Abstract
Background
Beta-cell monogenic forms of diabetes have strong support for precision medicine. We systematically analyzed evidence for precision treatments for GCK-related hyperglycemia, HNF1A-, HNF4A- and HNF1B-diabetes, and mitochondrial diabetes (MD) due to m.3243 A > G variant, 6q24-transient neonatal diabetes mellitus (TND) and SLC19A2-diabetes.
Methods
The search of PubMed, MEDLINE, and Embase for individual and group level data for glycemic outcomes using inclusion (English, original articles written after 1992) and exclusion (VUS, multiple diabetes types, absent/aggregated treatment effect measures) criteria. The risk of bias was assessed using NHLBI study-quality assessment tools. Data extracted from Covidence were summarized and presented as descriptive statistics in tables and text.
Results
There are 146 studies included, with only six being experimental studies. For GCK-related hyperglycemia, the six studies (35 individuals) assessing therapy discontinuation show no HbA1c deterioration. A randomized trial (18 individuals per group) shows that sulfonylureas (SU) were more effective in HNF1A-diabetes than in type 2 diabetes. Cohort and case studies support SU’s effectiveness in lowering HbA1c. Two cross-over trials (each with 15–16 individuals) suggest glinides and GLP-1 receptor agonists might be used in place of SU. Evidence for HNF4A-diabetes is limited. Most reported patients with HNF1B-diabetes (N = 293) and MD (N = 233) are on insulin without treatment studies. Limited data support oral agents after relapse in 6q24-TND and for thiamine improving glycemic control and reducing/eliminating insulin requirement in SLC19A2-diabetes.
Conclusion
There is limited evidence, and with moderate or serious risk of bias, to guide monogenic diabetes treatment. Further evidence is needed to examine the optimum treatment in monogenic subtypes.
Plain language summary
Monogenic diabetes is a type of diabetes caused by changes in genes that affect how the body makes or responds to insulin. Precision medicine (where knowledge of the gene change directs the selection of treatment) is available for some forms of monogenic diabetes. This study evaluated the published literature for several forms of monogenic diabetes to assess the level of evidence supporting specific precision treatments. Among the 146 small studies that we reviewed, only six compared different treatments. However, we found evidence supporting oral medications for some types of monogenic diabetes, and evidence that treatment is not needed for one particular type. Based on our results, we provide treatment recommendations for certain forms of monogenic diabetes and identify future directions for research to help us optimize precision medicine in monogenic diabetes.
Similar content being viewed by others
Introduction
In monogenic forms of diabetes, the underlying genetic cause has implications for the disease mechanism, treatment, and prognosis. Defining the underlying genetic etiology also defines the pathophysiology resulting in hyperglycemia; this greatly increases the chances of finding an optimal specific therapy or therapy for glucose lowering. Pathogenic variations in single genes can either result in reduced insulin secretion as seen in the beta-cell subtypes or reduced insulin action in the insulin-resistant subtypes. This systematic review relates to the treatment of beta-cell subtypes with the treatment of insulin-resistant subtypes being reviewed elsewhere1.
Beta-cell monogenic forms of diabetes have been the area of diabetes care where there is the strongest support for a precision medicine approach for treating hyperglycemia2,3. The supporting evidence usually came from initial case reports leading to follow-up with case series and in some cases experimental studies/trials. The evidence base is considered strong in the commonest subtypes of monogenic diabetes such as glucokinase (GCK)-related hyperglycemia, Hepatic Nuclear Factor 1 alpha (HNF1A)-diabetes, and ATP sensitive potassium channel (KATP)-neonatal diabetes (ND, related to pathogenic variants in KCNJ11 and ABCC8). Such evidence is yet to be shown for the more rare subtypes of monogenic diabetes. The varying levels of evidence combined with an expert opinion have led to recommendations for optimum treatment in international guidelines such as the International Society for Pediatric and Adolescent Diabetes (ISPAD)4, with the strongest support for sulfonylureas (SU) as first-line therapy for HNF1A-diabetes and Hepatic Nuclear Factor 4 alpha (HNF4A)-diabetes, no pharmacologic therapy for GCK-related hyperglycemia, insulin for Hepatic Nuclear Factor 1 beta (HNF1B)-diabetes and mitochondrial diabetes (MD) and high-dose SU for KATP-ND. A key point is that these clinical guidelines were developed mostly without a systematic review of all the available evidence.
