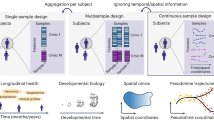
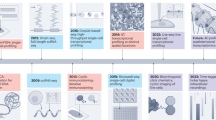
Abstract
Much like solving a game of Clue, understanding physiological mysteries involves answering who, what, when and where. Multiomics approaches delve into cellular and molecular identities (the who and what), whereas longitudinal data collection addresses the when. Spatial dimensions address the where. This Primer discusses current technologies enabling quantification across biological scales, emphasizing the importance of retaining the spatial dimension of that data. We outline experimental design considerations, including targeted versus untargeted approaches, sample types, biological scale and four main classes of molecule detection. Spatial analytics are explored, covering questioning approaches, analytical platforms, image segmentation and sampling. Example applications, reproducibility considerations, limitations and our outlook for the future are provided. Our goal is to unite spatial platforms and biological scales that not commonly brought together to encourage collaboration and innovation between diverse biological fields, offering a conceptional framework and an apples-to-apples comparison for understanding major technologies to foster cross-disciplinary dialogue.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
02 September 2024
A Correction to this paper has been published: https://doi.org/10.1038/s43586-024-00352-0
References
Walsh, L. & Quail, D. Decoding the tumor microenvironment with spatial technologies. Nat. Immunol. 24, 1982–1993 (2023).
Lewis, S. M. et al. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 18, 997–1012 (2021).
Bressan, D., Battistoni, G. & Hannon, G. J. The dawn of spatial omics. Science 381, eabq4964 (2023).
van Dam, S., Baars, M. J. D. & Vercoulen, Y. Multiplex tissue imaging: spatial revelations in the tumor microenvironment. Cancers 14, 3170 (2022).
Hayes, M. & Lim, A. Increase cell painting assay throughput using an automated workstation. Molecular Devices https://www.moleculardevices.com/en/assets/app-note/dd/img/increase-cell-painting-assay-throughput-using-an-automated-workstation (2022).
van Dijk, D. et al. Recovering gene interactions from single-cell data using data diffusion. Cell 174, 716–729.e27 (2018).
Väyrynen, J. P. et al. Spatial organization and prognostic significance of NK and NKT-like cells via multimarker analysis of the colorectal cancer microenvironment. Cancer Immunol. Res. 10, 215–227 (2022).
Magaki, S., Hojat, S. A., Wei, B., So, A. & Yong, W. H. An introduction to the performance of immunohistochemistry. Methods Mol. Biol. 1897, 289–298 (2019).
Kim, S.-W., Roh, J. & Park, C.-S. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J. Pathol. Transl. Med. 50, 411–418 (2016).
Meyerholz, D. K. & Beck, A. P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Invest. 98, 844–855 (2018).
Peters, S. The art of frozen tissue sectioning. Leica Biosystems https://www.leicabiosystems.com/us/knowledge-pathway/the-art-of-embedding-tissue-for-frozen-section/ (2024).
O’Rourke, M. B., Raymond, B. B. A., Djordjevic, S. P. & Padula, M. P. A versatile cost-effective method for the analysis of fresh frozen tissue sections via matrix-assisted laser desorption/ionisation imaging mass spectrometry. Rapid Commun. Mass Spectrom. 29, 637–644 (2015).
Shimma, S. & Sugiura, Y. Effective sample preparations in imaging mass spectrometry. Mass Spectrom. 3, S0029 (2014).
Coulter, H. D. & Terracio, L. Preparation of biological tissues for electron microscopy by freeze-drying. Anat. Rec. 187, 477–494 (1977).
Shao, F. et al. Frozen tissue preparation for high-resolution multiplex histological analyses of human brain specimens. J. Neurooncol. 135, 21–28 (2017).
Warram, J. M. et al. Antibody based imaging strategies of cancer. Cancer Metastasis Rev. 33, 809–822 (2014).
Livet, J. et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62 (2007).
Weber, K., Bartsch, U., Stocking, C. & Fehse, B. A multicolor panel of novel lentiviral ‘gene ontology’ (LeGO) vectors for functional gene analysis. Mol. Ther. 16, 698–706 (2008).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Refaat, A. et al. In vivo fluorescence imaging: success in preclinical imaging paves the way for clinical applications. J. Nanobiotechnol. 20, 450 (2022).
Molotkov, A. et al. 3D optical/CT as a preclinical companion imaging platform for glioblastoma drug development. Drug Deliv. 27, 1686–1694 (2020).
Olsen, C. & Ha, H. Evaluate cell migration with FluoroBlok Inserts on the SpectraMax MiniMax Cytometer. Molecular Devices https://www.moleculardevices.com/en/assets/app-note/br/evaluate-cell-migration-with-fluoroblok-inserts-on-the-spectramax-minimax-cytometer (2017).
Prigozhin, M. B. et al. Bright sub-20-nm cathodoluminescent nanoprobes for electron microscopy. Nat. Nanotechnol. 14, 420–425 (2019).
