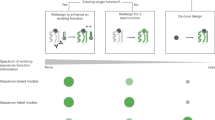
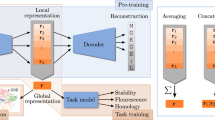
Abstract
Combining molecular modelling, machine-learned models and an increasingly detailed understanding of protein chemistry and physics, computational protein design and human expertise have been able to produce new protein structures, assemblies and functions that do not exist in nature. Currently, generative deep-learning-based methods, which exploit large databases of protein sequences and structures, are revolutionizing the field, leading to new capabilities, improved reliability and democratized access in protein design. This Primer provides an introduction to the main approaches in computational protein design, covering both physics-based and machine-learning-based tools. It aims to be accessible to biological, physical and computer scientists alike. Emphasis is placed on understanding the practical challenges arising from limitations in our fundamental understanding of protein structure and function and on recent developments and new ideas that may help transcend these.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
11 March 2025
In the version of the article initially published, Sophie Barbe’s email was incorrect and has now been amended in the HTML and PDF versions of the article.
References
Jiang, L. et al. De novo computational design of retro-aldol enzymes. Science 319, 1387–1391 (2008).
Arnold, F. H. Innovation by evolution: bringing new chemistry to life (nobel lecture). Angew. Chem. Int. Ed. 58, 14420–14426 (2019).
Winter, G. Harnessing evolution to make medicines (nobel lecture). Angew. Chem. Int. Ed. 58, 14438–14445 (2019).
Woolfson, D. N. A brief history of de novo protein design: minimal, rational, and computational. J. Mol. Biol. 433, 167160 (2021).
Chu, A. E., Lu, T. & Huang, P.-S. Sparks of function by de novo protein design. Nat. Biotechnol. 42, 203–215 (2024).
Arnold, F. H. Design by directed evolution. Acc. Chem. Res. 31, 125–131 (1998).
Arnold, F. H. Directed evolution: bringing new chemistry to life. Angew. Chem. Int. Ed. 57, 4143–4148 (2018).
Wang, Y. et al. Directed evolution: methodologies and applications. Chem. Rev. 121, 12384–12444 (2021).
Zeymer, C. & Hilvert, D. Directed evolution of protein catalysts. Annu. Rev. Biochem. 87, 131–157 (2018).
Korendovych, I. V. & DeGrado, W. F. De novo protein design, a retrospective. Q. Rev. Biophys. 53, e3 (2020).
Pan, X. & Kortemme, T. Recent advances in de novo protein design: principles, methods, and applications. J. Biol. Chem. 296, 100558 (2021).
Chen, K. & Arnold, F. H. Engineering new catalytic activities in enzymes. Nat. Catal. 3, 203–213 (2020).
Suleyman, M. & Bhaskar, M. The Coming Wave: Technology, Power, and the Twenty-first Century’s Greatest Dilemma (Crown, 2023).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Baek, M. et al. Efficient and accurate prediction of protein structure using RoseTTAFold2. Preprint at bioRxiv https://doi.org/10.1101/2023.05.24.542179 (2023).
Lin, Z. et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 379, 1123–1130 (2023).
Chai, C. D. et al. Chai-1: decoding the molecular interactions of life. Preprint at bioRxiv https://doi.org/10.1101/2024.10.10.615955 (2024).
Wohlwend, J. et al. Boltz-1 democratizing biomolecular interaction modeling. Preprint at bioRxiv https://doi.org/10.1101/2024.11.19.624167 (2024).
Wu, R. et al. High-resolution de novo structure prediction from primary sequence. Preprint at bioRxiv https://doi.org/10.1101/2022.07.21.500999 (2022).
Abramson, J. et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 630, 493–500 (2024).
Weijman, J. F. et al. Molecular architecture of the autoinhibited kinesin-1 lambda particle. Sci. Adv. 8, eabp9660 (2022).
Schweke, H. et al. An atlas of protein homo-oligomerization across domains of life. Cell 187, 999–1010.e15 (2024).
Shor, B. & Schneidman-Duhovny, D. CombFold: predicting structures of large protein assemblies using a combinatorial assembly algorithm and AlphaFold2. Nat. Methods 21, 477–487 (2024).
Krishna, R. et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 384, eadl2528 (2024).
Albanese, K. I. et al. Rationally seeded computational protein design of α-helical barrels. Nat. Chem. Biol. 20, 991–999 (2024).
Watson, J. L. et al. De novo design of protein structure and function with RFdiffusion. Nature 620, 1089–1100 (2023).
Dauparas, J. et al. Robust deep learning-based protein sequence design using ProteinMPNN. Science 378, 49–56 (2022).
Hsu, C. et al. Learning inverse folding from millions of predicted structures. In Proc. 39th International Conference on Machine Learning Vol. 162 (eds Chaudhuri, K. et al.) 8946–8970 (PMLR, 2022).
Akpinaroglu, D. et al. Structure-conditioned masked language models for protein sequence design generalize beyond the native sequence space. Preprint at bioRxiv https://doi.org/10.1101/2023.12.15.571823 (2023).
Gao, Z., Tan, C. & Li, S. Z. PiFold: toward effective and efficient protein inverse folding. In The Eleventh International Conference on Learning Representations, ICLR 2023 https://openreview.net/pdf?id=oMsN9TYwJ0j (OpenReview.net, 2023).
Ingraham, J. B. et al. Illuminating protein space with a programmable generative model. Nature 623, 1070–1078 (2023).
Ferruz, N., Schmidt, S. & Höcker, B. ProtGPT2 is a deep unsupervised language model for protein design. Nat. Commun. 13, 4348 (2022).
Hayes, T. et al. Simulating 500 million years of evolution with a language model. Science https://doi.org/10.1126/science.ads0018 (2024).
Sumida, K. H. et al. Improving protein expression, stability, and function with ProteinMPNN. J. Am. Chem. Soc. 146, 2054–2061 (2024).
Meador, K. et al. A suite of designed protein cages using machine learning and protein fragment-based protocols. Structure 32, 751–765.e11 (2024).
de Haas, R. J. et al. Rapid and automated design of two-component protein nanomaterials using ProteinMPNN. Proc. Natl Acad. Sci. USA 121, e2314646121 (2024).
Ma, B. et al. A top-down design approach for generating a peptide PROTAC drug targeting androgen receptor for androgenetic alopecia therapy. J. Med. Chem. 67, 10336–10349 (2024).
An, L. et al. Binding and sensing diverse small molecules using shape-complementary pseudocycles. Science 385, 276–282 (2024).
Winnifrith, A., Outeiral, C. & Hie, B. L. Generative artificial intelligence for de novo protein design. Curr. Opin. Struct. Biol. 86, 102794 (2024).
Carlini, N. et al. Extracting training data from diffusion models. In 32nd USENIX Security Symposium (eds Calandrino, J. A. & Troncoso, C.) 5253–5270 (USENIX Association, 2023).
Yang, K. K., Wu, Z. & Arnold, F. H. Machine-learning-guided directed evolution for protein engineering. Nat. Methods 16, 687–694 (2019).
Pierce, B. G. et al. ZDOCK server: interactive docking prediction of protein–protein complexes and symmetric multimers. Bioinformatics 30, 1771–1773 (2014).
Goverde, C. A., Wolf, B., Khakzad, H., Rosset, S. & Correia, B. E. De novo protein design by inversion of the AlphaFold structure prediction network. Protein Sci. 32, e4653 (2023).
Anfinsen, C. B. Principles that govern the folding of protein chains. Science 181, 223–230 (1973).
Vanommeslaeghe, K. et al. CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Lazaridis, T. & Karplus, M. Effective energy function for proteins in solution. Proteins 35, 133–152 (1999).
Berman, H. M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Alford, R. F. et al. The Rosetta All-atom energy function for macromolecular modeling and design. J. Chem. Theory Comput. 13, 3031–3048 (2017).
UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021).
Morcos, F. et al. Direct-coupling analysis of residue coevolution captures native contacts across many protein families. Proc. Natl Acad. Sci. USA 108, E1293–E1301 (2011).
wwPDB consortium. Protein Data Bank: the single global archive for 3D macromolecular structure data. Nucleic Acids Res. 47, D520–D528 (2019).
Defresne, M., Barbe, S. & Schiex, T. Scalable coupling of deep learning with logical reasoning. In Proc. Thirty-Second International Joint Conference on Artificial Intelligence (ed. Elkind, E.) 3615–3623 (International Joint Conferences on Artificial Intelligence Organization, 2023).
Tsuboyama, K. et al. Mega-scale experimental analysis of protein folding stability in biology and design. Nature 620, 434–444 (2023).
Lu, L. et al. De novo design of drug-binding proteins with predictable binding energy and specificity. Science 384, 106–112 (2024).
Glasscock, C. J. et al. Computational design of sequence-specific DNA-binding proteins. Preprint at bioRxiv https://doi.org/10.1101/2023.09.20.558720 (2023).
Vázquez Torres, S. et al. De novo design of high-affinity binders of bioactive helical peptides. Nature 626, 435–442 (2024).
Yang, E. C. et al. Computational design of non-porous pH-responsive antibody nanoparticles. Nat. Struct. Mol. Biol. 31, 1404–1412 (2024).
