Abstract
Age-related thymic involution precedes aging of all other organs in vertebrates and initiates the process of declining T cell diversity, which leads to eventual immune dysfunction. Whether FGF21, a liver-derived pro-longevity hormone that is also produced in thymic stroma, including by adipocytes, controls the mechanism of thymic demise is incompletely understood. Here, we demonstrate that elevation of FGF21 in thymic epithelial cells (TECs) and in adipocytes protects against thymic aging, whereas conditional hepatic overexpression did not impact thymic biology in aged mice. Notably, elevation of thymic FGF21 increased naïve CD8 T cells in aged animals and extended healthspan. Mechanistically, thymic FGF21 overexpression elevated TECs and reduced fibroadipogenic cells. Ablation of β-klotho, the obligatory co-receptor for FGF21 in Foxn1+ TECs, accelerated thymic aging, suggesting regulation of TECs by FGF21 is partially required for thymic lymphopoiesis. These findings establish that paracrine FGF21 improves thymic function and delays immune aging.
Similar content being viewed by others
Main
Once considered dispensable, the surgical removal of the thymus in adult humans is now known to accelerate immune aging and increase all-cause mortality1,2,3,4,5. One of the cardinal features of immunological aging is the loss of naïve T cell production due to degeneration of the thymus1,2,3,4. Notably, new T cell specificities can only be generated in a functional thymus and proliferation of pre-existing T cell clones in secondary lymphoid organs provides limited immune vigilance in older adults1,4. The “one-hoss shay”-based compressed morbidity analogy in gerontology research advocates healthspan and lifespan extension by maintaining the function of all organs to optimal levels during aging6. One biological impediment to this laudable goal is that aging of the thymus is fastest and is apparent at puberty, when ovarian and testicular function peaks2. By 40 years of age in healthy humans, approximately 80% of thymic space is replaced by adipocytes7. Thus, aging is associated with marked perturbations in the stromal cell microenvironment of the thymus8,9,10. This includes a reduction in lymphopoiesis-supporting thymic epithelial cells (TECs)8, an increase in fibroblasts9,10 and the emergence of adipocytes7,10. Notably, age-related loss of TECs and emergence of atypical age-associated TECs are considered to be one of the major causes of inability of the thymus to produce T cells11. The thymus, however, also retains a remarkable capacity of partial rejuvenation, suggesting that targeting the thymus may be an important therapeutic strategy to enhance immune function in older adults4. Interestingly, recent studies demonstrate that pro-longevity interventions such as caloric restriction in both mice10 and humans can enhance thymic function by reducing ectopic lipid to improve the intrathymic stromal cell microenvironment12. The thymus can also be partially and transiently rejuvenated by increasing endocrine hormones, such as growth hormone (GH)/insulin-like growth factor 1 (IGF-1) (refs. 13,14,15) and ghrelin16, and through reduction of gonadal steroids by increasing luteinizing hormone-releasing hormone or gonadotropin-releasing hormone17,18. Prior studies have demonstrated that restoration of TEC niches in aging is pivotal to thymic regeneration12,13,14,15,16,17,18,19. However, the identity and mechanism of TEC-derived growth factors that maintain or enhance thymic function is incompletely known19.
FGF21 belongs to the fibroblast growth factor (FGF) superfamily that regulate a myriad of fundamental processes such as growth, development, differentiation and proliferation and delays cellular aging by mechanisms that are not fully elucidated20,21,22,23,24. Our prior studies identified that FGF21, an endocrine hormone that increases lifespan, is primarily secreted by hepatocytes into circulation in response to various cellular stressors and is highly expressed in TECs24,25. The FGF family member FGF7/KGF is also known to enhance thymic function by acting on TECs26. Interestingly, global overexpression of FGF21 protects against age-related thymic involution25. However, transgenic FGF21-overexpressing mice display dwarfism, similar to long-lived animals with inhibition of the growth hormone-IGF-1 axis27,28. Thus, it remains unclear whether global FGF21-overexpression-induced growth suppression causes organismal tradeoffs that delay aging and indirectly improve thymic function in a non-cell-autonomous manner.
To determine whether endocrine or paracrine FGF21 impacts thymic aging, we developed hepatocyte-, TEC- and adipocyte-specific FGF21-overexpressing mice and ablated β-klotho (the obligate co-receptor for FGF21) in TECs to investigate the thymic involution process. Here, we show that albumin Cre-driven overexpression of FGF21 in the liver increases circulating FGF21 without causing growth retardation but does not affect thymic function in aged mice. Notably, FGF21 signaling via TECs and TEC- and adipocyte-specific FGF21 overexpression protects against age-related thymic involution.