Shahidi, R. et al. A serial multiplex immunogold labeling method for identifying peptidergic neurons in connectomes. eLife 4, e11147 (2015).
Lin, J.-R. et al. High-plex immunofluorescence imaging and traditional histology of the same tissue section for discovering image-based biomarkers. Nat. Cancer 4, 1036–1052 (2023).
Carstens, J. L. et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 8, 15095 (2017). This article presents the utility of spatial point processing of T cell infiltration as a prognostic factor in pancreatic cancers.
Ragan, T. et al. Serial two-photon tomography: an automated method for ex-vivo mouse brain imaging. Nat. Methods 9, 255–258 (2012).
Tsujikawa, T. et al. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Rep. 19, 203–217 (2017).
Guo, C. et al. Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance. Nature 623, 1053–1061 (2023).
Gerdes, M. J. et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc. Natl Acad. Sci. USA 110, 11982–11987 (2013).
Jhaveri, N. et al. Mapping the spatial proteome of head and neck tumors: key immune mediators and metabolic determinants in the tumor microenvironment. GEN Biotechnol. 2, 418–434 (2023).
Kinkhabwala, A. et al. MACSima imaging cyclic staining (MICS) technology reveals combinatorial target pairs for CAR T cell treatment of solid tumors. Sci. Rep. 12, 1911 (2022).
Neil, E. et al. Spatial protein and RNA analysis on the same tissue section using MICS technology. Preprint at bioRxiv https://doi.org/10.1101/2023.10.27.564191 (2023).
Saka, S. K. et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat. Biotechnol. 37, 1080–1090 (2019).
Jarosch, S. et al. Multimodal immune cell phenotyping in GI biopsies reveals microbiome-related T cell modulations in human GvHD. Cell Rep. Med. 4, 101125 (2023).
Lin, J.-R. et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife 7, 46 (2018).
Claes, B. S. R. et al. MALDI-IHC-guided in-depth spatial proteomics: targeted and untargeted MSI combined. Anal. Chem. 95, 2329–2338 (2023).
Lim, M. J. et al. MALDI HiPLEX-IHC: multiomic and multimodal imaging of targeted intact proteins in tissues. Front. Chem. 11, 1182404 (2023).
Yagnik, G., Liu, Z., Rothschild, K. J. & Lim, M. J. Highly multiplexed immunohistochemical MALDI-MS imaging of biomarkers in tissues. J. Am. Soc. Mass Spectrom. 32, 977–988 (2021).
Liu, Y. et al. High-plex protein and whole transcriptome co-mapping at cellular resolution with spatial CITE-seq. Nat. Biotechnol. 41, 1405–1409 (2023).
Hernandez, S. et al. Challenges and opportunities for immunoprofiling using a spatial high-plex technology: the Nanostring GeoMx® Digital Spatial Profiler. Front. Oncol. 12, 890410 (2022).
Llorens-Bobadilla, E. et al. Solid-phase capture and profiling of open chromatin by spatial ATAC. Nat. Biotechnol. 41, 1085–1088 (2023).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Nagendran, M. et al. 1457 Visium HD enables spatially resolved, single-cell scale resolution mapping of FFPE human breast cancer tissue. J. Immunother. Cancer https://doi.org/10.1136/jitc-2023-SITC2023.1457 (2023).
Rodriques, S. G. et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467 (2019).
Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019).
Wirth, J. et al. Spatial transcriptomics using multiplexed deterministic barcoding in tissue. Nat. Commun. 14, 1523 (2023).
Liu, Y. et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell 183, 1665–1681.e18 (2020).
Fu, X. et al. Polony gels enable amplifiable DNA stamping and spatial transcriptomics of chronic pain. Cell 185, 4621–4633.e17 (2022).
Cho, C.-S. et al. Microscopic examination of spatial transcriptome using Seq-Scope. Cell 184, 3559–3572.e22 (2021).
Wei, X. et al. Single-cell Stereo-seq reveals induced progenitor cells involved in axolotl brain regeneration. Science 377, eabp9444 (2022).
Borm, L. E. et al. Scalable in situ single-cell profiling by electrophoretic capture of mRNA using EEL FISH. Nat. Biotechnol. 41, 222–231 (2023).
Manesse, M., Patel, K. K., Bobrow, M. & Downing, S. R. The InSituPlex® staining method for multiplexed immunofluorescence cell phenotyping and spatial profiling of tumor FFPE samples. Methods Mol. Biol. 2055, 585–592 (2020).
Chen, J. et al. Molecular and spatial signatures of mouse embryonic endothelial cells at single-cell resolution. Circ. Res. 134, 529–546 (2024).
Wang, C. Y. et al. Molecular self-avoidance in synaptic neurexin complexes. Sci. Adv. 7, eabk1924 (2021).