Guo, A. B., Akpinaroglu, D., Kelly, M. J. S. & Kortemme, T. Deep learning guided design of dynamic proteins. Preprint at bioRxiv https://doi.org/10.1101/2024.07.17.603962 (2024).
Cross, J. A. et al. A de novo designed coiled coil-based switch regulates the microtubule motor kinesin-1. Nat. Chem. Biol. 20, 916–923 (2024).
Dou, J. et al. De novo design of a fluorescence-activating β-barrel. Nature 561, 485–491 (2018).
Cao, L. et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 370, 426–431 (2020).
Sesterhenn, F. et al. De novo protein design enables the precise induction of RSV-neutralizing antibodies. Science 368, eaay5051 (2020).
Bennett, N. R. et al. Atomically accurate de novo design of single-domain antibodies. Preprint at bioRxiv https://doi.org/10.1101/2024.03.14.585103 (2024).
Kajava, A. V. Tandem repeats in proteins: from sequence to structure. J. Struct. Biol. 179, 279–288 (2012).
Lupas, A. N. & Gruber, M. in Fibrous Proteins: Coiled-Coils, Collagen and Elastomers, Advances in Protein Chemistry 37–38 (Elsevier, 2005).
Woolfson, D. N. Understanding a protein fold: the physics, chemistry, and biology of α-helical coiled coils. J. Biol. Chem. 299, 104579 (2023).
Harbury, P. B., Plecs, J. J., Tidor, B., Alber, T. & Kim, P. S. High-resolution protein design with backbone freedom. Science 282, 1462–1467 (1998).
Huang, P.-S. et al. High thermodynamic stability of parametrically designed helical bundles. Science 346, 481–485 (2014).
Thomson, A. R. et al. Computational design of water-soluble α-helical barrels. Science 346, 485–488 (2014).
Dawson, W. M. et al. Coiled coils 9-to-5: rational de novo design of α-helical barrels with tunable oligomeric states. Chem. Sci. 12, 6923–6928 (2021).
Toda, M., Zhang, F. & Athukorallage, B. Elastic surface model for beta-barrels: geometric, computational, and statistical analysis. Proteins 86, 35–42 (2018).
Novotný, J., Bruccoleri, R. E. & Newell, J. Twisted hyperboloid (strophoid) as a model of β-barrels in proteins. J. Mol. Biol. 177, 567–573 (1984).
Naveed, H., Xu, Y., Jackups, R. Jr. & Liang, J. Predicting three-dimensional structures of transmembrane domains of β-barrel membrane proteins. J. Am. Chem. Soc. 134, 1775–1781 (2012).
Huang, P.-S. et al. De novo design of a four-fold symmetric TIM-barrel protein with atomic-level accuracy. Nat. Chem. Biol. 12, 29–34 (2016).
Marcos, E. et al. Principles for designing proteins with cavities formed by curved β sheets. Science 355, 201–206 (2017).
Kim, D. E. et al. Parametrically guided design of beta barrels and transmembrane nanopores using deep learning. Preprint at bioRxiv https://doi.org/10.1101/2024.07.22.604663 (2024).
Lasters, I., Wodak, S. J., Alard, P. & van Cutsem, E. Structural principles of parallel beta-barrels in proteins. Proc. Natl Acad. Sci. USA 85, 3338–3342 (1988).
Kumar, P., Paterson, N. G., Clayden, J. & Woolfson, D. N. De novo design of discrete, stable 310-helix peptide assemblies. Nature 607, 387–392 (2022).
Durairaj, J. et al. Uncovering new families and folds in the natural protein universe. Nature 622, 646–653 (2023).
Kuhlman, B. et al. Design of a novel globular protein fold with atomic-level accuracy. Science 302, 1364–1368 (2003).
Huang, P.-S. et al. RosettaRemodel: a generalized framework for flexible backbone protein design. PLoS ONE 6, e24109 (2011).
Koga, N. et al. Principles for designing ideal protein structures. Nature 491, 222–227 (2012).
Lin, Y.-R. et al. Control over overall shape and size in de novo designed proteins. Proc. Natl Acad. Sci. USA 112, E5478–85 (2015).
Jacobs, T. M. et al. Design of structurally distinct proteins using strategies inspired by evolution. Science 352, 687–690 (2016).
Pan, X. et al. Expanding the space of protein geometries by computational design of de novo fold families. Science 369, 1132–1136 (2020).
Harteveld, Z. et al. A generic framework for hierarchical de novo protein design. Proc. Natl Acad. Sci. USA 119, e2206111119 (2022).
Yang, C. et al. Bottom-up de novo design of functional proteins with complex structural features. Nat. Chem. Biol. 17, 492–500 (2021).
Zhou, J. & Grigoryan, G. Rapid search for tertiary fragments reveals protein sequence–structure relationships. Protein Sci. 24, 508–524 (2015).
Woolfson, D. N. et al. De novo protein design: how do we expand into the universe of possible protein structures? Curr. Opin. Struct. Biol. 33, 16–26 (2015).
Taylor, W. R. A ’periodic table’ for protein structures. Nature 416, 657–660 (2002).
Taylor, W. R., Chelliah, V., Hollup, S. M., MacDonald, J. T. & Jonassen, I. Probing the ‘dark matter’ of protein fold space. Structure 17, 1244–1252 (2009).
Minami, S. et al. Exploration of novel αβ-protein folds through de novo design. Nat. Struct. Mol. Biol. 30, 1132–1140 (2023).
Sakuma, K. et al. Design of complicated all-α protein structures. Nat. Struct. Mol. Biol. 31, 275–282 (2024).
Lipsh-Sokolik, R. et al. Combinatorial assembly and design of enzymes. Science 379, 195–201 (2023).
Kundert, K. & Kortemme, T. Computational design of structured loops for new protein functions. Biol. Chem. 400, 275–288 (2019).
Du, H. et al. A general platform for targeting MHC-II antigens via a single loop. Preprint at bioRxiv https://doi.org/10.1101/2024.01.26.577489 (2024).
Misson Mindrebo, L. et al. Fully synthetic platform to rapidly generate tetravalent bispecific nanobody-based immunoglobulins. Proc. Natl Acad. Sci. USA 120, e2216612120 (2023).
Yu, Y. & Lutz, S. Circular permutation: a different way to engineer enzyme structure and function. Trends Biotechnol. 29, 18–25 (2011).
Schellman, C. & Jaenicke, R. in The AlphaL Conformation at the Ends of Helices (ed. Jaenicke, R.) (Elsevier, 1980).
Thornton, J. M., Sibanda, B. L., Edwards, M. S. & Barlow, D. J. Analysis, design and modification of loop regions in proteins. Bioessays 8, 63–69 (1988).
Aurora, R. & Rose, G. D. Helix capping. Protein Sci. 7, 21–38 (1998).
Richardson, J. S. & Richardson, D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science 240, 1648–1652 (1988).
Wilmot, C. M. & Thornton, J. M. Analysis and prediction of the different types of β-turn in proteins. J. Mol. Biol. 203, 221–232 (1988).
Brunet, A. P. et al. The role of turns in the structure of an alpha-helical protein. Nature 364, 355–358 (1993).
Efimov, A. V. Patterns of loop regions in proteins. Curr. Opin. Struct. Biol. 3, 379–384 (1993).
Aurora, R., Srinivasan, R. & Rose, G. D. Rules for alpha-helix termination by glycine. Science 264, 1126–1130 (1994).
Harper, E. T. & Rose, G. D. Helix stop signals in proteins and peptides: the capping box. Biochemistry 32, 7605–7609 (1993).
Engel, D. E. & DeGrado, W. F. Alpha-alpha linking motifs and interhelical orientations. Proteins 61, 325–337 (2005).
Hill, R. B., Raleigh, D. P., Lombardi, A. & DeGrado, W. F. De novo design of helical bundles as models for understanding protein folding and function. Acc. Chem. Res. 33, 745–754 (2000).
Canutescu, A. A. & Dunbrack, R. L. Jr. Cyclic coordinate descent: a robotics algorithm for protein loop closure. Protein Sci. 12, 963–972 (2003).
Cortés, J., Siméon, T., Remaud-Siméon, M. & Tran, V. Geometric algorithms for the conformational analysis of long protein loops. J. Comput. Chem. 25, 956–967 (2004).
Barozet, A., Chacón, P. & Cortés, J. Current approaches to flexible loop modeling. Curr. Res. Struct. Biol. 3, 187–191 (2021).
Mandell, D. J., Coutsias, E. A. & Kortemme, T. Sub-angstrom accuracy in protein loop reconstruction by robotics-inspired conformational sampling. Nat. Methods 6, 551–552 (2009).
Barozet, A. et al. MoMA-LoopSampler: a web server to exhaustively sample protein loop conformations. Bioinformatics 38, 552–553 (2022).
Jiang, H. et al. De novo design of buttressed loops for sculpting protein functions. Nat. Chem. Biol. 20, 974–980 (2024).
Aguilar Rangel, M. et al. Fragment-based computational design of antibodies targeting structured epitopes. Sci. Adv. 8, eabp9540 (2022).
Mann, S. I., Nayak, A., Gassner, G. T., Therien, M. J. & DeGrado, W. F. De novo design, solution characterization, and crystallographic structure of an abiological Mn-porphyrin-binding protein capable of stabilizing a Mn(V) species. J. Am. Chem. Soc. 143, 252–259 (2021).