Results
Hepatocyte-derived endocrine FGF21 is dispensable for thymic function during aging
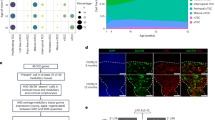
Our prior studies found that FGF21 expression in TECs declines with aging25 and that mice with global increase in FGF21 displayed delayed age-related thymic involution25. Our previous studies were done with an Apoe-Fgf21 model in which FGF21 was overexpressed at pharmacologic levels from embryonic stage21,25. These Apoe-Fgf21Tg mice, which have circulating FGF21 levels that are ~100-fold higher than those that occur under physiologic conditions, were markedly smaller in size21,24. To overcome this issue, we generated CAG-LacZ-Fgf21-eGFP transgenic mice (Fig. 1a). This transgene consists of the synthetic CAG promoter and the Fgf21 gene separated by a loxP-STOP-loxP cassette23. In the presence of Cre recombinase, the STOP cassette is excised and transcription of Fgf21 is enabled (Fig. 1a). To validate the CAG-LacZ-Fgf21-eGFP mice, we crossed them with albumin (Alb)-Cre mice, which express Cre recombinase exclusively in hepatocytes23. Compared to control littermates, AlbCre-iFGF21Tg mice have approximately fivefold higher circulating levels of FGF21 (Fig. 1a), which is much lower than those in Apoe-Fgf21 mice and comparable to levels which occur in response to prolonged fasting or ketogenic or low-protein diets20,22. Importantly, this increase in FGF21 did not cause dwarfism or affect thymic size relative to body weight in 2 year old mice (Fig. 1b). Compared to 24-month-old control littermates, the age-matched AlbCre-iFGF21Tg mice had no change in naive or effector-memory (E/M) T cells. Interestingly, 24-month-old AlbCre-iFGF21Tg mice had a significant increase in CD8 naive (CD62L+CD44lo) cells and a reduction in age-induced expansion of E/M cells (CD62L−CD44hi) but no changes in thymic cellularity (Fig. 1b,c). Consistent with the absence of changes in total thymocyte numbers in aged AlbCre-iFGF21Tg mice, there was no significant difference in the earliest thymocyte progenitors and double-negative (DN) or double-positive (DP) or CD4 and CD8 single-positive thymocytes (Fig. 1d). Furthermore, hepatocyte-derived elevation of FGF21 did not affect medullary and cortical TECs (mTECs and cTECs, respectively) (Fig. 1e). Together, these data demonstrate that up to fivefold elevation of endocrine hepatocyte-derived FGF21 is not sufficient to impact the thymic aging process.
a, Strategy for generation of liver-specific FGF21 transgenic (Tg) mice. This transgene consists of the synthetic CAG promoter and the Fgf21 gene separated by a loxP-STOP-loxP cassette. In the presence of Cre recombinase, the STOP cassette is excised and transcription of Fgf21 is enabled in liver (created with BioRender.com). FGF21 expression in serum in 24-month-old AlbCre-iFGF21Tg mice (P = 0.0002). b, Body wight, thymic weight and total thymic cellularity normalized to body weight (P = 0.023) in 24-month-old mice. WT, wild-type. c, Splenocytes were stained with CD4, CD8, CD62L and CD44 to identify naive (CD4/CD8+CD62L+CD44−) (P = 0.048) and E/M (CD4/CD8+ CD62L-CD44+) T cells (P = 0.040). Representative fluorescence-activated cell sorting (FACS) dot plots and quantification from control and 24-month-old mice AlbCre-iFGF21Tg mice are shown. d, Thymocytes from aged mice were stained to identify ETPs (LinloCD117+CD25−) and DN2 (LinloCD117+CD25+) and DN3 (LinloCD117−CD25+) cells. e, Thymic cells were analyzed for mTECs (CD45− EpCAM+MHCII+Ly5.1+ /UEA1) and cTECs (CD45− EpCAM+MHCII+Ly5.1- /UEA1) in 24-month-old mice (n = 6/group). Data are presented as means ± standard error of the mean (s.e.m.). Two-tailed paired and unpaired t-tests were performed for statistical analysis (*P < 0.05, ***P < 0.001). Ctl, control.
TEC-derived FGF21 protects against thymic aging
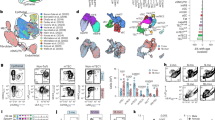
To determine the impact of FGF21 on mechanisms of thymic involution and the role of local expression of FGF21 during thymic aging processes, we next investigated peripheral immune cell changes by thymic epithelial-driven FGF21 overexpression. CAG-LacZ-Fgf21-eGFP mice were crossed with Foxn1Cre mice, which express Cre recombinase exclusively in TECs and keratinocytes (Fig. 2a). Analysis of CD45−Epcam+ TECs revealed a twofold increase of FGF21, with no changes in non-TEC compartments (Fig. 2c). Consistent with our prior results25, FGF21 was not expressed in CD45+ immune cells in the thymus (Fig. 2c). Notably, Foxn1+TEC overexpression of FGF21 did not increase circulating FGF21 levels in 12- to 24-month-old mice (Fig. 2c), suggesting minimal contribution from Foxn1+ skin epithelial cells or keratinocyte toward the circulation. Interestingly, immunohistological analyses of serial sections of 24-month-old control littermates and Foxn1Cre-iFGF21Tg mice revealed that TEC-specific FGF21 overexpression protected against age-related thymic involution (Fig. 2b). The 24-month-old Foxn1Cre-iFGF21Tg mice displayed preservation of cortical and medullary cellularity, maintenance of corticomedullary junctions and substantial reduction in ectopic thymic adipocytes (Fig. 2b), the key hallmarks of thymic involution2,3,4. Consistent with improved thymic architecture, compared to controls, 24-month-old Foxn1Cre-iFGF21Tg mice had greater thymic size (Fig. 2d,e), weight (Fig. 2f) and total thymocyte counts (Fig. 2g), whereas adult 12-month-old mice had no change in thymic cellularity (Extended Data Fig. 2a), suggesting an age-specific effect. Moreover, 24-month-old Foxn1Cre-iFGF21Tg mice had a significant increase in the frequency and numbers of naive CD8 T cells, DP and DN3, with a reduction in E/M T cells in the spleen, with no change in CD4 cell frequencies (Fig. 2h) and earliest thymic progenitors (ETPs), DN and DN2 (Extended Data Fig. 1a).