Eser, P. Ö. et al. Oncogenic switch and single-agent MET inhibitor sensitivity in a subset of EGFR-mutant lung cancer. Sci. Transl. Med. 13, eabb3738 (2021).
Janesick, A. et al. High resolution mapping of the tumor microenvironment using integrated single-cell, spatial and in situ analysis. Nat. Commun. 14, 8353 (2023).
He, S. et al. High-plex imaging of RNA and proteins at subcellular resolution in fixed tissue by spatial molecular imaging. Nat. Biotechnol. 40, 1794–1806 (2022).
Wang, H. et al. Systematic benchmarking of imaging spatial transcriptomics platforms in FFPE tissues. Preprint at bioRxiv https://doi.org/10.1101/2023.12.07.570603 (2023). The authors compare similar spatial transcriptomics platforms for performance, consistency and capability to capture transcripts.
Manshouri, T. et al. GLI1 activates pro-fibrotic pathways in myelofibrosis fibrocytes. Cell Death Dis. 13, 481 (2022).
Nagarajan, M. B., Tentori, A. M., Zhang, W. C., Slack, F. J. & Doyle, P. S. Spatially resolved and multiplexed microRNA quantification from tissue using nanoliter well arrays. Microsyst. Nanoeng. 6, 1–9 (2020).
Zhao, T. et al. Spatial genomics enables multi-modal study of clonal heterogeneity in tissues. Nature 601, 85–91 (2022).
Li, Z. et al. Single-cell lipidomics with high structural specificity by mass spectrometry. Nat. Commun. 12, 2869 (2021).
Maciel, L. Í. L. et al. Desorption electrospray ionization and matrix-assisted laser desorption/ionization as imaging approaches for biological samples analysis. Anal. Bioanal. Chem. 415, 4125–4145 (2023).
Moore, J. L. & Charkoftaki, G. A guide to MALDI imaging mass spectrometry for tissues. J. Proteome Res. 22, 3401–3417 (2023). An introduction to matrix-assisted laser desorption ionization mass spectrometry imaging highlighting what types of analyses are possible with fresh frozen versus formalin-fixed paraffin-embedded tissue.
Gessel, M. M., Norris, J. L. & Caprioli, R. M. MALDI imaging mass spectrometry: spatial molecular analysis to enable a new age of discovery. J. Proteom. 107, 71–82 (2014).
Jia, F., Zhao, X. & Zhao, Y. Advancements in ToF-SIMS imaging for life sciences. Front. Chem. 11, 1237408 (2023).
Vickerman, C. J. Molecular imaging and depth profiling by mass spectrometry — SIMS, MALDI or DESI? Analyst 136, 2199–2217 (2011).
He, M. J. et al. Comparing DESI-MSI and MALDI-MSI mediated spatial metabolomics and their applications in cancer studies. Front. Oncol. 12, 891018 (2022).
Claude, E., Towers, M. & Ballantyne, J. Full spectrum molecular imaging on the SYNAPT XS: combined imaging techniques yield comprehensive results. Waters Corporation https://www.waters.com/content/dam/waters/en/app-notes/2020/720006851/720006851-en.pdf (2020).
Waters Corporation. MS Imaging – the SELECT SERIES™ MALDI and MRT. www.waters.com/webassets/cms/library/docs/720007652en.pdf (2022).
Oetjen, J. et al. Novel MALDI Imaging solution empowered by a timsTOF flex and dedicated bioinformatics pipeline for identification of lipids from tissue. https://www.bruker.com/en/applications/academia-life-science/imaging/maldi-imaging/SpatialOMx/_jcr_content/root/sections/section_1751684075/sectionpar/search.download-asset.pdf/10b76c66-2228-4ab5-bbbe-1f5937c336ee/1869079-lcms-156-maldi-imaging-by-timstof-flex-ebook-rev-01.pdf (2019).
Müller, L. et al. A high-throughput MALDI-TOF MS biochemical screen for small molecule inhibitors of the antigen aminopeptidase ERAP1. SLAS Discov. 28, 3–11 (2023).
Takahashi, Y. et al. Pharmacokinetic analysis by mass spectrometric imaging and highly sensitive quantification of metabolites by micro LC/MS. in 70th ASMS Conference on Mass Spectrometry and Allied Topics (ASMS, 2022).
Spengler, B. et al. High Resolution in Mass and Space: AP-MALDI Imaging Using Orbitrap-based Instrumentation (ASMS, 2012).
Satoh, T., Kubo, A., Shimma, S. & Toyoda, M. Mass spectrometry imaging and structural analysis of lipids directly on tissue specimens by using a spiral orbit type tandem time-of-flight mass spectrometer, spiralTOF-TOF. Mass Spectrom. 1, A0013 (2012).
Aichler, M. & Walch, A. MALDI imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab. Invest. 95, 422–431 (2015).