Anishchenko, I. et al. De novo protein design by deep network hallucination. Nature 600, 547–552 (2021).
Wang, J. et al. Scaffolding protein functional sites using deep learning. Science 377, 387–394 (2022).
Szegedy, C. et al. Going deeper with convolutions. In Proc. 2015 IEEE Conf. Computer Vision and Pattern Recognition (IEEE, 2015).
Yeh, A. H.-W. et al. De novo design of luciferases using deep learning. Nature 614, 774–780 (2023).
Wicky, B. I. M. et al. Hallucinating symmetric protein assemblies. Science 378, 56–61 (2022).
Frank, C. et al. Scalable protein design using optimization in a relaxed sequence space. Science 386, 439–445 (2024).
Frank, C., Schiwietz, D., Fuß, L., Ovchinnikov, S. & Dietz, H. Alphafold2 refinement improves designability of large de novo proteins. Preprint at bioRxiv https://doi.org/10.1101/2024.11.21.624687 (2024).
Ho, J., Jain, A. & Abbeel, P. Denoising diffusion probabilistic models. In Advances in Neural Information Processing Systems 33 (eds Larochelle, H. et al.) (NeurIPS, 2020).
Song, Y. et al. Score-based generative modeling through stochastic differential equations. In 9th International Conference on Learning Representations, ICLR 2021 https://openreview.net/forum?id=PxTIG12RRHS (OpenReview.net, 2021).
Lin, Y., Lee, M., Zhang, Z. & AlQuraishi, M. Out of many, one: designing and scaffolding proteins at the scale of the structural universe with Genie 2. Preprint at https://arxiv.org/abs/2405.15489 (2024).
Yim, J. et al. SE(3) diffusion model with application to protein backbone generation. In Proc. Mahine Learning Research https://proceedings.mlr.press/v202/yim23a.html (OpenReview.net, 2023).
Yim, J. et al. Fast protein backbone generation with SE(3) flow matching. Preprint at https://arxiv.org/abs/2310.05297 (2023).
Wang, C. et al. Proteus: exploring protein structure generation for enhanced designability and efficiency. In Proc. 41st International Conference on Machine Learning https://openreview.net/forum?id=IckJCzsGVS (OpenReview.net, 2024).
Huguet, G. et al. Sequence-augmented SE(3)-flow matching for conditional protein backbone generation. In Thirty-Eighth Annual Conference on Neural Information Processing Systems https://openreview.net/forum?id=paYwtPBpyZ (OpenReview.net, 2024).
Campbell, A., Yim, J., Barzilay, R., Rainforth, T. & Jaakkola, T. S. Generative flows on discrete state-spaces: enabling multimodal flows with applications to protein co-design. In Proc. Forty-first International Conference on Machine Learning https://openreview.net/forum?id=kQwSbv0BR4 (OpenReview.net, 2024).
Ren, M., Zhu, T. & Zhang, H. CarbonNovo: joint design of protein structure and sequence using a unified energy-based model. In Forty-first International Conference on Machine Learning, ICML 2024 https://openreview.net/forum?id=FSxTEvuFa7 (OpenReview.net, 2024).
Chu, A. E. et al. An all-atom protein generative model. Proc. Natl Acad. Sci. USA 121, e2311500121 (2024).
Lisanza, S. L. et al. Multistate and functional protein design using RoseTTAFold sequence space diffusion. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02395-w (2024).
Qu, W. et al. P(all-atom) is unlocking new path for protein design. Preprint at bioRxiv https://doi.org/10.1101/2024.08.16.608235 (2024).
Dahiyat, B. I., Sarisky, C. A. & Mayo, S. L. De novo protein design: towards fully automated sequence selection. J. Mol. Biol. 273, 789–796 (1997).
Lovell, S. C., Word, J. M., Richardson, J. S. & Richardson, D. C. The penultimate rotamer library. Proteins 40, 389–408 (2000).
Shapovalov, M. V. & Dunbrack, R. L. Jr. A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure 19, 844–858 (2011).
Cooper, M. C., de Givry, S. & Schiex, T. Graphical models: queries, complexity, algorithms. In Proc. 37th International Symposium on Theoretical Aspects of Computer Science Vol. 154 (STACS 2020) (eds Paul, C. & Bläser, M.) 4:1–4:22 (Schloss Dagstuhl — Leibniz-Zentrum für Informatik, 2020).
Hallen, M. A. et al. OSPREY 3.0: open-source protein redesign for you, with powerful new features. J. Comput. Chem. 39, 2494–2507 (2018).
Hallen, M. A. & Donald, B. R. Protein design by provable algorithms. Commun. ACM 62, 76–84 (2019).
Allouche, D. et al. Computational protein design as an optimization problem. Artif. Intell. 212, 59–79 (2014).
Pierce, N. A. & Winfree, E. Protein design is NP-hard. Protein Eng. 15, 779–782 (2002).
Simoncini, D. et al. Guaranteed discrete energy optimization on large protein design problems. J. Chem. Theory Comput. 11, 5980–5989 (2015).
Khatri, B., Majumder, P., Nagesh, J., Penmatsa, A. & Chatterjee, J. Increasing protein stability by engineering the n → π* interaction at the β-turn. Chem. Sci. 11, 9480–9487 (2020).
Boyken, S. E. et al. De novo design of protein homo-oligomers with modular hydrogen-bond network-mediated specificity. Science 352, 680–687 (2016).
Pavlovicz, R. E., Park, H. & DiMaio, F. Efficient consideration of coordinated water molecules improves computational protein–protein and protein–ligand docking discrimination. PLoS Comput. Biol. 16, e1008103 (2020).
Ruffini, M. et al. Guaranteed diversity and optimality in cost function network based computational protein design methods. Algorithms 14, 168 (2021).
Colom, M. S. et al. Complete combinatorial mutational enumeration of a protein functional site enables sequence–landscape mapping and identifies highly-mutated variants that retain activity. Protein Sci. 33, e5109 (2024).
DiMaio, F., Leaver-Fay, A., Bradley, P., Baker, D. & André, I. Modeling symmetric macromolecular structures in Rosetta3. PLoS ONE 6, e20450 (2011).
Defresne, M., Barbe, S. & Schiex, T. Protein design with deep learning. Int. J. Mol. Sci. 22, 11741 (2021).
Goverde, C. A. et al. Computational design of soluble and functional membrane protein analogues. Nature 631, 449–458 (2024).
Jing, B., Eismann, S., Suriana, P., Townshend, R. J. L. & Dror, R. O. Learning from protein structure with geometric vector perceptrons. In 9th International Conference on Learning Representations, ICLR 2021 https://openreview.net/forum?id=1YLJDvSx6J4 (OpenReview.net, 2021).
Young, G. & Householder, A. S. Discussion of a set of points in terms of their mutual distances. Psychometrika 3, 19–22 (1938).
Corso, G., Stark, H., Jegelka, S., Jaakkola, T. & Barzilay, R. Graph neural networks. Nat. Rev. Methods Primers 4, 17 (2024).
Krapp, L. F., Meireles, F. A., Abriata, L. A. & Peraro, M. D. Context-aware geometric deep learning for protein sequence design. Nat. Commun. 15, 6273 (2024).
Dessaux, D. et al. Designing symmetrical multi-component proteins using a hybrid generative AI approach. Preprint at bioRxiv https://doi.org/10.1101/2024.06.13.598662 (2024).
Li, A. J. et al. Neural network-derived Potts models for structure-based protein design using backbone atomic coordinates and tertiary motifs. Protein Sci. 32, e4554 (2023).
Silva, L. A., Meynard-Piganeau, B., Lucibello, C. & Feinauer, C. Uncovering sequence diversity from a known protein structure. Preprint at https://arxiv.org/abs/2406.11975 (2024).
Durante, V., Katsirelos, G. & Schiex, T. Efficient low rank convex bounds for pairwise discrete graphical models. In Proc. Machine Learning Research Vol. 162 (eds Chaudhuri, K.) 5726–5741 (PMLR, 2022).
Liu, Y. et al. Rotamer-free protein sequence design based on deep learning and self-consistency. Nat. Comput. Sci. 2, 451–462 (2022).
Liu, J., Guo, Z., You, H., Zhang, C. & Lai, L. All-atom protein sequence design based on geometric deep learning. Angew. Chem. Int. Ed. 63, e202411461 (2024).
Dauparas, J. et al. Atomic context-conditioned protein sequence design using LigandMPNN. Preprint at bioRxiv https://doi.org/10.1101/2023.12.22.573103 (2023).
Krapp, L. F. et al. Context-aware geometric deep learning for protein sequence design. Nat. Commun. 15, 6273 (2024).
Baldwin, E., Hajiseyedjavadi, O., Baase, W. & Matthews, B. The role of backbone flexibility in the accommodation of variants that repack the core of T4 lysozyme. Science 262, 1715–1718 (1993).
Bordner, A. & Abagyan, R. Large-scale prediction of protein geometry and stability changes for arbitrary single point mutations. Proteins Struct. Funct. Bioinf. 57, 400–413 (2004).
Boehr, D. D., Nussinov, R. & Wright, P. E. The role of dynamic conformational ensembles in biomolecular recognition. Nat. Chem. Biol. 5, 789–796 (2009).