a, Strategy for generation of TEC-specific FGF21 transgenic mice (created with BioRender.com). b, Representative hematoxylin and eosin (H&E) histology images of thymus in 24-month-old mice. TEC-specific overexpression of FGF21 increases cortical cellularity and protects against thinning of cortex and expansion of medulla. Scale bars, 200 μm (left) or 20 μm (right). Notably, FoxnCre-iFGF21Tg mice show reduced ectopic lipid in thymus and adipocytes. m, medulla; c, cortex. c, Expression of FGF21 in TECs of control and Foxn1Cre-FGF21Tg-overexpressing mice. Quantitative polymerase chain reaction (Q-PCR) analysis for FGF21 gene expression in various cell types and serum FGF21 levels in 12- and 24-month-old wilt-type and FoxN1Cre:iFGF21 mice (12 months, n = 8–9, 24 m, wild-type n = 25, 24 months Tg n = 19). The median, 25th and 75th percentiles (boundaries of boxes), and 5th and 95th percentiles (whiskers above and below boxplots) are indicated in the boxplots (P = 0.030). d–g, Characterization of thymic involution of aged mice. Shown are thymic mass (P = 0.014), body weight (P = 0.035), and cellularity (P = 0.009) (n = 6–13/group). h, FACS quantification, frequencies and numbers of naive (CD62L+CD44−) (P = 0.022) and E/M (CD62L−CD44+) (P = 0.039) T cells in spleen of 24-month-old control and iFGF21-FoxN1Cre mice (n = 6–7/group). Data are presented as means ± s.e.m. Two-tailed paired and unpaired t-tests were performed for statistical analysis (*P < 0.05, ** P < 0.01).
Increased FGF21 production in TECs improves the thymic microenvironment and enhances healthspan during aging
Age-related loss of TECs is considered to be one of the major causes of the inability of the thymus to produce T cells11,19,29. We found that Foxn1Cre-iFGF21 mice have both an increased frequency and numbers of medullary and cortical TECs during aging (Fig. 3a). In addition to the loss of TECs, thymic aging is associated with the emergence of fibroadipogenic cells that are linked to the process of epithelial-mesenchymal transition11,30. We have previously reported that a decrease in thymic fibroadipogenic cells is associated with increased thymopoiesis10. Conversely, genetic gain of function of proadipogenic signaling in mesenchymal origin cells reduces thymopoiesis31. Consistent with these studies and the well-known role of FGF21 in increasing fatty acid oxidation32, we found that compared to control mice, 24-month-old Foxn1Cre-iFGF21 mice showed a significant reduction in lipid-containing fibroadipogenic cells (Fig. 3b). Compared to controls, immunoblot analyses of thymi from 24-month-old Foxn1Cre-iFGF21 mice confirmed a twofold increase in FGF21, with significant increase in IGF-1 and trend toward higher IL-7 protein concentrations (Fig. 3c). We isolated the enriched EpCAM+ TECs from control and aged Foxn1Cre-iFGF21 mice and found that TEC-specific increase of FGF21 significantly increased the expression of thymic growth factors Il7, scf and Igf1, and we observed a trend toward increased Foxn1 (Fig. 3d and Extended Data Fig. 1b). Moreover, consistent with the reduced number of ectopic adipocytes in thymus (Fig. 2b) and the decrease in fibroadipogenic cells, the increase of TEC-derived FGF21 lowered the age-related increase in Pparg, the master regulator of adipogenesis (Fig. 3d).
a, FACS quantification and gating to identify and quantify mTECs (P = 0.047) and cTECs (P = 0.005) in enzymatically digested thymi from 24-month-old control and Foxn1Cre:iFGF21-FoxN1Cre mice (n = 10/group). b, Representative FACS plots and quantification of CD45−PDGFRα+ lipid-containing fibroadipogenic cells from 24-month-old mice (P = 0.01) (n = 4–8/group). c, Immunoblot analyses and quantification of FGF21 (P = 0.008), IL-7 and IGF-1 (P = 0.028) protein in the thymi derived from 24-month-old age-matched controls and Foxn1Cre:iFGF21Tg mice (n = 3/group). d, Real-time PCR analysis of growth factors, Il7 (P = 0.001), Scf (P = 0.018), Igf1 P = 0.013) and the proadipogenic regulator Pparg (P = 0.025) in EpCAM+ TECs (n = 10/group). e, Healthspan test results of 24-month-old mice; rotarod measured motor coordination, frailty and grip strength in 24-month-old control and Foxn1Cre:iFGF21Tg mice (n = 12/group). Data presented as means ± s.e.m. Two-tailed paired and unpaired t-tests were performed for statistical analysis (*P < 0.05, **P < 0.01, ****P < 0.0001).
Interestingly, recent findings from a study of >1,000 people aged 9–96 years in two validation cohorts identified that an increase in naive CD8 T cells and immune-inflammatory clock (iAGE) is a strong predictor of longevity and healthspan33. Moreover, decline of T cell diversity is also associated with age-related disease34. Therefore, we next investigated whether preservation of thymic function and increase in naive CD8 T cell frequency induced by the elevation of thymic FGF21 impacts healthspan. The rotarod test that assays for a decline in sensorimotor, neuromuscular function, motor coordination and frailty showed significant improvements in 24-month-old old Foxn1Cre-iFGF21 mice. Similarly, compared to control mice, the TEC-specific FGF21 elevation significantly improved grip strength in older mice (Fig. 3e). Together, these data demonstrate that an elevation of thymic FGF21 improves thymic function and delays measures of organismal aging.