Zavalin, A., Yang, J., Hayden, K., Vestal, M. & Caprioli, R. M. Tissue protein imaging at 1 μm laser spot diameter for high spatial resolution and high imaging speed using transmission geometry MALDI TOF MS. Anal. Bioanal. Chem. 407, 2337–2342 (2015).
Munjoma, N. et al. Improved detection and identification of lipids using the Xevo G3 QToF mass spectrometer (ASMS, 2023).
Shrestha, B., Towers, M., Olivos, H., Midley, A. & Claude, E. Spatial distribution of isobaric lipids using high-resolution ion mobility with the DESI XS (ICMS, 2021).
Waters Corporation. MS Imaging — DESI XS and SELECT SERIES MRT (Waters Corporation, 2021).
Waters Corporation. Targeted MS imaging solution with DESI XS and Xevo TQ absolute brochure. Waters Corporation https://www.waters.com/webassets/cms/library/docs/720007975en.pdf (2023).
Eberlin, L. S. et al. DESI then MALDI mass spectrometry imaging of lipid and protein distributions in single tissue sections. Anal. Chem. 83, 8366–8371 (2011).
Sans, M. et al. Integrated spatial transcriptomics and lipidomics of precursor lesions of pancreatic cancer identifies enrichment of long chain sulfatide biosynthesis as an early metabolic alteration. Preprint at. bioRxiv https://doi.org/10.1101/2023.08.14.553002 (2023).
Wilkinson, D. J. Historical and contemporary stable isotope tracer approaches to studying mammalian protein metabolism. Mass Spectrom. Rev. 37, 57–80 (2018).
Chen, Y.-D. et al. Microscopy-guided subcellular proteomic discovery by high-speed ultra-content photo-biotinylation. Preprint at bioRxiv https://doi.org/10.1101/2023.12.27.573388 (2023).
Bortolomeazzi, M. et al. A SIMPLI (Single-cell Identification from MultiPLexed Images) approach for spatially-resolved tissue phenotyping at single-cell resolution. Nat. Commun. 13, 781 (2022).
Pemberton, J., Kala, S., Dikshit, A. & Hupple, C. 1490 High-plex co-detection of RNA and protein to explore tumor-immune interactions utilizing RNAscope with imaging mass cytometry. J. Immunother. Cancer 11, 1654 (2023).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Keren, L. et al. MIBI-TOF: a multiplexed imaging platform relates cellular phenotypes and tissue structure. Sci. Adv. 5, eaax5851 (2019).
Iyer, A., Hamers, A. A. J. & Pillai, A. B. CyTOF® for the masses. Front. Immunol. 13, 815828 (2022).
Kuett, L. et al. Three-dimensional imaging mass cytometry for highly multiplexed molecular and cellular mapping of tissues and the tumor microenvironment. Nat. Cancer 3, 122–133 (2022).
Ma, T. et al. Single-cell glycomics analysis by CyTOF-Lec reveals glycan features defining cells differentially susceptible to HIV. eLife 11, e78870 (2022).
Vaquero, J. J. & Kinahan, P. Positron emission tomography: current challenges and opportunities for technological advances in clinical and preclinical imaging systems. Annu. Rev. Biomed. Eng. 17, 385–414 (2015).
Amirrashedi, M., Zaidi, H. & Ay, M. R. Towards quantitative small-animal imaging on hybrid PET/CT and PET/MRI systems. Clin. Transl. Imaging 8, 243–263 (2020).
Goorden, M. C. et al. VECTor: a preclinical imaging system for simultaneous submillimeter SPECT and PET. J. Nucl. Med. 54, 306–312 (2013).
SOFIE. SOFIE-GNEXT PET/CT. SOFIE https://sofie.com/wp-content/uploads/2022/09/SOFIE-GNEXT-Brochure-Digital.pdf (2024).
Szanda, I. et al. National Electrical Manufacturers Association NU-4 performance evaluation of the PET component of the NanoPET/CT preclinical PET/CT scanner. J. Nucl. Med. 52, 1741–1747 (2011).
Molinos, C. et al. Low-dose imaging in a new preclinical total-body PET/CT scanner. Front. Med. 6, 88 (2019).
MR Solutions. MR solutions’ preclinical PET/CT product range molecular imaging. MR Solutions https://www.mrsolutions.com/molecular-imaging/molecular-imaging/pet-ct-molecular-imaging/ (2021).
Prieto, E. et al. Performance evaluation of a preclinical SPECT/CT system for multi-animal and multi-isotope quantitative experiments. Sci. Rep. 12, 18161 (2022).
Lukas, M., Kluge, A., Beindorff, N. & Brenner, W. Multi-isotope capabilities of a small-animal multi-pinhole SPECT system. J. Nucl. Med. 61, 152–161 (2020).
MR Solutions. MRS*SPECT/CT/MR — MR solutions. MR Solutions https://www.mrsolutions.com/molecular-imaging/molecular-imaging/spect-ct-mr/ (2021).