Sonaglioni, D. et al. Dynamic personality of proteins and effect of the molecular environment. J. Phys. Chem. Lett. 15, 5543–5548 (2024).
Gaillard, T., Panel, N. & Simonson, T. Protein side chain conformation predictions with an MMGBSA energy function. Proteins Struct. Funct. Bioinf. 84, 803–819 (2016).
Murphy, G. S. et al. Increasing sequence diversity with flexible backbone protein design: the complete redesign of a protein hydrophobic core. Structure 20, 1086–1096 (2012).
Khatib, F. et al. Algorithm discovery by protein folding game players. Proc. Natl Acad. Sci. USA 108, 18949–18953 (2011).
Tyka, M. D. et al. Alternate states of proteins revealed by detailed energy landscape mapping. J. Mol. Biol. 405, 607–618 (2011).
Loshbaugh, A. L. & Kortemme, T. Comparison of Rosetta flexible-backbone computational protein design methods on binding interactions. Proteins Struct. Funct. Bioinf. 88, 206–226 (2020).
Ollikainen, N., de Jong, R. M. & Kortemme, T. Coupling protein side-chain and backbone flexibility improves the re-design of protein–ligand specificity. PLoS Comput. Biol. 11, e1004335 (2015).
Smith, C. A. & Kortemme, T. Backrub-like backbone simulation recapitulates natural protein conformational variability and improves mutant side-chain prediction. J. Mol. Biol. 380, 742–756 (2008).
Sun, M. G. & Kim, P. M. Data driven flexible backbone protein design. PLoS Comput. Biol. 13, e1005722 (2017).
Simoncini, D., Zhang, K. Y., Schiex, T. & Barbe, S. A structural homology approach for computational protein design with flexible backbone. Bioinformatics 35, 2418–2426 (2019).
Gainza, P., Roberts, K. E. & Donald, B. R. Protein design using continuous rotamers. PLoS Comput. Biol. 8, e1002335 (2012).
Hallen, M. A., Keedy, D. A. & Donald, B. R. Dead-end elimination with perturbations (deeper): a provable protein design algorithm with continuous sidechain and backbone flexibility. Proteins Struct. Funct. Bioinf. 81, 18–39 (2013).
Hallen, M. A. & Donald, B. R. Cats (coordinates of atoms by Taylor series): protein design with backbone flexibility in all locally feasible directions. Bioinformatics 33, i5–i12 (2017).
Zuckerman, D. M. Statistical Physics of Biomolecules: An Introduction (CRC Press, 2010).
Jou, J. D., Holt, G. T., Lowegard, A. U. & Donald, B. R. Minimization-aware recursive k*: a novel, provable algorithm that accelerates ensemble-based protein design and provably approximates the energy landscape. J. Comput. Biol. 27, 550–564 (2020).
Viricel, C., de Givry, S., Schiex, T. & Barbe, S. Cost function network-based design of protein–protein interactions: predicting changes in binding affinity. Bioinformatics 34, 2581–2589 (2018).
Ojewole, A. A., Jou, J. D., Fowler, V. G. & Donald, B. R. Bbk*(branch and bound over k*): a provable and efficient ensemble-based protein design algorithm to optimize stability and binding affinity over large sequence spaces. J. Comput. Biol. 25, 726–739 (2018).
Silver, N. W. et al. Efficient computation of small-molecule configurational binding entropy and free energy changes by ensemble enumeration. J. Chem. Theory Comput. 9, 5098–5115 (2013).
Kamisetty, H., Ramanathan, A., Bailey-Kellogg, C. & Langmead, C. J. Accounting for conformational entropy in predicting binding free energies of protein–protein interactions. Proteins Struct. Funct. Bioinf. 79, 444–462 (2011).
Valiant, L. G. The complexity of enumeration and reliability problems. SIAM J. Comput. 8, 410–421 (1979).
Nisonoff, H. Efficient Partition Function Estimation in Computational Protein Design: Probabilistic Guarantees and Characterization of a Novel Algorithm. PhD thesis, Duke University, Durham (2015).
Viricel, C., Simoncini, D., Barbe, S. & Schiex, T. Guaranteed weighted counting for affinity computation: beyond determinism and structure. In Principles and Practice of Constraint Programming: 22nd International Conference, CP 2016, Toulouse, France, September 5–9, 2016, Proceedings Vol. 22, 733–750 (Springer, 2016).
Havranek, J. J. & Harbury, P. B. Automated design of specificity in molecular recognition. Nat. Struct. Biol. 10, 45–52 (2003).
Desjarlais, J. R. & Handel, T. M. Side-chain and backbone flexibility in protein core design. J. Mol. Biol. 290, 305–318 (1999).
Hu, X., Wang, H., Ke, H. & Kuhlman, B. High-resolution design of a protein loop. Proc. Natl Acad. Sci. USA 104, 17668–17673 (2007).
Murphy, P. M., Bolduc, J. M., Gallaher, J. L., Stoddard, B. L. & Baker, D. Alteration of enzyme specificity by computational loop remodeling and design. Proc. Natl Acad. Sci. USA 106, 9215–9220 (2009).
Davis, I. W., Arendall, W. B., Richardson, D. C. & Richardson, J. S. The backrub motion: how protein backbone shrugs when a sidechain dances. Structure 14, 265–274 (2006).
Friedland, G. D., Linares, A. J., Smith, C. A. & Kortemme, T. A simple model of backbone flexibility improves modeling of side-chain conformational variability. J. Mol. Biol. 380, 757–774 (2008).
Ollikainen, N., Smith, C. A., Fraser, J. S. & Kortemme, T. in Methods in Enzymology Vol. 523, 61–85 (Elsevier, 2013).
Fu, X., Apgar, J. R. & Keating, A. E. Modeling backbone flexibility to achieve sequence diversity: the design of novel α-helical ligands for Bcl-xL. J. Mol. Biol. 371, 1099–1117 (2007).
Fung, H. K., Floudas, C. A., Taylor, M. S., Zhang, L. & Morikis, D. Toward full-sequence de novo protein design with flexible templates for human beta-defensin-2. Biophys. J. 94, 584–599 (2008).
Sala, D., Engelberger, F., Mchaourab, H. & Meiler, J. Modeling conformational states of proteins with AlphaFold. Curr. Opin. Struct. Biol. 81, 102645 (2023).
Del Alamo, D., Sala, D., Mchaourab, H. S. & Meiler, J. Sampling alternative conformational states of transporters and receptors with AlphaFold2. eLife 11, e75751 (2022).
Wayment-Steele, H. K. et al. Predicting multiple conformations via sequence clustering and AlphaFold2. Nature 625, 832–839 (2024).
Stein, R. A. & Mchaourab, H. S. SPEACH_AF: sampling protein ensembles and conformational heterogeneity with AlphaFold2. PLoS Comput. Biol. 18, e1010483 (2022).
Kalakoti, Y. & Wallner, B. AFsample2: predicting multiple conformations and ensembles with AlphaFold2. Preprint at bioRxiv https://doi.org/10.1101/2024.05.28.596195 (2024).
Bryant, P. & Noé, F. Structure prediction of alternative protein conformations. Nat. Commun. 15, 7328 (2024).
Jing, B. et al. Eigenfold: generative protein structure prediction with diffusion models. Preprint at https://arxiv.org/abs/2304.02198 (2023).
Zheng, S. et al. Predicting equilibrium distributions for molecular systems with deep learning. Nat. Mach. Intell. 6, 558–567 (2024).
Lu, J., Zhong, B. & Tang, J. Score-based enhanced sampling for protein molecular dynamics. In ICML 2023 Workshop on Structured Probabilistic Inference & Generative Modeling https://openreview.net/forum?id=NO3QwxuHv9#all (2023).
Jing, B., Berger, B. & Jaakkola, T. S. AlphaFold meets flow matching for generating protein ensembles. In NeurIPS 2023 Workshop on Generative AI and Biology https://openreview.net/pdf?id=yQcebEgQfH (OpenReview.net, 2024).
Albergo, M. S. & Vanden-Eijnden, E. Building normalizing flows with stochastic interpolants. In The Eleventh International Conference on Learning Representations, ICLR 2023 https://openreview.net/forum?id=li7qeBbCR1t (OpenReview.net, 2023).
Davey, J. A. & Chica, R. A. Multistate approaches in computational protein design. Protein Sci. 21, 1241–1252 (2012).
Karimi, M. & Shen, Y. iCFN: an efficient exact algorithm for multistate protein design. Bioinformatics 34, i811–i820 (2018).
Vucinic, J., Simoncini, D., Ruffini, M., Barbe, S. & Schiex, T. Positive multistate protein design. Bioinformatics 36, 122–130 (2020).
Davey, J. A., Damry, A. M., Euler, C. K., Goto, N. K. & Chica, R. A. Prediction of stable globular proteins using negative design with non-native backbone ensembles. Structure 23, 2011–2021 (2015).
Davey, J. A. & Chica, R. A. Multistate computational protein design with backbone ensembles. Methods Mol. Biol. 1529, 161–179 (2017).
Sauer, M. F., Sevy, A. M., Crowe, J. E. Jr. & Meiler, J. Multi-state design of flexible proteins predicts sequences optimal for conformational change. PLoS Comput. Biol. 16, e1007339 (2020).