Adipocyte-derived FGF21 protects against thymic aging independently of adiponectin
In contrast to a young thymus, in which thymocytes are the major contributors to the thymic microenvironment, adipocytes constitute the majority of cells in the aged thymic space2,4,7,12. Importantly, thymic adipocytes are present in several areas of the thymus, including the subcapsular area, trabeculae, septae, cortex, medulla, corticomedullary junction and perivascular space, which suggests that adipocytes are important tissue-resident thymic cells7. In addition, the para-aortic adipose tissue lies in close apposition with the thymus, similar to the fat that surrounds the lymph nodes7,12. Thus, adipocyte-derived secreted factors can likely impact the thymic microenvironment. Therefore, to investigate whether elevation of FGF21 from adipocytes impacts the thymus, we developed a transgenic mouse model with inducible overexpression of FGF21 specifically in adipocytes using a doxycycline-dependent inducible Tet-on system (Fig. 4a). Moreover, as >30% of older adults are overweight4, we fed control and Adn-iFGF21Tg mice with high-fat diet (HFD) and evaluated immune and metabolic measures at 6 and 18 months of age. Consistent with prior studies suggesting that FGF21 increases fat utilization, Adn-iFGF21Tg mice were protected from adiposity and showed a significant increase in the thymic somatic index (Fig. 4b). T cell subsets in 6-month-old control and Adn-iFGF21Tg mice did not show significant differences (Fig. 4c). Interestingly, compared to controls, 3-month-old Adn-iFGF21Tg mice on HFD weighed significantly less but had no change in thymic mass and cellularity (Extended Data Fig. 2b). However, adipocyte overexpression of FGF21 protected mice from prolonged HFD feeding together with aging-induced thymic involution, as determined by reduced intrathymic adipocytes, well-defined corticomedullary cellularity and maintenance of corticomedullary junctions (Fig. 4d). Notably, evaluation of these mice fed long-term HFD until 18 months of age revealed greater protection from weight gain, an improved thymic somatic index (Fig. 4e) and a marked protection from age and adiposity-associated thymic involution (Fig. 4f). Consistent with these findings, elevation of adipocyte-derived FGF21 in 18-month-old HFD-fed mice also increased the number of naive CD4 and CD8 T cells with a reduction in the E/M population, suggesting maintenance of T cell diversity (Fig. 4g). These results suggest that an elevation of adipocyte-secreted FGF21 can protect against HFD-induced thymic involution into advanced age.
a, Mouse model of inducible adipocyte-specific FGF21 expression using a Tet-on system that allows protein expression specific to adipocytes in a doxycycline-inducible manner (created with BioRender.com). b, Thymus weight/body weight (P < 0.0001) ratio (somatic index, P = 0.0005) were determined in 6-month-old adipose-specific inducible overexpression of FGF21 Adn:iFGF21Tg mice fed HFD (n = 4/group). dox, doxycycline. c, FACS quantification of CD4 and CD8 naïve and E/M cells in 6-month-old Adn-iFGF21Tg mice fed HFD (n = 4/group). d, Formalin-fixed paraffin-embedded sections of thymi derived from 6-month-old control and Adn:iFGF21Tg mice fed HFD stained with H&E display reduced ectopic adipocytes and maintenance of corticomedullary junctions in adipocyte-specific FGF21Tg mice. Scale bars, 200 μm (top) or 20 μm (bottom). e, Thymus weight/body weight (P < 0.0001) ratio (somatic index, P = 0.005) in 18-month-old control and Adn:iFGF21Tg mice fed HFD (n = 5–6/group). m, medulla; c, cortex; a, adipocytes. f, Histological examination of the structure of thymus in 18-month-old control and And:iFGF21Tg mice fed HFD (magnification, ×4). g, FACS quantification of naive T cells (CD4+ and CD8+CD62L+CD44−) 0(CD4+, P = 0.046, CD8+, P = 0.009) and E/M (CD4+ and CD8+CD62L−CD44+, P = 0.05) T cells from splenocyte of 18-month-old control and Adn:iFGF21 Tg mice (n = 8–9/group). Data are presented as means ± s.e.m. Two-tailed paired and unpaired t-tests were performed for statistical analysis (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Adiponectin is secreted from adipocytes and is closely related to C1q/TNF-related proteins35,36. Our prior results have demonstrated that FGF21 stimulates adiponectin secretion to mediate some of its beneficial effects on energy expenditure37,38, including ceramide-induced thymic inflammation39. To determine if adiponectin mediated the elevated FGF21-induced prothymic effects, we deleted adiponectin from transgenic mice that overexpress FGF21 in adipocytes (Adipoq−/−:Adn-iFgf21Tg; Fig. 5a). Interestingly, when fed HFD, compared to adiponectin-deficient mice, Adipoq−/−:Adn-iFgf21Tg mice were protected from adiposity, similar to overexpression of FGF21 in adipocytes (Fig. 5a). Adipoq−/−:Adn-iFgf21Tg mice had a 5- to 10-fold increase in intrathymic FGF21 levels (Fig. 5b). Furthermore, the analysis of perithymic fat pad revealed approximately 50-fold higher FGF21 expression in Adipoq−/−:Adn-iFgf21Tg mice (Fig. 5b). Notably, despite deletion of adiponectin, FGF21 overexpression in adipocytes protected mice from HFD-aging-induced thymic lipoatrophy, as evidenced by improved corticomedullary cellularity and reduced intrathymic adipocytes (Fig. 5c). Consistent with these data, compared to control 6- and 18-month-old Adipoq−/− mice fed HFD, Adipoq−/−:Adn-iFgf21Tg mice had an increase in naive CD8 T cells and a reduction in E/M cells (Fig. 5d,e). Together, these results demonstrate that adipocyte-derived FGF21 serves as a potent prothymic growth factor that protects against age-adiposity-associated thymic involution, independently of adiponectin.