Sánchez, F. et al. ALBIRA: a small animal PET/SPECT/CT imaging system. Med. Phys. 40, 051906 (2013).
Krishnamoorthy, S. et al. Performance evaluation of the MOLECUBES β-CUBE — a high spatial resolution and high sensitivity small animal PET scanner utilizing monolithic LYSO scintillation detectors. Phys. Med. Biol. 63, 155013 (2018).
Braeckman, K., Neyt, S., Deprez, K., Vandeghinste, B. & Mollet, P. In vivo use of a compact high sensitivity mouse collimator. J. Nucl. Med. 63, 3326 (2022).
Cai, Z. et al. Early detection of increased marrow adiposity with age in rats using Z-spectral MRI at ultra-high field (7 T). NMR Biomed. 35, e4633 (2022).
Klohs, J. & Herrmann, S. Preclinical MRI of Neurological Diseases — From Morphological Imaging to the Mapping of Tissue Properties. White Paper https://ok.bruker.com/social-doc/Twitter/BBIO-Imaging/Bruker/uct,mri,nmi,bbio,bbioimaging,Imaging/ui/FZpNEq/ (2023).
MR Solutions. Preclinical MRI 3 T–4.7 T–7 T–9.4 T — Variable field — Cryogen-free. MR Solutions https://www.mrsolutions.com/mr-imaging/mr-imaging/mr-dry-magnet-cryogen-free/ (2021).
Nagy, K. et al. Performance evaluation of the small-animal nanoScan PET/MRI system. J. Nucl. Med. 54, 1825–1832 (2013).
Gsell, W. et al. Characterization of a preclinical PET insert in a 7 tesla MRI scanner: beyond NEMA testing. Phys. Med. Biol. 65, 245016 (2020).
Yang, M. et al. Multiplexed PET probes for imaging breast cancer early response to VEGF121/rGel treatment. Mol. Pharm. 8, 621–628 (2011).
Parot, V. et al. A new approach for multiplexed PET imaging. IEEE Nucl. Sci. Symposium Med. Imaging Conf. (IEEE, 2014).
Yang, J. J. et al. Rational design of protein-based MRI contrast agents. J. Am. Chem. Soc. 130, 9260–9267 (2008).
Kupče, Ē., Mote, K. R., Webb, A., Madhu, P. K. & Claridge, T. D. W. Multiplexing experiments in NMR and multi-nuclear MRI. Prog. Nucl. Magnet. Reson. Spectrosc. 124–125, 1–56 (2021).
Adams, S. R. et al. Multicolor electron microscopy for simultaneous visualization of multiple molecular species. Cell Chem. Biol. 23, 1417–1427 (2016).
Scotuzzi, M. et al. Multi-color electron microscopy by element-guided identification of cells, organelles and molecules. Sci. Rep. 7, 45970 (2017).
Sibai, M. et al. The spatial landscape of cancer hallmarks reveals patterns of tumor ecology. Preprint at bioRxiv https://doi.org/10.1101/2022.06.18.496114 (2023).
Dries, R. et al. Advances in spatial transcriptomic data analysis. Genome Res. 31, 1706–1718 (2021).
Liao, J. et al. De novo analysis of bulk RNA-seq data at spatially resolved single-cell resolution. Nat. Commun. 13, 6498 (2022).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 e21 (2019).
Ben-Chetrit, N. et al. Integration of whole transcriptome spatial profiling with protein markers. Nat. Biotechnol. 41, 788–793 (2023).
Palla, G. et al. Squidpy: a scalable framework for spatial omics analysis. Nat. Methods 19, 171–178 (2022).
Dries, R. et al. Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol. 22, 78 (2021).
Chidester, B., Zhou, T., Alam, S. & Ma, J. SPICEMIX enables integrative single-cell spatial modeling of cell identity. Nat. Genet. 55, 78–88 (2023).
Kiemen, A. L. et al. CODA: quantitative 3D reconstruction of large tissues at cellular resolution. Nat. Methods 19, 1490–1499 (2022).
Feng, H., Lin, L. & Chen, J. scDIOR: single cell RNA-seq data IO software. BMC Bioinformatics 23, 16 (2022).
Bosisio, F. M. et al. Next-generation pathology using multiplexed immunohistochemistry: mapping tissue architecture at single-cell level. Front. Oncol. 12, 918900 (2022).
Baars, M. J. D. et al. MATISSE: a method for improved single cell segmentation in imaging mass cytometry. BMC Biol. 19, 99 (2021).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Stirling, D. R. et al. CellProfiler 4: improvements in speed, utility and usability. BMC Bioinformatics 22, 433 (2021).
Palhares Viana, M. & Rafelski, S. A new open source toolkit for segmenting 3D intracellular structures in microscopy images. Biophys. J. 116, 290a (2019).
Bannon, D. et al. DeepCell Kiosk: scaling deep learning-enabled cellular image analysis with Kubernetes. Nat. Methods 18, 43–45 (2021).