Ambroggio, X. I. & Kuhlman, B. Computational design of a single amino acid sequence that can switch between two distinct protein folds. J. Am. Chem. Soc. 128, 1154–1161 (2006).
Sevy, A. M., Jacobs, T. M., Crowe, J. E. Jr. & Meiler, J. Design of protein multi-specificity using an independent sequence search reduces the barrier to low energy sequences. PLoS Comput. Biol. 11, e1004300 (2015).
Leaver-Fay, A., Jacak, R., Stranges, P. B. & Kuhlman, B. A generic program for multistate protein design. PLoS ONE 6, e20937 (2011).
Allen, B. D. & Mayo, S. L. An efficient algorithm for multistate protein design based on faster. J. Comput. Chem. 31, 904–916 (2010).
Negron, C. & Keating, A. E. in Methods in Enzymology Vol. 523, 171–190 (Elsevier, 2013).
Fromer, M., Yanover, C. & Linial, M. Design of multispecific protein sequences using probabilistic graphical modeling. Proteins Struct. Funct. Bioinf. 78, 530–547 (2010).
Fromer, M. et al. SPRINT: side-chain prediction inference toolbox for multistate protein design. Bioinformatics 26, 2466–2467 (2010).
Yanover, C., Fromer, M. & Shifman, J. M. Dead-end elimination for multistate protein design. J. Comput. Chem. 28, 2122–2129 (2007).
Hallen, M. A. & Donald, B. R. COMETS (constrained optimization of multistate energies by tree search): a provable and efficient protein design algorithm to optimize binding affinity and specificity with respect to sequence. J. Comput. Biol. 23, 311–321 (2016).
Traoré, S. et al. Fast search algorithms for computational protein design. J. Comput. Chem. 37, 1048–1058 (2016).
Löffler, P., Schmitz, S., Hupfeld, E., Sterner, R. & Merkl, R. Rosetta: MSF: a modular framework for multi-state computational protein design. PLoS Comput. Biol. 13, e1005600 (2017).
Nazet, J., Lang, E. & Merkl, R. Rosetta:MSF:NN: boosting performance of multi-state computational protein design with a neural network. PLoS ONE 16, e0256691 (2021).
Eisenstein, M. Seven technologies to watch in 2022. Nature 601, 658–661 (2022).
Porebski, B. T. & Buckle, A. M. Consensus protein design. Protein Eng. Des. Sel. 29, 245–251 (2016).
Plückthun, A. Designed ankyrin repeat proteins (DARPins): binding proteins for research, diagnostics, and therapy. Annu. Rev. Pharmacol. Toxicol. 55, 489–511 (2015).
Pabo, C. O., Peisach, E. & Grant, R. A. Design and selection of novel Cys2His2 zinc finger proteins. Annu. Rev. Biochem. 70, 313–340 (2001).
Spence, M. A., Kaczmarski, J. A., Saunders, J. W. & Jackson, C. J. Ancestral sequence reconstruction for protein engineers. Curr. Opin. Struct. Biol. 69, 131–141 (2021).
Voet, A. R. D. et al. Computational design of a self-assembling symmetrical β-propeller protein. Proc. Natl Acad. Sci. USA 111, 15102–15107 (2014).
Reynolds, K. A., Russ, W. P., Socolich, M. & Ranganathan, R. in Methods in Enzymology 213–235 (Elsevier, 2013).
Brender, J. R., Shultis, D., Khattak, N. A. & Zhang, Y. An evolution-based approach to DE novo protein design. Methods Mol. Biol. 1529, 243–264 (2017).
Russ, W. P. et al. An evolution-based model for designing chorismate mutase enzymes. Science 369, 440–445 (2020).
Schmitz, S., Ertelt, M., Merkl, R. & Meiler, J. Rosetta design with co-evolutionary information retains protein function. PLoS Comput. Biol. 17, e1008568 (2021).
Malbranke, C., Bikard, D., Cocco, S., Monasson, R. & Tubiana, J. Machine learning for evolutionary-based and physics-inspired protein design: current and future synergies. Curr. Opin. Struct. Biol. 80, 102571 (2023).
Fram, B. et al. Simultaneous enhancement of multiple functional properties using evolution-informed protein design. Nat. Commun. 15, 5141 (2024).
Verkuil, R. et al. Language models generalize beyond natural proteins. Preprint at bioRxiv https://doi.org/10.1101/2022.12.21.521521 (2022).
Madani, A. et al. Large language models generate functional protein sequences across diverse families. Nat. Biotechnol. 41, 1099–1106 (2023).
Munsamy, G. et al. Conditional language models enable the efficient design of proficient enzymes. Preprint at bioRxiv https://doi.org/10.1101/2024.05.03.592223 (2024).
Winski, A. et al. AlphaFold2 captures the conformational landscape of the HAMP signaling domain. Protein Sci. 33, e4846 (2024).
Akdel, M. et al. A structural biology community assessment of AlphaFold2 applications. Nat. Struct. Mol. Biol. 29, 1056–1067 (2022).
McDonald, E. F., Jones, T., Plate, L., Meiler, J. & Gulsevin, A. Benchmarking AlphaFold2 on peptide structure prediction. Structure 31, 111–119.e2 (2023).
Castorina, L. V., Petrenas, R., Subr, K. & Wood, C. W. PDBench: evaluating computational methods for protein-sequence design. Bioinformatics 39, btad027 (2023).
Dallago, C. et al. FLIP: benchmark tasks in fitness landscape inference for proteins. In Thirty-fifth Conference on Neural Information Processing Systems Datasets and Benchmarks Track (Round 2) https://openreview.net/forum?id=p2dMLEwL8tF (OpenReview.net, 2021).
Notin, P. et al. ProteinGym: large-scale benchmarks for protein fitness prediction and design. in 37th Conference on Neural Information Processing Systems (NeurIPS 2023) (eds Oh, A. et al.), Vol. 36, 64331–64379 (Curran Associates, Inc., 2023).
Zhang, Y. & Skolnick, J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Arun, K. S., Huang, T. S. & Blostein, S. D. Least-squares fitting of two 3-D point sets. IEEE Trans. Patt. Anal. Mach. Intell. 9, 698–700 (1987).
Li, S. C., Bu, D., Xu, J. & Li, M. Finding nearly optimal GDT scores. J. Comput. Biol. 18, 693–704 (2011).
Mariani, V., Biasini, M., Barbato, A. & Schwede, T. lDDT: a local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 29, 2722–2728 (2013).
Wallner, B. AFsample: improving multimer prediction with AlphaFold using massive sampling. Bioinformatics 39, btad573 (2023).
Roney, J. P. & Ovchinnikov, S. State-of-the-art estimation of protein model accuracy using AlphaFold. Phys. Rev. Lett. 129, 238101 (2022).
Bennett, N. R. et al. Improving de novo protein binder design with deep learning. Nat. Commun. 14, 2625 (2023).
Liu, C. et al. Diffusing protein binders to intrinsically disordered proteins. Preprint at bioRxiv https://doi.org/10.1101/2024.07.16.603789 (2024).
Wu, K. et al. Sequence-specific targeting of intrinsically disordered protein regions. Preprint at bioRxiv https://doi.org/10.1101/2024.07.15.603480 (2024).
Manfredi, M. et al. Alpha&ESMhFolds: a web server for comparing AlphaFold2 and ESMFold models of the human reference proteome. J. Mol. Biol. 436, 168593 (2024).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2010).
Corso, G., Stärk, H., Jing, B., Barzilay, R. & Jaakkola, T. DiffDock: diffusion steps, twists, and turns for molecular docking. In The Eleventh International Conference on Learning Representations https://openreview.net/forum?id=kKF8_K-mBbS (ICLR 2023).
Moretti, R., Bender, B. J., Allison, B. & Meiler, J. Rosetta and the design of ligand binding sites. Methods Mol. Biol. 1414, 47–62 (2016).
Basu, S. & Wallner, B. DockQ: a quality measure for protein–protein docking models. PLoS ONE 11, e0161879 (2016).
Dominguez, C., Boelens, R. & Bonvin, A. M. J. J. HADDOCK: a protein–protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 125, 1731–1737 (2003).
Kanitkar, T. R. et al. Methods for molecular modelling of protein complexes. Methods Mol. Biol. 2305, 53–80 (2021).
Radom, F., Plückthun, A. & Paci, E. Assessment of ab initio models of protein complexes by molecular dynamics. PLoS Comput. Biol. 14, e1006182 (2018).
Wang, L. et al. Accurate and reliable prediction of relative ligand binding potency in prospective drug discovery by way of a modern free-energy calculation protocol and force field. J. Am. Chem. Soc. 137, 2695–2703 (2015).
Chipot, C. Free energy methods for the description of molecular processes. Annu. Rev. Biophys. 52, 113–138 (2023).
Barros, E. P. et al. Improving the efficiency of ligand-binding protein design with molecular dynamics simulations. J. Chem. Theory Comput. 15, 5703–5715 (2019).
Chevalier, A. et al. Massively parallel de novo protein design for targeted therapeutics. Nature 550, 74–79 (2017).
Childers, M. C. & Daggett, V. Insights from molecular dynamics simulations for computational protein design. Mol. Syst. Des. Eng. 2, 9–33 (2017).
Gainza, P. et al. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17, 184–192 (2020).