Adiponectin knockout (KO) mice (ADNK) and mice lacking adiponectin and overexpressing FGF21 in adipocytes were fed HFD and aged for 18 months. a, Body weight and thymic size (P = 0.0001, n = 10–13/group). b, Q-PCR analyses of FGF21 in thymi (P = 0.021) and perithymic fat pads (P = 0.0006) (n = 7–9/group). c, Representative H&E-stained sections from 18-month-old adiponectin KO control and adiponectin KO-iFGF21 Tg mice fed HFD. Cortical regions (c) and medullary areas (m) are shown. Scale bar, 200 μm (left) or 20 μm (right). n = 5–9 per group. d, Representative flow cytometry analysis of immune cell populations in spleen. d,e, Quantification of naive T cells (CD4+and CD8+CD62L+CD44−) (CD8+, P = 0.044) and E/M (CD4+ and CD8+CD62L−CD44+) (CD4+, P = 0.001; CD8+, P = 0.0006) T cells from splenocytes of 6-month-old (n = 4/group) (d) and 18-month-old (e) adiponectin KO and ADNK:Adn:iFGF21 double Tg mice (n = 10–12/group). Data are presented as means ± s.e.m. Two-tailed paired and unpaired t-tests were performed for statistical analysis (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
β-Klotho expression in TECs is partially required for FGF21-dependent protection from thymic aging
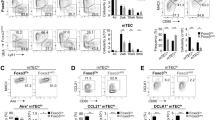
FGF21 elicits its biological effects by binding to its obligate co-receptor β-klotho in complex with FGF receptor 1c (FGFR1c), FGFR2c or FGFR3c, but not FGFR420,32,40. We previously reported that FGF21 and its co-receptor, β-klotho (KLB (protein)) and FGF receptors are expressed in thymic stromal cells and not in immune cells25. To evaluate the direct role of FGF21 signaling on TECs in a model of aging we conditionally ablated β-klotho (Klb (gene)) from Foxn1 origin TECs (Fig. 6a). Ablation of Klb from TECs did not affect thymic cellularity in 6- and 12-month-old adult mice (Extended Data Fig. 2c,d). However, compared to control littermates, upon aging, the 24-month-old mice lacking Klb in TECs had diminished thymic cellularity (Fig. 6b). Additional analyses revealed that this reduction was associated with reduced frequency of ETPs and DN cells (Fig. 6c) and TECs (Fig. 6d) in thymus of 24-month-old Foxn1Cre:Klbfl/fl mice. Interestingly, ablation of β-klotho in TECs significantly reduced the frequency of age-related naive CD4 and CD8 T cells from CD8+ cells in spleen and increased the frequency of E/M T cells (Fig. 6e). Together, these data suggest that homeostatic FGF21 action on TECs is partially required and is not essential for maintenance of thymic function during aging.
a, Strategy for generation of TEC-specific-β-klotho KO mice. We generated the conditional mutant mouse by crossing the KLB floxed strain with FoxN1cre (created with BioRender.com). b, Thymic cellularity from 24-month-old control and Foxn1Cre:Klbfl/fl mice (P = 0.002, n = 10/group). c, Elimination of KLB in TECs accelerated decline of ETPs in aged mice. Shown are representative FACS plots to identify ETPs (LinloCD117+CD25) in thymi derived from 24-month-old control and Foxn1Cre:Klbfl/fl mice (ETP, P = 0.004, DN = 0.002, n = 6–10/group). d, Thymic cells from aged mice were stained to quantify mTEChi (P = 0.04), mTEClo (P = 0.001), cTEChi (P = 0.04) and cTEClo (P = 0.003) subsets in 24-month-old control and Foxn1Cre:Klbfl/fl mice (n = 4/group). e, Representative FACS dot plots of splenocytes stained with CD4, CD8, CD62L and CD44 to identify naïve (CD8+, P = 0.04) and E/M T cells (CD4+, P = 0.003, CD8+, P = 0.0002) (n = 9/group). Data are presented as means ± s.e.m. Two-tailed paired and unpaired t-tests were performed for statistical analysis (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Discussion
With age, thymic function declines precipitously at puberty when ovarian and testicular function peaks for sexual maturation1,2,3,4,5. The diminished ability of the thymus to produce naive T cells with progressive aging remains a fundamental and puzzling phenomenon for immunology and, to date, an intractable clinical condition that contributes to immune dysfunction in older adults1,2,3,4,5. Age-related thymic involution is associated with reduced immune surveillance, increased risk and severity of emerging infections, certain cancers, vaccination failures and delayed T cell reconstitution in patients undergoing hematopoietic stem cell transplantation29,34. Therefore, the ability to enhance thymopoiesis is thought to be central to the rejuvenation of T cell-mediated immune surveillance in older adults4,29. Importantly, these clinical data further underscore critical role of thymic function in healthspan and lifespan5. The rejuvenation of thymic function with age or delay of thymic aging in humans remains a worthy goal. Aging is known to cause loss of Lin−CD25−CD117+ ETPs that give rise to T cells in thymus41. In addition, loss of TECs8,42 and emergence of fibroadipogenic cells10,11 and adipocytes31 impair thymic function in aging. In addition, the thymus reconstitution in aged mice can be facilitated by in vitro-generated T cell progenitors43. Here, we identify that intrathymic elevation of TEC- and adipocyte-derived FGF21 protects against thymic involution induced by aging and obesity.
The FGF family member FGF7 (KGF) has been implicated in the maintenance of TEC function in aging26. Notably, FGF21 ligates a cell surface receptor complex composed of conventional tyrosine kinase FGFR and requires β-klotho as an obligate co-receptor protein for its signaling activity40. Although hepatocytes are the predominant endocrine source of FGF21, we have found that on a per cell basis, TECs express 3-4-fold higher FGF21 levels than liver25. Moreover, FGF21 has also been proposed to be a myokine and adipokine32. Notably, FGF21 and KLB are not expressed by immune cells, suggesting stromal cells are the major target cells25. Consistent with prior studies suggesting that elevation of FGF21 signaling increases fatty acid oxidation and reduces ectopic lipid32, mice overexpressing FGF21 in TECs and adipocytes had reduced ectopic intrathymic lipid. Interestingly, our studies provide evidence that paracrine FGF21 that elevates intrathymic levels in lymphopoietic niches may be critical for enhancing thymic function during aging. The mechanism of FGF21 action to increase TECs and reduce fibroadipogenic cells requires future investigations. However, our data suggest that FGF21 action on TECs via β-klotho plays only a partial role in thymic function as adult mice lacking KLB in TECs did not show impairment of thymopoiesis. Notably, upon aging, deficient FGF21 signaling on TECs partakes in thymic involution processes. These results suggest FGF21 may act on mesenchymal, fibroadipogenic, adipocyte precursors or sympathetic nerves in the thymus.