DigitalSlideArchive/HistomicsTK. Digital Slide Archive (2024).
Lee, M. Y. et al. CellSeg: a robust, pre-trained nucleus segmentation and pixel quantification software for highly multiplexed fluorescence images. BMC Bioinformatics 23, 46 (2022).
Rueden, C. T. et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529 (2017).
Bankhead, P. et al. QuPath: open source software for digital pathology image analysis. Sci. Rep. 7, 16878 (2017).
Navikas, V. et al. Semi-automated approaches for interrogating spatial heterogeneity of tissue samples. Sci. Rep. 14, 5025 (2024).
Escobar Díaz Guerrero, R., Carvalho, L., Bocklitz, T., Popp, J. & Oliveira, J. L. Software tools and platforms in digital pathology: a review for clinicians and computer scientists. J. Pathol. Inf. 13, 100103 (2022). A high-level view of the current state of computational pathology introducing readers to both legacy and novel user-friendly pathology image analysis software comparing and contrasting their features for users to determine what is appropriate for their particular use case.
Durkee, M. S., Abraham, R., Clark, M. R. & Giger, M. L. Artificial intelligence and cellular segmentation in tissue microscopy images. Am. J. Pathol. 191, 1693–1701 (2021).
Bera, K., Schalper, K. A., Rimm, D. L., Velcheti, V. & Madabhushi, A. Artificial intelligence in digital pathology — new tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 16, 703–715 (2019).
Yuan, Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb. Perspect. Med. 6, a026583 (2016).
Barua, S. et al. Spatial interaction of tumor cells and regulatory T cells correlates with survival in non-small cell lung cancer. Lung Cancer 117, 73–79 (2018).
Maley, C. C., Koelble, K., Natrajan, R., Aktipis, A. & Yuan, Y. An ecological measure of immune-cancer colocalization as a prognostic factor for breast cancer. Breast Cancer Res. 17, 131 (2015).
Feng, Z. et al. Multispectral imaging of formalin-fixed tissue predicts ability to generate tumor-infiltrating lymphocytes from melanoma. J. Immunother. Cancer 3, 1–11 (2015).
Natrajan, R. et al. Microenvironmental heterogeneity parallels breast cancer progression: a histology–genomic integration analysis. PLoS Med. 13, e1001961 (2016).
Parra, E. R. Methods to determine and analyze the cellular spatial distribution extracted from multiplex immunofluorescence data to understand the tumor microenvironment. Front. Mol. Biosci. 8, 668340 (2021). A highly detailed review to a novice attempting to incorporate spatial analytical workflows in their assessment (such as the quantification of cell–cell infiltration and clustering at the tissue level), specifically on pathology-imaging-based data sets.
Haase, P. Spatial pattern analysis in ecology based on Ripley’s K-function: introduction and methods of edge correction. J. Vegetation Sci. 6, 575–582 (1995).
Schürch, C. M. et al. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell 182, 1341–1359.e19 (2020).
Summers, H. D., Wills, J. W. & Rees, P. Spatial statistics is a comprehensive tool for quantifying cell neighbor relationships and biological processes via tissue image analysis. Cell Rep. Methods 2, 100348 (2022).
Biancalani, T. et al. Deep learning and alignment of spatially resolved single-cell transcriptomes with Tangram. Nat. Methods 18, 1352–1362 (2021).
Zhu, J. & Sabatti, C. Integrative spatial single-cell analysis with graph-based feature learning. Preprint at bioRxiv https://doi.org/10.1101/2020.08.12.248971 (2020).
Svensson, V., Teichmann, S. A. & Stegle, O. SpatialDE: identification of spatially variable genes. Nat. Methods 15, 343–346 (2018).
Baranwal, M., Krishnan, S., Oneka, M., Frankel, T. & Rao, A. CGAT: cell graph attention network for grading of pancreatic disease histology images. Front. Immunol. 12, 727610 (2021).
Maseda, F., Cang, Z. & Nie, Q. DEEPsc: a deep learning-based map connecting single-cell transcriptomics and spatial imaging data. Front. Genet. 12, 636743 (2021).
Dezem, F. S. et al. A machine learning one-class logistic regression model to predict stemness for single cell transcriptomics and spatial omics. BMC Genomics 24, 717 (2023).
Burguet, J. & Andrey, P. Edge correction for intensity estimation of 3D heterogeneous point processes from replicated data. Spat. Stat. 36, 100421 (2020).
Stoyan, D. Spatial point patterns: methodology and applications with R. Biometric. J. 59, 219–220 (2017).
Lara, O. D. et al. Tumor core biopsies adequately represent immune microenvironment of high-grade serous carcinoma. Sci. Rep. 9, 17589 (2019).
Izawa, T. et al. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer 92, 1807–1817 (2001).
Trujillo, K. A., Jones, A. C., Griffith, J. K. & Bisoffi, M. Markers of field cancerization: proposed clinical applications in prostate biopsies. Prostate Cancer 2012, 302894 (2012).