Gainza, P. et al. De novo design of protein interactions with learned surface fingerprints. Nature 617, 176–184 (2023).
Gligorijević, V. et al. Structure-based protein function prediction using graph convolutional networks. Nat. Commun. 12, 3168 (2021).
Sanderson, T., Bileschi, M. L., Belanger, D. & Colwell, L. J. ProteInfer, deep neural networks for protein functional inference. eLife 12, e80942 (2023).
Brandes, N., Ofer, D., Peleg, Y., Rappoport, N. & Linial, M. ProteinBERT: a universal deep-learning model of protein sequence and function. Bioinformatics 38, 2102–2110 (2022).
Khersonsky, O. et al. Automated design of efficient and functionally diverse enzyme repertoires. Mol. Cell 72, 178–186.e5 (2018).
Weinstein, J. Y. et al. Designed active-site library reveals thousands of functional GFP variants. Nat. Commun. 14, 2890 (2023).
Kumar, N. & Skolnick, J. EFICAz2.5: application of a high-precision enzyme function predictor to 396 proteomes. Bioinformatics 28, 2687–2688 (2012).
Somarowthu, S., Yang, H., Hildebrand, D. G. C. & Ondrechen, M. J. High-performance prediction of functional residues in proteins with machine learning and computed input features. Biopolymers 95, 390–400 (2011).
Somarowthu, S. & Ondrechen, M. J. POOL server: machine learning application for functional site prediction in proteins. Bioinformatics 28, 2078–2079 (2012).
Tong, W., Wei, Y., Murga, L. F., Ondrechen, M. J. & Williams, R. J. Partial order optimum likelihood (POOL): maximum likelihood prediction of protein active site residues using 3D structure and sequence properties. PLoS Comput. Biol. 5, e1000266 (2009).
Song, J. et al. PREvaIL, an integrative approach for inferring catalytic residues using sequence, structural, and network features in a machine-learning framework. J. Theor. Biol. 443, 125–137 (2018).
Zou, Z., Tian, S., Gao, X. & Li, Y. MlDEEPre: multi-functional enzyme function prediction with hierarchical multi-label deep learning. Front. Genet. 9, 714 (2018).
Feehan, R., Franklin, M. W. & Slusky, J. S. G. Machine learning differentiates enzymatic and non-enzymatic metals in proteins. Nat. Commun. 12, 3712 (2021).
Feehan, R., Copeland, M., Franklin, M. W. & Slusky, J. S. G. MAHOMES II: a webserver for predicting if a metal binding site is enzymatic. Protein Sci. 32, e4626 (2023).
van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. 42, 243–246 (2024).
Kim, W. et al. Rapid and sensitive protein complex alignment with Foldseek-multimer. Preprint at bioRxiv https://doi.org/10.1101/2024.07.15.603480 (2024).
Holm, L. in Methods in Molecular Biology (ed. Clifton, N. J.) 29–42 (Springer US, 2020).
Shindyalov, I. N. & Bourne, P. E. Protein structure alignment by incremental combinatorial extension (CE) of the optimal path. Protein Eng. Des. Sel. 11, 739–747 (1998).
Johnson, S. R. et al. Computational scoring and experimental evaluation of enzymes generated by neural networks. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02214-2 (2024).
Stam, M. J. & Wood, C. W. DE-STRESS: a user-friendly web application for the evaluation of protein designs. Protein Eng. Des. Sel. 34, gzab029 (2021).
Goldenzweig, A. et al. Automated structure- and sequence-based design of proteins for high bacterial expression and stability. Mol. Cell 63, 337–346 (2016).
Marques, S. M., Planas-Iglesias, J. & Damborsky, J. Web-based tools for computational enzyme design. Curr. Opin. Struct. Biol. 69, 19–34 (2021).
Hon, J. et al. SoluProt: prediction of soluble protein expression in Escherichia coli. Bioinformatics 37, 23–28 (2021).
Ding, Z. et al. MPEPE, a predictive approach to improve protein expression in E. coli based on deep learning. Comput. Struct. Biotechnol. J. 20, 1142–1153 (2022).
Thumuluri, V. et al. NetSolP: predicting protein solubility in E. coli using language models. Bioinformatics 38, 941–946 (2021).
Walker, J. M. The Proteomics Protocols Handbook (Humana Press, 2005).
Cock, P. J. A. et al. Biopython: freely available python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Schavemaker, P. E., Śmigiel, W. M. & Poolman, B. Ribosome surface properties may impose limits on the nature of the cytoplasmic proteome. eLife 6, e30084 (2017).
Yagi, S. et al. Seven amino acid types suffice to create the core fold of RNA polymerase. J. Am. Chem. Soc. 143, 15998–16006 (2021).
Berger, S. et al. Preclinical proof of principle for orally delivered Th17 antagonist miniproteins. Cell 187, 4305–4317.e18 (2024).
Structural Genomics Consortium et al. Protein production and purification. Nat. Methods 5, 135–146 (2008).
Wingfield, P. T. Overview of the purification of recombinant proteins. Curr. Protocols Protein Sci. https://doi.org/10.1002/0471140864.ps0601s80 (2015).
Du, M. et al. 1Progress, applications, challenges and prospects of protein purification technology. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2022.1028691 (2022).
Stemmer, W. P., Crameri, A., Ha, K. D., Brennan, T. M. & Heyneker, H. L. Single-step assembly of a gene and entire plasmid from large numbers of oligodeoxyribonucleotides. Gene 164, 49–53 (1995).
Gould, N., Hendy, O. & Papamichail, D. Computational tools and algorithms for designing customized synthetic genes. Front. Bioeng. Biotechnol. 2, 41 (2014).
Langan, R. A. et al. De novo design of bioactive protein switches. Nature 572, 205–210 (2019).
Miles, A. J., Janes, R. W. & Wallace, B. A. Tools and methods for circular dichroism spectroscopy of proteins: a tutorial review. Chem. Soc. Rev. 50, 8400–8413 (2021).
Micsonai, A. et al. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl Acad. Sci. USA 112, E3095–E3103 (2015).
Koga, R. et al. Robust folding of a de novo designed ideal protein even with most of the core mutated to valine. Proc. Natl Acad. Sci. USA 117, 31149–31156 (2020).
Gao, K., Oerlemans, R. & Groves, M. R. Theory and applications of differential scanning fluorimetry in early-stage drug discovery. Biophys. Rev. 12, 85–104 (2020).
Lössl, P., van de Waterbeemd, M. & Heck, A. Jr. The diverse and expanding role of mass spectrometry in structural and molecular biology. EMBO J. 35, 2634–2657 (2016).
Lanucara, F., Holman, S. W., Gray, C. J. & Eyers, C. E. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem. 6, 281–294 (2014).
Karch, K. R., Snyder, D. T., Harvey, S. R. & Wysocki, V. H. Native mass spectrometry: recent progress and remaining challenges. Annu. Rev. Biophys. 51, 157–179 (2022).
Figueroa, M. et al. The unexpected structure of the designed protein Octarellin V.1 forms a challenge for protein structure prediction tools. J. Struct. Biol. 195, 19–30 (2016).
Yagi, S. & Tagami, S. An ancestral fold reveals the evolutionary link between RNA polymerase and ribosomal proteins. Nat. Commun. 15, 5938 (2024).
Porter, L. L., Artsimovitch, I. & Ramírez-Sarmiento, C. A. Metamorphic proteins and how to find them. Curr. Opin. Struct. Biol. 86, 102807 (2024).
Bhattacharya, S. et al. NMR-guided directed evolution. Nature 610, 389–393 (2022).
Jaskolski, M., Dauter, Z. & Wlodawer, A. A brief history of macromolecular crystallography, illustrated by a family tree and its nobel fruits. FEBS J. 281, 3985–4009 (2014).
Wlodawer, A., Minor, W., Dauter, Z. & Jaskolski, M. Protein crystallography for non-crystallographers, or how to get the best (but not more) from published macromolecular structures. FEBS J. 275, 1–21 (2008).
Wlodawer, A., Minor, W., Dauter, Z. & Jaskolski, M. Protein crystallography for aspiring crystallographers or how to avoid pitfalls and traps in macromolecular structure determination. FEBS J. 280, 5705–5736 (2013).
Saibil, H. R. Cryo-EM in molecular and cellular biology. Mol. Cell 82, 274–284 (2022).
Jacques, D. A. & Trewhella, J. Small-angle scattering for structural biology — expanding the frontier while avoiding the pitfalls. Protein Sci. 19, 642–657 (2010).
Skou, S., Gillilan, R. E. & Ando, N. Synchrotron-based small-angle X-ray scattering of proteins in solution. Nat. Protoc. 9, 1727–1739 (2014).
Byer, A. S., Pei, X., Patterson, M. G. & Ando, N. Small-angle X-ray scattering studies of enzymes. Curr. Opin. Chem. Biol. 72, 102232 (2023).
Kobayashi, N. et al. Self-assembling nano-architectures created from a protein nano-building block using an intermolecularly folded dimeric de novo protein. J. Am. Chem. Soc. 137, 11285–11293 (2015).
Morris, R., Black, K. A. & Stollar, E. J. Uncovering protein function: from classification to complexes. Essays Biochem. 66, 255–285 (2022).