A recent study using deep learning and data analyses of immune cell profiles of 1,001 individuals aged 8 to 96 years identified that loss of naive CD8 T cells serves as a robust measure of healthy versus unhealthy aging33. Interestingly, this study confirmed that loss of naive CD8 cells serves as a robust biological clock and predictor of longevity. In support of these findings, thymic FGF21-induced elevation of naive CD8 T cells in the spleen was associated with improved healthspan in aged mice. It remains unclear whether elevated thymic activity that increases the number of naive CD8 T cells confers longevity. Future studies may be required to determine whether longevity in humans is linked to better thymic function and export of naive CD8 cells. In summary, our findings using multiple genetic mouse models demonstrate that elevation of intrathymic FGF21 via paracrine action on thymic stroma is an important mechanism that controls age-related thymic involution.
Methods
Experimental procedures
Mice and animal care
All mice were on the C57BL/6 J genetic background, AlbCre-iFGF21Tg, FoxN1Cre-iFGF21Tg and KLBfl/fl FoxN1Cre mice were obtained from S. Kliewer and D. Mangelsdorf (UT Southwestern). TEC-specific-β-klotho KO mice were generated by crossing the KLB floxed strain with FoxN1cre. FoxN1Cre-iFGF21Tg and AlbCre-iFGF21Tg mice were generated for TEC-specific and liver-specific transgenic mice. Adipocyte-specific inducible transgenic mice, ADN-iFGF21Tg and ADNK-iFGF21Tg (Adipoq−/−:And-iFGF21Tg) were generated by the P. E. Scherer laboratory (UT Southwestern). We previously generated the adiponectin promoter-rtTA mice that were crossed with TRE- FGF21 or TRE-Cre constructs, thus providing a doxycycline-inducible model.
Mice were fed a 60% HFD containing 600 mg kg−1 doxycycline (BioServ) at 8 weeks of age until mice reached 6 or 18 months of age. Mice were housed in a pathogen-free facility with a 12-h light/12-h dark cycle in ventilated cage racks that deliver HEPA-filtered air to each cage with free access to food and sterile water though a hydropac system at Yale School of Medicine, which offers maximum biosecurity. The hydropac water delivery system has one-way water flow, so unlike water bottles and autowater pipes, there is no source of water contamination from bacteria derived from mouse saliva. Sentinel mice in our animal rooms were negative for currently tested standard mouse pathogens (ectromelia virus, epizootic diarrhea of infant mice, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, murine hepatitis virus, murine norovirus, metapneumovirus, minute virus of mice, pneumovirus of mice, mammalian orhtoreovirus type 3, Theiler’s murine encephalomyelitis virus and Sendai virus), whereas the studies were performed (Research Animal Diagnostic Laboratory). All experiments and animal use were conducted in compliance with the National Institute of Animal Care and Use Committee at Yale University.
Thymocyte and total thymic stromal cell isolation
For thymocyte isolation, thymic were harvested and minced into small pieces though the 100 μM filter. For thymic stromal cells isolation, thymus was digested with 0.125% collage D (Roche) with 0.125% DNase for 10 min at 37 °C three times. After final digestion, cells were washed and removed red blood cells by ACK buffer and then pelleted by centrifugation to collect the thymic stromal cells for flow cytometry.
Thymic stromal cell isolation (EpCam+ selection)
For positive selection of EpCam+ cells from whole thymus was incubated with biotinylated anti-mouse EpCam antibody (eBioscience) in isolation buffer (PBS, 0.1% BSA, 2 mM EDTA, pH 7.4). Then, cells were washed and incubated with prewashed magnetic Dynabeads Biotin binder (Life Technologies, 11047) to isolate the EpCam+ fraction.
Flow cytometry
Thymocyte and stromal cells were collected from enzymatic digestion, washed and pelleted by centrifugation and then resuspended in FACS buffer (2% BSA, 0.09% sodium azide). Thymocyte cells were stained with live/dead viability dye (Invitrogen), incubated with Fc block (CD16/32) and then stained for surface markers, including CD25 and CD117 (c-Kit), and a dump channel that included CD11b, Gr1, NK1.1, CD11c, βTCR, TER-119, CD3, CD8, γδTCR and CD45R (B220) for ETP, DN and DP cells. Stromal cells were stained with CD45.2, MHCII, Ly51 and EpCam for TECs. For fibroblasts and droplets, cells were stained with CD45.2, EpCam, ERTR7 and LipidTOX, followed by incubation for 30 min on ice in the dark. Cells were then fixed with 1% paraformaldehyde. All antibodies were purchased from eBioscience, BioLegend and BD. Flow cytometry was performed on a BD LSR II, and results were analyzed by FlowJo using DIVA software (v8.0.I).
FGF21 measurements
The collected blood was incubated at room temperature for 2 h and centrifuged at 900g at 4°C for 20 min. FGF21 protein levels were measured in serum by ELISA (R&D) according to the manufacturer’s instructions. Data were analyzed using a four-parameter logistic regression method.
Gene expression analysis (Q-PCR)
Quantitative real-time RT-PCR was performed from total RNA of whole thymus and sorted TECs (mTECs and cTECs) and perithymic fat in STAT-60 and extracted using the RNeasy Plus Tissue Mini or Micro Kit (QIAGEN) following the manufacturer’s instructions. Total RNA was digested by DNase (Invitrogen). RNA concentrations of the samples were then measured, and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time RT-PCR analyses were completed in duplicate on a LightCycler 480 II (Roche) with the SYBR Green PCR kit as instructed by the manufacturer (Applied Biosystems). Specific genes were analyzed by deltaCT values normalized to GAPDH or 18S. A list of the quantitative real-time RT-PCR primers used is shown in Supplementary Table 1.
Histology
For thymic structure, tissues were collected in 10% formalin, embedded in paraffin and then cut into 5- to 7-μm-thick sections. Thymi were stained with H&E. The images were acquired and analyzed using a Keyence BZ-x microscope.