Zhang, X., Cui, X. & Zhu, F. The effects of sample size on omics study: from the perspective of robustness and diagnostic accuracy. IOP Conf. Ser. Earth Environ. Sci. 252, 022127 (2019).
Lee, J., Yoo, M. & Choi, J. Recent advances in spatially resolved transcriptomics: challenges and opportunities. BMB Rep. 55, 113–124 (2022).
Neary-Zajiczek, L. et al. Minimum resolution requirements of digital pathology images for accurate classification. Med. Image Anal. 89, 102891 (2023).
Dakshinamoorthy, G. et al. Abstract 490: highly multiplexed single-cell spatial analysis of tissue specimens using CODEX. Cancer Res. 79, 490 (2019).
Athieniti, E. & Spyrou, G. M. A guide to multi-omics data collection and integration for translational medicine. Comput. Struct. Biotechnol. J. 21, 134–149 (2023).
Vandereyken, K., Sifrim, A., Thienpont, B. & Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 24, 494–515 (2023).
Cheng, M. et al. Spatially resolved transcriptomics: a comprehensive review of their technological advances, applications, and challenges. J. Genet. Genomics 50, 625–640 (2023).
Deng, Y., Bai, Z. & Fan, R. Microtechnologies for single-cell and spatial multi-omics. Nat. Rev. Bioeng. 1, 769–784 (2023).
Fortmann, S. D. et al. Fixation before dissociation using a deep eutectic solvent preserves in vivo states and phospho-signaling in single-cell sequencing. Preprint at bioRxiv https://doi.org/10.1101/2023.02.13.528370 (2023).
Pontén, F., Jirström, K. & Uhlen, M. The Human Protein Atlas — a tool for pathology. J. Pathol. 216, 387–393 (2008).
Lomakin, A. et al. Spatial genomics maps the structure, nature and evolution of cancer clones. Nature 611, 1–9 (2022).
Ferri-Borgogno, S. et al. Molecular, metabolic, and subcellular mapping of the tumor immune microenvironment via 3D targeted and non-targeted multiplex multi-omics analyses. Cancers 16, 846 (2024). This article provides a proof of concept to analytically combine multimodal spatial analyses.
Dilmetz, B. A. et al. Novel technical developments in mass spectrometry imaging in 2020: a mini review. Anal. Sci. Adv. 2, 225–237 (2021).
Ferri-Borgogno, S. et al. Spatial transcriptomics depict ligand–receptor crosstalk heterogeneity at the tumor–stroma interface in long-term ovarian cancer survivors. Cancer Res. 83, 1503–1516 (2023). This article uses spatial analyses to quantify secreted cytokine expression.
Kidd, S. et al. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS ONE 7, e30563 (2012).
Kang, Y. et al. 3D imaging analysis on an organoid-based platform guides personalized treatment in pancreatic ductal adenocarcinoma. J. Clin. Invest. 132, e151604 (2022).
Sekera, E. R., Akkaya-Colak, K. B., Lopez, A., Mihaylova, M. M. & Hummon, A. B. Mass spectrometry imaging and histology for the analysis of budding intestinal organoids. Anal. Chem. 96, 4251–4258 (2024).
Wang, Y. & Hummon, A. B. MS imaging of multicellular tumor spheroids and organoids as an emerging tool for personalized medicine and drug discovery. J. Biol. Chem. 297, 101139 (2021).
Zanotelli, V. R. et al. A quantitative analysis of the interplay of environment, neighborhood, and cell state in 3D spheroids. Mol. Syst. Biol. 16, e9798 (2020).
Berg, H. F. et al. Patient-derived organoids reflect the genetic profile of endometrial tumors and predict patient prognosis. Commun. Med. 1, 1–14 (2021).
Warchol, S. et al. Visinity: visual spatial neighborhood analysis for multiplexed tissue imaging data. IEEE Trans. Visual. Comput. Graph. 29, 1–11 (2022).
Zhao, E. et al. Spatial transcriptomics at subspot resolution with BayesSpace. Nat. Biotechnol. 39, 1375–1384 (2021).
Krishnan, S. N., Mohammed, S., Frankel, T. L. & Rao, A. GaWRDenMap: a quantitative framework to study the local variation in cell–cell interactions in pancreatic disease subtypes. Sci. Rep. 12, 3708 (2022).
Ahmed, J. & Yasin, A. S. Graph neural operators for classification of spatial transcriptomics data. Preprint at http://arxiv.org/abs/2302.00658 (2023).
Yuan, Y. & Bar-Joseph, Z. GCNG: graph convolutional networks for inferring gene interaction from spatial transcriptomics data. Genome Biol. 21, 300 (2020).
Hu, J. et al. SpaGCN: integrating gene expression, spatial location and histology to identify spatial domains and spatially variable genes by graph convolutional network. Nat. Methods 18, 1342–1351 (2021).