Zhou, M., Li, Q. & Wang, R. Current experimental methods for characterizing protein–protein interactions. ChemMedChem 11, 738–756 (2016).
Poluri, K. M., Gulati, K. & Sarkar, S. Experimental Methods for Determination of Protein–Protein Interactions 197–264 (Springer Singapore, 2021).
Bisswanger, H. Enzyme assays. Perspect. Sci. 1, 41–55 (2014).
Chong, S. Overview of Cell-free Protein Synthesis: Historic Landmarks, Commercial Systems, and Expanding Applications 16.30.1–16.30.11 (John Wiley & Sons, Inc., 2014).
Alfi, A. et al. Cell-free mutant analysis combined with structure prediction of a lasso peptide biosynthetic protease B2. ACS Synth. Biol. 11, 2022–2028 (2022).
Taguchi, H. & Niwa, T. Reconstituted cell-free translation systems for exploring protein folding and aggregation. J. Mol. Biol. 436, 168726 (2024).
Thornton, E. L. et al. Applications of cell free protein synthesis in protein design. Protein Sci. 33, e5148 (2024).
Zielonka, S. & Krah, S. (eds) in Methods in Molecular Biology 1st edn (ed. Clifton, N. J.) (Humana Press, 2019).
Newton, M. S., Cabezas-Perusse, Y., Tong, C. L. & Seelig, B. In vitro selection of peptides and proteins — advantages of mRNA display. ACS Synth. Biol. 9, 181–190 (2020).
Gantz, M., Mathis, S. V., Nintzel, F. E. H., Lio, P. & Hollfelder, F. On synergy between ultrahigh throughput screening and machine learning in biocatalyst engineering. Faraday Discuss. 252, 89–114 (2024).
Park, C. & Marqusee, S. Pulse proteolysis: a simple method for quantitative determination of protein stability and ligand binding. Nat. Methods 2, 207–212 (2005).
Rocklin, G. J. et al. Global analysis of protein folding using massively parallel design, synthesis, and testing. Science 357, 168–175 (2017).
Linsky, T. W. et al. Sampling of structure and sequence space of small protein folds. Nat. Commun. 13, 7151 (2022).
Araya, C. L. & Fowler, D. M. Deep mutational scanning: assessing protein function on a massive scale. Trends Biotechnol. 29, 435–442 (2011).
Forrer, P., Jung, S. & Pluckthun, A. Beyond binding: using phage display to select for structure, folding and enzymatic activity in proteins. Curr. Opin. Struct. Biol. 9, 514–520 (1999).
Seelig, B. & Szostak, J. W. Selection and evolution of enzymes from a partially randomized non-catalytic scaffold. Nature 448, 828–831 (2007).
Layton, C. J., McMahon, P. L. & Greenleaf, W. J. Large-scale, quantitative protein assays on a high-throughput DNA sequencing chip. Mol. Cell 73, 1075–1082.e4 (2019).
Markin, C. J. et al. Revealing enzyme functional architecture via high-throughput microfluidic enzyme kinetics. Science 373, eabf8761 (2021).
Lee, J. et al. A broadly generalizable stabilization strategy for sarbecovirus fusion machinery vaccines. Nat. Commun. 15, 5496 (2024).
Boyoglu-Barnum, S. et al. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature 592, 623–628 (2021).
Walls, A. C. et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2. Cell 183, 1367–1382.e17 (2020).
Parkinson, J., Hard, R. & Wang, W. The RESP AI model accelerates the identification of tight-binding antibodies. Nat. Commun. 14, 454 (2023).
Mason, D. M. et al. Optimization of therapeutic antibodies by predicting antigen specificity from antibody sequence via deep learning. Nat. Biomed. Eng. 5, 600–612 (2021).
Makowski, E. K. et al. Co-optimization of therapeutic antibody affinity and specificity using machine learning models that generalize to novel mutational space. Nat. Commun. 13, 3788 (2022).
Shanker, V. R., Bruun, T. U. J., Hie, B. L. & Kim, P. S. Inverse folding of protein complexes with a structure-informed language model enables unsupervised antibody evolution. Preprint at bioRxiv https://doi.org/10.1101/2023.12.19.572475 (2023).
Shanehsazzadeh, A. et al. Unlocking de novo antibody design with generative artificial intelligence. Preprint at bioRxiv https://doi.org/10.1101/2023.01.08.523187 (2023).
Mahajan, S. P., Ruffolo, J. A., Frick, R. & Gray, J. J. Hallucinating structure-conditioned antibody libraries for target-specific binders. Front. Immunol. 13, 999034 (2022).
Giordano-Attianese, G. et al. A computationally designed chimeric antigen receptor provides a small-molecule safety switch for T-cell therapy. Nat. Biotechnol. 38, 426–432 (2020).
Sesterhenn, F. et al. Boosting subdominant neutralizing antibody responses with a computationally designed epitope-focused immunogen. PLoS Biol. 17, e3000164 (2019).
Dawson, W. M. et al. Differential sensing with arrays of de novo designed peptide assemblies. Nat. Commun. 14, 383 (2023).
Quijano-Rubio, A. et al. De novo design of modular and tunable protein biosensors. Nature 591, 482–487 (2021).
Zhang, J. Z. et al. Thermodynamically coupled biosensors for detecting neutralizing antibodies against SARS-CoV-2 variants. Nat. Biotechnol. 40, 1336–1340 (2022).
Ng, A. H. et al. Modular and tunable biological feedback control using a de novo protein switch. Nature 572, 265–269 (2019).
Lee, G. R. et al. Small-molecule binding and sensing with a designed protein family. Preprint at bioRxiv https://doi.org/10.1101/2023.11.01.565201 (2023).
Rhys, G. G. et al. De novo designed peptides for cellular delivery and subcellular localisation. Nat. Chem. Biol. 18, 999–1004 (2022).
Huddy, T. F. et al. Blueprinting extendable nanomaterials with standardized protein blocks. Nature 627, 898–904 (2024).
Wargacki, A. J. et al. Complete and cooperative in vitro assembly of computationally designed self-assembling protein nanomaterials. Nat. Commun. 12, 883 (2021).
Kratochvil, H. T. et al. Transient water wires mediate selective proton transport in designed channel proteins. Nat. Chem. 15, 1012–1021 (2023).
Scott, A. J. et al. Constructing ion channels from water-soluble α-helical barrels. Nat. Chem. 13, 643–650 (2021).
Shimizu, K. et al. De novo design of a nanopore for single-molecule detection that incorporates a β-hairpin peptide. Nat. Nanotechnol. 17, 67–75 (2022).
Zhang, S. et al. Bottom-up fabrication of a proteasome-nanopore that unravels and processes single proteins. Nat. Chem. 13, 1192–1199 (2021).
Courbet, A. et al. Computational design of mechanically coupled axle-rotor protein assemblies. Science 376, 383–390 (2022).
Cao, L. et al. Design of protein-binding proteins from the target structure alone. Nature 605, 551–560 (2022).
Lauko, A. et al. Computational design of serine hydrolases. Preprint at bioRxiv https://doi.org/10.1101/2024.08.29.610411 (2024).
Schnettler, J. D. et al. Selection of a promiscuous minimalist cAMP phosphodiesterase from a library of de novo designed proteins. Nat. Chem. 16, 1200–1208 (2024).
Röthlisberger, D. et al. Kemp elimination catalysts by computational enzyme design. Nature 453, 190–195 (2008).
Siegel, J. B. et al. Computational design of an enzyme catalyst for a stereoselective bimolecular Diels–Alder reaction. Science 329, 309–313 (2010).
Bjelic, S. et al. Computational design of enone-binding proteins with catalytic activity for the Morita–Baylis–Hillman reaction. ACS Chem. Biol. 8, 749–757 (2013).
Rajagopalan, S. et al. Design of activated serine-containing catalytic triads with atomic-level accuracy. Nat. Chem. Biol. 10, 386–391 (2014).
Khersonsky, O. et al. Evolutionary optimization of computationally designed enzymes: Kemp eliminases of the KE07 series. J. Mol. Biol. 396, 1025–1042 (2010).
Khersonsky, O. et al. Optimization of the in-silico-designed Kemp eliminase KE70 by computational design and directed evolution. J. Mol. Biol. 407, 391–412 (2011).
Khersonsky, O. et al. Bridging the gaps in design methodologies by evolutionary optimization of the stability and proficiency of designed kemp eliminase KE59. Proc. Natl Acad. Sci. USA 109, 10358–10363 (2012).
Blomberg, R. et al. Precision is essential for efficient catalysis in an evolved Kemp eliminase. Nature 503, 418–421 (2013).
Giger, L. et al. Evolution of a designed retro-aldolase leads to complete active site remodeling. Nat. Chem. Biol. 9, 494–498 (2013).
Preiswerk, N. et al. Impact of scaffold rigidity on the design and evolution of an artificial Diels-Alderase. Proc. Natl Acad. Sci. USA 111, 8013–8018 (2014).
Obexer, R. et al. Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase. Nat. Chem. 9, 50–56 (2017).
Crawshaw, R. et al. Engineering an efficient and enantioselective enzyme for the Morita–Baylis–Hillman reaction. Nat. Chem. 14, 313–320 (2022).
Lux, M. W., Strychalski, E. A. & Vora, G. J. Advancing reproducibility can ease the ‘hard truths’ of synthetic biology. Synth. Biol. 8, ysad014 (2023).
