H.Z. and N.K.). K.B.S. is supported by the National Institutes of Health (R01GM117145). Q.Z. is the Connie and Bob Lurie Fellow of the Damon Runyon Cancer Research Foundation (DRG-2434-21). B.M.K. was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and funded by the Ministry of Science and ICT (No. NRF-2017M3A9F6029755).
Author information
Authors and Affiliations
Contributions
Introduction (J.A.H. and J.E.M.); Experimentation (J.A.H., L.X., N.K., H.Z., J.D. and J.E.M.), Applications (J.A.H., N.K., Q.Z., E.J.C., B.M.K., K.B.S., H.Z. and J.E.M.); Reproducibility and data deposition (J.A.H. and J.E.M.); Limitations and optimizations (J.A.H. and J.E.M.); Outlook (J.A.H. and J.E.M.); Overview of the Primer (J.A.H. and J.E.M.). J.A.H. and J.E.M. coordinated the compilation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Details of patents covering material related to the content of this Primer can be found in the Supplementary information. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Nicholas Ball, Tae Kyo Park and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Dispersity
-
A measure of the heterogeneity of a polymer sample.
- Organosuperbase
-
Strong organic base with a basicity greater than that of proton sponge (pKBH+ 18.6 in MeCN).
- Orthogonality
-
The selective reactivity of functional groups only when exposed to specific reaction conditions, while remaining untouched in all other instances.
- SuFExability
-
The propensity of an S–F-containing functional group to undergo catalyst-mediated exchange.
- SuFExable
-
Functional groups containing sulfur-fluoride bonds that are compatible electrophiles in the sulfur fluoride exchange (SuFEx) reaction (such as sulfonyl fluorides, fluorosulfates and iminosulfur oxydifluorides).
- SuFExed
-
The incorporation of a sulfur fluoride exchangeable (SuFExable) group to the native structure of a molecule (such as ligand and drug).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Homer, J.A., Xu, L., Kayambu, N. et al. Sulfur fluoride exchange. Nat Rev Methods Primers 3, 58 (2023). https://doi.org/10.1038/s43586-023-00241-y
Published:
DOI: https://doi.org/10.1038/s43586-023-00241-y
This article is cited by
-
Reductive sulfinylation by nucleophilic chain isomerization of sulfonylpyridinium
Nature Communications (2025)
-
Diversity oriented clicking for modular synthesis
Nature Reviews Methods Primers (2025)
-
Intramolecular chalcogen bonding activated SuFEx click chemistry for efficient organic-inorganic linking
Nature Communications (2024)
-
Iterative SuFEx approach for sequence-regulated oligosulfates and its extension to periodic copolymers
Nature Communications (2024)
-
Computational analysis of modular diazotransfer reactions for the development of predictive reactivity models and diazotransfer reagents
Nature Synthesis (2024)