Dong, K. & Zhang, S. Deciphering spatial domains from spatially resolved transcriptomics with an adaptive graph attention auto-encoder. Nat. Commun. 13, 1739 (2022).
Long, Y. et al. Spatially informed clustering, integration, and deconvolution of spatial transcriptomics with GraphST. Nat. Commun. 14, 1155 (2023).
Abdelaal, T., Mourragui, S., Mahfouz, A. & Reinders, M. J. T. SpaGE: spatial gene enhancement using scRNA-seq. Nucleic Acids Res. 48, e107 (2020).
Pang, M., Su, K. & Li, M. Leveraging information in spatial transcriptomics to predict super-resolution gene expression from histology images in tumors. Preprint at bioRxiv https://doi.org/10.1101/2021.11.28.470212 (2021).
Acknowledgements
J.L.C. was supported by the University of Alabama at Birmingham start-up funds. A.R. and S.N.K. were supported by CCSG Bioinformatics Shared Resource 5 P30 CA046592, a gift from Agilent technologies and a Precision Health Investigator award from U-M Precision Health to A.R. along with L. Rozek and M. Sartor. S.N.K. and A.R. were partially supported by the NCI Grant R37-CA214955. S.N.K. and A.R. were also partially supported by the University of Michigan (U-M) start-up institutional research funds. S.N.K., S.F.-B. and A.R. were also supported by a Research Scholar Grant from the American Cancer Society (RSG-16-005-01). E.H.S. was supported by a Cancer Prevention and Research Institute of Texas (CPRIT) award RP190617. A.G.S. was supported by NIH funding R01CA279143, R01CA276540, R01CA240589 and the Preclinical Imaging Facility (5P30CA013148).
Author information
Authors and Affiliations
Contributions
Introduction (J.L.C., S.F.-B. and J.K.B.); Experimentation (J.L.C., S.N.K., A.R., A.G.S., E.H.S., S.F.-B. and J.K.B.); Results (J.L.C., S.N.K., A.R., S.F.-B. and J.K.B.); Applications (J.L.C. and J.K.B.); Reproducibility and data deposition (J.L.C., A.G.S., E.H.S. and J.K.B.); Limitations and optimizations (J.L.C., A.G.S. and J.K.B.); Outlook (J.L.C., E.H.S., S.F.-B. and J.K.B.); overview of the Primer (J.L.C.).
Corresponding authors
Ethics declarations
Competing interests
A.R. serves as a member for Voxel Analytics, LLC and consults for Genophyll, LLC. J.K.B. consults for Standard BioTools, Fortis Labs and Biogenex. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Yvonne Vercoulen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
2023 NIH Data Management and Sharing policy: https://oir.nih.gov/sourcebook/intramural-program-oversight/intramural-data-sharing/2023-nih-data-management-sharing-policy
Aylward.org: https://www.aylward.org/notes/open-access-medical-image-repositories
Giotto: https://giottosuite.readthedocs.io/en/latest
Image Data Resource: https://idr.openmicroscopy.org/
METASPACE: https://metaspace2020.eu/
NIH Scientific Data Sharing: https://sharing.nih.gov/data-management-and-sharing-policy/sharing-scientific-data/repositories-for-sharing-scientific-data
Open Science policy: https://research-and-innovation.ec.europa.eu/strategy/strategy-2020-2024/our-digital-future/open-science_en
PRIDE: https://www.ebi.ac.uk/pride/
Registry of Research Data Repositories: https://re3data.org
Seurat: https://satijalab.org/seurat
Squidpy: https://squidpy.readthedocs.io/en/stable
The Cancer Imaging Archive: https://www.cancerimagingarchive.net/
Supplementary information
Glossary
- Antigen retrieval
-
Process of reversing crosslinking resultant from fixation to make antigens accessible to antibodies. Common methods include heat-mediated retrieval using neutral pH or high pH buffers or enzymatic/proteolytic digestion.
- Autofluorescence
-
The light emitted by biological substances, specifically within 330–500 nm bands, which are often subtracted from optical-based detection methods by default.
- Drop-out
-
A phenomenon in which cells should express a certain marker but do not because the sample was too small to detect it. This is common in single-cell sequencing, but can be applied to any method functioning at the edges of detection.
- Image registration
-
The process in which cyclic or multimodal images have the pixels aligned to match the regions of the same tissue imaged at different times/across serial sections of the same tissue.
- Image segmentation
-
Partitioning of the image into objects or segments (organelle, cell, tissue or organ) that are biologically relevant or significant based on individual image pixel information.
- Multiplexing
-
Analysis of two or more molecules at the same time.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carstens, J.L., Krishnan, S.N., Rao, A. et al. Spatial multiplexing and omics. Nat Rev Methods Primers 4, 54 (2024). https://doi.org/10.1038/s43586-024-00330-6
Accepted:
Published:
DOI: https://doi.org/10.1038/s43586-024-00330-6