Behavioral test (rotarod and grip strength)
The rotarod test is a functional assay widely used for measuring balance and coordination in rodents that has shown age-related sensitivity in mice. This test was performed with an AccuRotor rotarod (AccuScan Instruments). The mouse was placed on top of the rotating cylinder at the slowest speed and permitted locomotion until falling. Each mouse received training three times and was then tested for a total three trials with readout recorded and mean time spent on the rotarod over three trials.
The settings used were maximum speed 400 (x0.1 rpm) and time to maximum speed 300 s. The grip strength test was performed using a grip strength meter (TSE Systems). Maximum strength (gram force) of forelimb of mice holding a bar in the meter was measured in five trials. Because variability in performance can occur throughout the trial, a normative claim (good versus bad) was used for each recoding, and then good readings for three trials were aligned and averaged for each mouse.
Western blotting
Tissues were harvested followed by snap freezing in liquid nitrogen, and tissues were homogenized in RIPA buffer (Sigma) with protease inhibitors (Sigma). Cell lysates were prepared by harvesting in RIPA buffer with protease inhibitors. Equal amount of protein was run on SDS-PAGE gel (Invitrogen) after quantification of protein concentration using the DC protein assay (Bio-Rad) and transferred to nitrocellulose membrane. Primary antibodies and appropriate secondary antibodies were used to probe blots. The bands were detected by chemiluminescent visualization (ChemiDoc MPTM Imaging System, Bio-Rad). Primary antibodies were incubated in 5% milk for 1 h and subsequently incubated overnight at 4 °C. Host-matched secondary antibodies were incubated for 1 h at room temperature. The following primary antibodies were used for experiments: antibodies to FGF21(1:1,000, RnD), IL-7 (1:1,000, Cell Signaling Technology), IGF-1 (1:1,000, Cell Signaling Technology) and β-actin (1:1,000, Cell Signaling Technology). The secondary antibodies used were goat anti-rabbit IgG (H + L), HRP, goat anti-mouse IgG (H + L) and HRP (Invitrogen, Sigma). ImageJ was used for densitometry analysis.
Statistics and reproducibility
A two-tailed Student’s t-test was used to test for differences between genotypes. For all experiments, a P < 0.05 was considered significant (*P < 0.05, **P < 0.005, ***P < 0.001, ****P < 0.0001). The number of biological replicates and independent experimental repeats is indicated in each figure legend corresponding to each experiment. Data are shown as mean ± s.e.m. GraphPad Prism was used for all statistical tests for analysis of experimental results. Data collection and analysis were not performed blind to the conditions of the experiments. To verify the reproducibility of our findings, experiments were performed using at least two biological replicates. Some mice were excluded from the experiments due to animal health and conditions if they developed a movement disorder, lost weight abnormally or reached a humane endpoint.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Dorshkind, K., Montecino-Rodriguez, E. & Signer, R. A. The ageing immune system: is it ever too old to become young again? Nat. Rev. Immunol. 9, 57–62 (2009).
Steinmann, G. G. Changes in the human thymus during aging. Curr Top Pathol 75, 43–88 (1986).
Lynch, H. E. et al. Thymic involution and immune reconstitution. Trends Immunol 30, 366–373 (2009).
Dixit, V. D. Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence. Semin. Immunol. 24, 321–330 (2012).
Kooshesh, K. A., Foy, B. H., Sykes, D. B., Gustafsson, K. & Scadden, D. T. Health consequences of thymus removal in adults. N. Engl. J. Med. 389, 406–417 (2023).
Kennedy, D. Longevity, quality, and the one-hoss shay. Science 305, 1369 (2004).
Dixit, V. D. Thymic fatness and approaches to enhance thymopoietic fitness in aging. Curr. Opin. Immunol. 22, 521–528 (2010).
Gray, D. H. et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 108, 3777–3785 (2006).
Yang, H. et al. Obesity accelerates thymic aging. Blood 114, 3803–3812 (2009).
Yang, H., Youm, Y. H. & Dixit, V. D. Inhibition of thymic adipogenesis by caloric restriction is coupled with reduction in age-related thymic involution. J. Immunol. 183, 3040–3052 (2009).
Kousa, A. I. et al. Age-related epithelial defects limit thymic function and regeneration. Nat. Immunol. 25, 1593–1606 (2024).
Spadaro, O. et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677 (2022).
Taub, D. D., Murphy, W. J. & Longo, D. L. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr. Opin. Pharmacol. 10, 408–424 (2010).
Montecino-Rodriguez, E., Clark, R. & Dorshkind, K. Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology 139, 4120–4126 (1998).
Napolitano, L. A. et al. Growth hormone enhances thymic function in HIV-1-infected adults. J. Clin. Invest. 118, 1085–1098 (2008).
Dixit, V. D. et al. Ghrelin promotes thymopoiesis during aging. J. Clin. Invest. 117, 2778–2790 (2007).
Dixit, V. D., Sridaran, R., Edmonsond, M. A., Taub, D. & Thompson, W. E. Gonadotropin-releasing hormone attenuates pregnancy-associated thymic involution and modulates the expression of antiproliferative gene product prohibitin. Endocrinology 144, 1496–1505 (2003).
Sutherland, J. S. et al. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 175, 2741–2753 (2005).
Petrie, H. T. & Zuniga-Pflucker, J. C. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu. Rev. Immunol. 25, 649–679 (2007).
Kliewer, S. A. & Mangelsdorf, D. J. A dozen years of discovery: insights into the physiology and pharmacology of FGF21. Cell Metab 29, 246–253 (2019).
Inagaki, T. et al. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metab. 8, 77–83 (2008).
Bookout, A. L. et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 19, 1147–1152 (2013).
Abu-Odeh, M. et al. FGF21 promotes thermogenic gene expression as an autocrine factor in adipocytes. Cell Rep. 35, 109331 (2021).