The systematic review will allow a comprehensive assessment of the strength of the evidence for the specific recommendations for precision medicine approaches. To our knowledge there is only one area of beta-cell monogenic diabetes where robust systematic reviews have been used – this is in the glycemic treatment of KATP-ND with high-dose SU5 and the partial response of neurological features in KATP-ND to high-dose SU therapy6. We therefore decided to systematically review the evidence of a precision medicine approach with an optimal glucose-lowering therapy for all the common subtypes of beta-cell monogenic diabetes except for the KATP-ND (Table 1).
This systematic review is written on behalf of the American Diabetes Association (ADA)/European Association for the Study of Diabetes (EASD) Precision Medicine in Diabetes Initiative (PMDI) as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine7. The PMDI was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD) to address the burgeoning need for better diabetes prevention and care through precision medicine8. The evidence for whom to test for monogenic diabetes, how to test them, and how to interpret a gene variant, as well as for underpinning the link between the genetic test result and prognostics are covered as separate systematic reviews in this series, by other members of the PMDI addressing precision diagnostics and prognostics for monogenic diabetes9,10.
We aimed to provide a systematic review of the evidence for precision medicine in beta-cell monogenic diabetes to answer several questions. What is the optimal glucose-lowering therapy in the three commonest subtypes of autosomal dominant familial diabetes also known as Maturity Onset Diabetes of the Young (MODY): GCK-related hyperglycemia, HNF1A-diabetes, and HNF4A-diabetes? What is the optimal glucose-lowering therapy in the two commonest subtypes of syndromic diabetes: HNF1B-diabetes and Mitochondrial Diabetes (MD) due to m.3243 A > G in the MT-TL1 gene? Are there alternatives to insulin therapy in 6q24-related transient neonatal diabetes (6q24-TND); and does thiamine supplementation improve glycemia in SLC19A2-diabetes also known as Thiamine-Responsive Megaloblastic Anemia (TRMA) syndrome? While there is some support for no treatment in GCK-related hyperglycemia and sulphonylureas for HNF1A-diabetes, overall there is limited evidence to guide the treatment in monogenic diabetes with most studies being non-randomized and small.
Methods
Protocols for systematic reviews were developed and registered in Prospero (https://www.crd.york.ac.uk/prospero/; for MODY (CRD42021279872); syndromic diabetes (CRD42021250955) and neonatal diabetes (CRD42023399408). The study was reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines11.
Search strategy
We comprehensively reviewed the literature associated with glycemic treatment outcomes, searching in PubMed, MEDLINE, and Embase separately for (1) GCK-related hyperglycemia, HNF1A-diabetes, and HNF4A-diabetes; (2a) HNF1B-diabetes; (2b) MD with m.3243 A > G variant; (3a) 6q24-TND; (3b) SLC19A2-diabetes. The Online Mendelian Inheritance in Man codes (and Orphanet rare disease nomenclature ORPHA codes) for the diseases are (1) #125851 (552), #600496 (552), #125850 (552), respectively; (2a) #137920 (552); (2b) #520000 (225); (3a) #601410 (99886), and (3b) #249270 (49827).
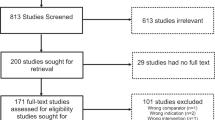
Filtering and selection of studies for full-text review and data extraction were recorded using Covidence (https://www.covidence.org). The search strategies and the PRISMA summaries for the searches are shown in Supplemental Tables 1–5 and Supplemental Figs. 1–4. At least two authors independently reviewed the titles and abstracts to filter out the articles for full-text review. Two authors independently reviewed full-text articles and either a third author (regarding GCK-related hyperglycemia, HNF1A-diabetes, and HNF4A-diabetes) or the two reviewers jointly (HNF1B-diabetes, MD, 6q24-TND, SLC19A2-diabetes) resolved discrepancies. One author for each search performed the data extraction using a standardized form and two reviewed it. Data were extracted from text, tables, or figures from the main and supplemental documents. Data extracted for each study included first author, publication year, country, study design, number of participants, age at diagnosis of diabetes and at the time of study, sex, diabetes duration, treatment information, and glycemic data.