Koehler Leman, J. et al. Better together: elements of successful scientific software development in a distributed collaborative community. PLoS Comput. Biol. 16, e1007507 (2020).
Koehler Leman, J. et al. Ensuring scientific reproducibility in bio-macromolecular modeling via extensive, automated benchmarks. Nat. Commun. 12, 6947 (2021).
Sandve, G. K., Nekrutenko, A., Taylor, J. & Hovig, E. Ten simple rules for reproducible computational research. PLoS Comput. Biol. 9, e1003285 (2013).
Moreau, D., Wiebels, K. & Boettiger, C. Containers for computational reproducibility. Nat. Rev. Methods Primers 3, 50 (2023).
Wilson, G. et al. Good enough practices in scientific computing. PLoS Comput. Biol. 13, e1005510 (2017).
Gibney, E. Not all ‘open source’ AI models are actually open: here’s a ranking. Nature https://doi.org/10.1038/d41586-024-02012-5 (2024).
Liesenfeld, A. & Dingemanse, M. Rethinking open source generative AI: open washing and the EU AI act. In The 2024 ACM Conference on Fairness, Accountability, and Transparency (ACM, 2024).
Hsia, Y. et al. Design of a hyperstable 60-subunit protein dodecahedron [corrected]. Nature 535, 136–139 (2016).
Alberstein, R. G., Guo, A. B. & Kortemme, T. Design principles of protein switches. Curr. Opin. Struct. Biol. 72, 71–78 (2022).
Cerasoli, E., Sharpe, B. K. & Woolfson, D. N. ZiCo: a peptide designed to switch folded state upon binding zinc. J. Am. Chem. Soc. 127, 15008–15009 (2005).
Zhu, J. & Lu, P. Computational design of transmembrane proteins. Curr. Opin. Struct. Biol. 74, 102381 (2022).
Chakravarty, D. & Porter, L. L. AlphaFold2 fails to predict protein fold switching. Protein Sci. 31, e4353 (2022).
Zambaldi, V. et al. De novo design of high-affinity protein binders with AlphaProteo. Preprint at https://arxiv.org/abs/2409.08022 (2024).
Lu, H. et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 604, 662–667 (2022).
Raissi, M., Perdikaris, P. & Karniadakis, G. E. Physics-informed neural networks: a deep learning framework for solving forward and inverse problems involving nonlinear partial differential equations. J. Comput. Phys. 378, 686–707 (2019).
Jones, J. A., Andreas, M. P. & Giessen, T. W. Exploring the extreme acid tolerance of a dynamic protein nanocage. Biomacromolecules 24, 1388–1399 (2023).
Groenhof, G. Introduction to QM/MM simulations. Methods Mol. Biol. 924, 43–66 (2013).
Majewski, M. et al. Machine learning coarse-grained potentials of protein thermodynamics. Nat. Commun. 14, 5739 (2023).
Johnston, B. et al. Molecularnodes: v4.2.9 for Blender 4.2+. Zenodo https://doi.org/10.5281/zenodo.14241983 (2024).
Fleuret, F. The little Book of Deep Learning https://fleuret.org/public/lbdl.pdf (Université de Genève, 2023).
Vijayakumar, A. K. et al. Diverse beam search for improved description of complex scenes. In Proc. Thirty-Second AAAI Conference on Artificial Intelligence (eds McIlraith, S. A. & Weinberger, K. Q.) https://doi.org/10.1609/aaai.v32i1.12340 (AAAI Press, 2018).
Acknowledgements
Protein structures were rendered using the ‘Molecular Nodes’ add-on for blender.org408. This work was supported by the French ‘Investing for the Future — PIA3’ programme under the Grant agreement ANR-23-IACL-0002, by the French National Research Agency, under the Grant agreement ANR-22-CE45-0025-01, by the RCUK | Biotechnology and Biological Sciences Research Council (BBSRC) under the Grant agreement BB/V004220/1 and by the National Science Foundation under the Grant agreement 2019598.
Author information
Authors and Affiliations
Contributions
Introduction (K.I.A., D.N.W. and T.S.); Experimentation (K.I.A., D.N.W., S.B. and T.S.); Results (K.I.A. and S.T.); Applications (K.I.A., S.B. and T.S.); Reproducibility and data deposition (K.I.A., S.T. and T.S.); Limitations and optimizations (K.I.A. and T.S.); Outlook (T.S., D.N.W. and S.T.); conceptualization of the Primer (T.S.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Responsible AI x Biodesign: https://responsiblebiodesign.ai/
Supplementary information
Glossary
- EC number
-
The Enzyme Commission (EC) number is a unique four-digit numerical classification system used to identify and categorize enzymes based on their catalytic function.
- Embeddings
-
Numerical internal vector representations of some input data that can be extracted from deep-learning networks trained on this type of data.
- Epistasis
-
Interactions between amino acid residues that influence the structure, function or stability of the protein.
- Forward folding
-
Forward folding consists of predicting the structure of a designed protein sequence to check whether it is predicted to fold in the targeted structure.
- Generative model
-
A probabilistic model of data \(P(X),P(X,Y)\,{\rm{or}}\,P(X|Y)\) that can be sampled to generate more data-like objects. The realism of the generated objects depends both on the training data and the ability of the model to capture the complex dependencies that exist.
- Graphical model
-
Mathematical models that represent complex numerical functions of many variables as a combination, usually the sum, of many functions involving few variables, as do pairwise decomposable energy functions.
- Inductive bias
-
Set of assumptions that a machine-learning model makes about the nature of data and the relationships within it, which influences how the model learns and generalizes from training data to new, unseen data. These assumptions are baked into the model’s architecture, learning algorithm and training process.
- Interface predicted alignment error
-
(ipAE). Specific to multimer predictions, measuring the average pAE of interchain residue pairs.
- Interface predicted template modelling score
-
Specific to multimer predictions, measuring the accuracy of the predicted relative positions of the subunits forming the protein–protein complex.
- Inverse-folding problem
-
The problem of finding a sequence that will fold onto a given backbone structure.
- Multiple sequence alignment
-
(MSAs). An arrangement of several biological sequences in a way that highlights regions of similarity, indicative of evolutionary relationships, functional similarities or structural similarities between the sequences.
- Multistate design
-
(MSD). A design approach in which multiple states of the designed protein are simultaneously taken into account during sequence design.
- Oligomeric state
-
Number of peptide or protein subunits that interact non-covalently to form a functional protein assembly.
- Out-of-distribution
-
Data that are significantly different from the data a model was trained on.
- Position-specific score matrix
-
(PSSM). A 20 × ℓ matrix with one column for every position of a multiple sequence alignment, where each column vector contains the \(\log ({f}_{{\rm{aa}}})\), in which \({f}_{{\rm{aa}}}\) is the frequency of amino acid aa in the multiple sequence alignment column.
- Predicted alignment error
-
(pAE). A measure of how confident the structure prediction software is in the relative position of two residues within a predicted structure.
- Predicted local distance difference test
-
(pLDDT). Scaled from 0 to 100, this test measures the confidence in the local structure, predicting how well the prediction would agree with an experimental structure. It is based on the local distance difference test Cα, which is a score that does not rely on superposition but instead measures the correctness of the local distances.
- Predicted template modelling score
-
(pTM). A prediction of how well the modelling software has predicted the overall structure.
- Probability distribution
-
For discrete objects such as sequences, a probability distribution maps each object x from the considered collection of objects to its probability \(P(x)\). The sum of all the probabilities of all objects in the collection must sum to 1. This can be guaranteed by normalizing the distribution.
- Protein language models
-
Probabilistic models of protein sequences that assign a probability to protein sequences.
- Rational design
-
Human design following rules of thumb, physical and expert protein knowledge. May be computer-assisted, using molecular dynamics, for example.
- Scoring functions
-
A combination of probabilistic information provided by physical energy with statistical information extracted from data, assembled to estimate the probability of observing a protein in a given conformation in a computationally tractable form.
- Theozyme
-
A theozyme is a theoretical minimal active site model composed of a calculated transition state, including key functional groups from amino acid side chains needed for transition state stabilization of the substrate.
- Topology
-
Protein topology is a property of a protein that does not change under deformation (without breaking a bond). In biology, this is extended to include mutual orientation of secondary structures (α-helices, β-strands, etc.) in the protein structure.
- Transition state
-
The highest-energy intermediate state that briefly exists during a chemical reaction. Enzymes accelerate reactions by lowering this energy barrier.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albanese, K.I., Barbe, S., Tagami, S. et al. Computational protein design. Nat Rev Methods Primers 5, 13 (2025). https://doi.org/10.1038/s43586-025-00383-1
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43586-025-00383-1
This article is cited by
-
De novo protein design: a transformative frontier in clinical protein applications
Journal of Translational Medicine (2026)
-
Sequence-based generative AI design of versatile tryptophan synthases
Nature Communications (2026)
-
Keeping the light on: understanding methods as AI redraws the map
Nature Reviews Methods Primers (2026)
-
An update for AlphaFold3 versus experimental structures: assessing the precision of small molecule binding in GPCRs
Acta Pharmacologica Sinica (2025)
-
Chemoenzymatic cascade depolymerization of plastics
Communications Chemistry (2025)