Zhang, Y. et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1, e00065 (2012).
Youm, Y. H., Horvath, T. L., Mangelsdorf, D. J., Kliewer, S. A. & Dixit, V. D. Prolongevity hormone FGF21 protects against immune senescence by delaying age-related thymic involution. Proc. Natl Acad. Sci. USA 113, 1026–1031 (2016).
Rossi, S. W. et al. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood 109, 3803–3811 (2007).
Brown-Borg, H. M. & Bartke, A. GH and IGF1: roles in energy metabolism of long-living GH mutant mice. J. Gerontol. A Biol. Sci. Med. Sci. 67, 652–660 (2012).
Vallejo, A. N. et al. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc. Natl Acad. Sci. USA 106, 11252–11257 (2009).
Chidgey, A., Dudakov, J., Seach, N. & Boyd, R. Impact of niche aging on thymic regeneration and immune reconstitution. Semin. Immunol. 19, 331–340 (2007).
Youm, Y. H. et al. Deficient ghrelin receptor-mediated signaling compromises thymic stromal cell microenvironment by accelerating thymic adiposity. J. Biol. Chem. 284, 7068–7077 (2009).
Youm, Y. H. et al. Thiazolidinedione treatment and constitutive-PPARgamma activation induces ectopic adipogenesis and promotes age-related thymic involution. Aging Cell 9, 478–489 (2010).
Kharitonenkov, A. & Adams, A. C. Inventing new medicines: the FGF21 story. Mol. Metab. 3, 221–229 (2014).
Sayed, N. et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 1, 598–615 (2021).
Goronzy, J. J. & Weyand, C. M. T cell development and receptor diversity during aging. Curr. Opin. Immunol. 17, 468–475 (2005).
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Turer, A. T. & Scherer, P. E. Adiponectin: mechanistic insights and clinical implications. Diabetologia 55, 2319–2326 (2012).
Lin, Z. et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17, 779–789 (2013).
Holland, W. L. et al. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 17, 790–797 (2013).
Youm, Y. H. et al. The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 1, 56–68 (2012).
Lee, S. et al. Structures of beta-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 553, 501–505 (2018).
Bhandoola, A., von Boehmer, H., Petrie, H. T. & Zuniga-Pflucker, J. C. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity 26, 678–689 (2007).
Cowan, J. E., Takahama, Y., Bhandoola, A. & Ohigashi, I. Postnatal involution and counter-involution of the thymus. Front. Immunol. 11, 897 (2020).
Mohtashami, M., Li, Y. R., Lee, C. R. & Zuniga-Pflucker, J. C. Thymus reconstitution in young and aged mice is facilitated by in vitro-generated progenitor T cells. Front. Immunol. 13, 926773 (2022).
Acknowledgements
We thank S. Kliewer and D. Mangelsdorf for sharing the FGF21 transgenic and β-klotho floxed mice. This research is supported in part by National Institutes of Health grant NIA:P01AG051459.
Author information
Authors and Affiliations
Contributions
Y.-H.Y. contributed to design and performed majority of experiments and data analysis and prepared the figures and manuscript. C.G. contributed to generation of ADN-iFGF21Tg and ADNK-iFGF21Tg (Adipoq−/−:And-iFGF21Tg) mice and performed mouse phenotyping. Y.Z. generated conditional FGF21 transgenic mice and performed FGF21 protein analysis. T.D. performed the mouse phenotyping and healthspan analyses. P.E.S. generated the adipocyte-specific FGF21Tg mouse model and adiponectin-deficient mice, supervised the analyses and participated in design and analyses of data from these animal models. V.D.D. conceived the project, supervised the study, guided the data analyses and interpretation and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Yousuke Takahama, Bing Wu and Juan Carlos Zúñiga-Pflücker for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Foxn1+TEC overexpression of FGF21 did not changes in ETP DN, DP, DN2 and DN3 population.
(a) Representative flow cytometry analysis of immune cell population in thymus (CD4, CD8, ETP, DN, DP, DN2 and DN3) in 24-month-old WT and FoxN1cre-iFGF21Tg mice (DN3, p = 0.031, DP, p = 0.027), (n = 4/group). (b) Real-time PCR analysis of FGF21 level and growth factors in whole thymus and thymic epithelial cells (EpCam+) from 24-month-old FoxN1Cre-iFGF21Tg mice (p = 0.046). Data are presented as means ± SEM. Two-tailed paired and unpaired t-tests were performed for statistical analysis * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001.
Extended Data Fig. 2 No change in thymic involution upon FGF21 overexpression and ablation of FGF21 signaling in Foxn1+TECs.
(a) The thymic mass, body weight and thymocyte plus thymic stromal cell numbers in 12-month-old control and Foxn1Cre:iFGF21Tg mice (p = 0.015), (n = 12/group). (b) The thymic mass, body weight and thymocyte plus thymic stromal cell numbers in 3-month-old control and Adn:iFGF21Tg mice (p = <0.0001), (n = 6/group). (c) The thymic mass, body weight and thymocyte plus thymic stromal cell numbers in 6 and 12-month-old control and Foxn1Cre:Klbfl/fl mice (n = 4/group). (d) Thymic size of control animals and mice lacking KLB in thymic epithelial cells. Data are presented as means ± SEM. Two-tailed paired and unpaired t-tests were performed for statistical analysis * P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001.
Supplementary information
Supplementary Information
Supplementary Table 1.
Source data
Source Data Fig. 1
Western blots.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Youm, YH., Gliniak, C., Zhang, Y. et al. Enhanced paracrine action of FGF21 in stromal cells delays thymic aging. Nat Aging 5, 576–587 (2025). https://doi.org/10.1038/s43587-025-00813-5
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s43587-025-00813-5
This article is cited by
-
Metabolic regulation of immunological aging
Nature Aging (2025)
-
FGF21 keeps the thymus young
Nature Aging (2025)