Inclusion and exclusion criteria
We included English language original articles (case reports, case series, cross-sectional studies, experimental studies, trials) written after 1992 (following the initial molecular characterization of monogenic causes of diabetes) concerning the treatment of hyperglycemia in human individuals diagnosed with monogenic diabetes subtypes of interest. Individuals with variants of unknown significance, multiple types of diabetes, and those lacking measures of treatment effect were excluded. Studies or data within studies that aggregated treatment effects for multiple monogenic diabetes types were also excluded. We included studies reporting on more than one monogenic diabetes type of interest with partially incomplete data, as long as data could be fully extracted for at least one monogenic diabetes subtype.
Risk of bias and evidence appraisal
We used NHLBI study-quality assessment tools to assess the risk of bias (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) and the methods outlined by Sherifali et al., to assess the level of evidence and to grade recommendations12.
Data synthesis
Data were extracted from Covidence into Microsoft Excel. Data were summarized using Microsoft Excel. Data are presented as mean ± standard deviation, median [interquartile range, IQR], median (range), or as percentages.
Meta-analysis was not carried out due to the heterogeneity in study design as well as the high level of risk of bias in included studies, particularly case reports and series that make up a substantial portion of the literature.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Risk of bias
Most studies across all diabetes types were rated as having moderate or serious risk of bias related to the study design (mainly comprising observational case reports and case series) and selection of the study population and outcome variables (with genetic diagnoses not based on non-targeted population screening). Whenever a response or a non-response to a drug was reported, it was unclear whether other factors significantly or moderately contributed to the reported response.
What is the optimal glucose-lowering therapy in the three commonest subtypes of autosomal dominant familial diabetes (MODY): GCK-related hyperglycemia, HNF1A-diabetes, and HNF4A-diabetes?
There were 2389 unique studies identified by the literature search (Supplemental Fig. 1). After the title and abstract review, 136 studies remained for full-text review. Data was extracted from 33 articles in total, including six experimental studies (two designed as superiority trials), of which four were randomized controlled studies, 25 case reports or series, or cohort studies, one cross-sectional study, and one study presenting both cross-sectional and cohort data. There were 12 studies that contributed to data on treatment for GCK-related hyperglycemia, 23 studies for HNF1A-diabetes, and three studies for HNF4A-diabetes (with several studies contributing data to more than one diabetes subtype). The key summaries of these data are in Tables 2–5 and Supplemental Tables 6–7. The overall level of evidence was low and the risk of bias was high for most studies. The randomized controlled trials did not include power calculations.
What is the optimal glucose-lowering therapy in GCK-related hyperglycemia?
We originally posed the question of what is the impact of pharmacological and non-pharmacological glucose-lowering therapy in GCK-related hyperglycemia. However, we identified only one case report that presented data on active pharmacological intervention13 (Supplemental Table 6) and one study on dietary intervention14 (Table 2), and the rest of the 10 studies15,16,17,18,19,20,21,22,23,24 (Table 4 and Supplemental Table 6) assessed the impact of stopping anti-hyperglycemic agents on HbA1c or glucose or assessed the stability of HbA1c over time on no therapy. Thus, we concentrated on analyzing the evidence to support or refute non-treatment of GCK-related hyperglycemia.
Most studies on GCK-related hyperglycemia looked at within individual stability of HbA1c over time without glucose-lowering therapy (175 individuals from case reports and cohort data, range of time between 3–126 months)15,17,19,21,22,24 or after cessation of pharmacologic therapy following genetic diagnosis (35 individuals from case reports and cohort data)16,20,21,23,24. All studies showed stability of HbA1c with no significant change including when glucose-lowering therapy of either oral hypoglycemic agents (OHA) or insulin was discontinued (Table 4, Supplemental Table 6). Only a single case report suggested adding therapy might lower HbA1c13 (Supplemental Table 6).
A single large cross-sectional cohort study (n = 799) showed no differences in HbA1c between observational non-randomized treated and untreated groups24 (Table 4).
There were no randomized long-term treatment trials in GCK-related hyperglycemia. We identified a single experimental cross-over study for GCK carriers14 that assessed the impact of high-carbohydrate (60%) vs. low-carbohydrate (25%) unstandardized diet on mean glucose levels (MBG) and time spent above target postprandial blood glucose level (7.8 mmol/L) as measured by continuous glucose monitor (CGM) in 10 GCK subjects (Table 2). The duration of each intervention was brief (2 days with 1 day washout period). A statistically significant difference in mean glucose level (0.78 mmol/L) and time above target (11.7%) was found after high-carbohydrate diet if the analysis was restricted to the seven patients with an initial HbA1c above 6.5% (a non-predefined analysis). No comparison groups such as individuals without diabetes or with type 2 diabetes were included.
What is the optimal glucose-lowering therapy in HNF1A-diabetes and HNF4A-diabetes?
We analyzed studies for evidence of SU as an effective glucose-lowering therapy for HNF1A-diabetes and HNF4A-diabetes. We also assessed evidence for alternative or augmentative non-insulin therapies.
A striking observation was that all the trials or experimental studies (4 trials, 1 uncontrolled study, 76 individuals)25,26,27,28,29, and almost all the observational data (18 studies, 182 individuals)21,23,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 were for HNF1A-diabetes. While five studies included both HNF1A-diabetes and HNF4A-diabetes, only three studies (16 individuals) had HNF4A-diabetes data that could be extracted21,22,34.
In HNF1A-diabetes a single superiority, randomized cross-over study tested using SU in comparison with type 2 diabetes25 (Table 3). This was the only study in monogenic beta-cell diabetes to have a comparative group with type 2 diabetes, hence truly testing if it was a specific precision approach. The study compared the fasting plasma glucose (FPG) response in individuals with HNF1A-diabetes (N = 18) and type 2 diabetes (N = 18) to therapy with SU (gliclazide) or metformin for 6 weeks. The study groups were matched for body-mass index and FPG after the wash-out period off-treatment but not for sex or age. Gliclazide was superior to metformin in lowering fasting glucose in HNF1A-diabetes but not in type 2 diabetes (5-fold greater response to SU than metformin in HNF1A-diabetes; no difference in type 2 diabetes).
Glinides have been proposed as an alternative to long-acting SU, for example in instances of problematic hypoglycemia. There was one randomized, placebo-controlled cross-over study that compared the acute effects of premeal dosing of SU (1.25 mg glibenclamide), glinide (30 mg nateglinide) or placebo on glucose excursions during and after a standardized meal and exercise in 15 participants26 (Table 3). When comparing glibenclamide and nateglinide response to the standardized meal, peak insulin occurred earlier and plasma glucose levels (peak and up to 140 min) were lower with nateglinide. Exercise after the meal resulted in hypoglycemia (glucose <3.5 mmol/L) in 6 of 15 participants after glibenclamide while there were no episodes of hypoglycemia after nateglinide. Among the case studies, nine individuals from six studies initiating SU or glinide therapy showed an average HbA1c decrement of 1.3% after an average of 12.2 months of treatment31,34,35,36,40,44 (Supplemental Table 7).
We analyzed whether HNF1A patients treated with insulin therapy can successfully transfer to SU. In a prospective study on HNF1A-diabetes (n = 27) and HNF4A-diabetes (n = 7) not on SU at diagnosis, 25 of the 31 patients on insulin discontinued insulin but only 12 (48%) achieved an HbA1c of 7.5%23 (Table 5). Good glycemia was associated with a shorter diabetes duration and lower HbA1c at transfer. An uncontrolled study of eight individuals with HNF1A-diabetes assessed the success of transitioning from insulin that had been used since diabetes diagnosis (median insulin dose 0.5 units per kg; median duration of insulin treatment 20 years) to SU (gliclazide, median dose 80 mg daily)29 (Table 5). All patients were able to discontinue insulin. The HbA1c response was variable with a median reduction of 0.8% and six of eight improving but one individual had a worsening of HbA1c by 3.2%. Case-level data also show that seven individuals from five studies transitioning from insulin to SU showed an average HbA1c decrement of 1.35% after an average of 6.2 months of treatment with SU33,34,38,42,44 (Supplemental Table 7).
Two randomized, double-blinded cross-over studies compared alternative and augmentative regimens to SU monotherapy in HNF1A-diabetes (Table 3). Sixteen patients were enrolled in a trial comparing the change in FPG and risk of hypoglycemia during 6 weeks of SU monotherapy (glimepiride) and 6 weeks of glucagon-like peptide-1 receptor agonist (GLP1RA) monotherapy (liraglutide) with a 1-week washout between medications27. Among the 15 patients who completed the trial, FPG and post-prandial glucose were lowered by both treatments without a statistically significant difference between them. The number of mild hypoglycemic episodes (glucose <3.9 mmol/L) was markedly higher with glimepiride therapy (18 events) compared to liraglutide (1 event). The second superiority trial compared the effects of 16 weeks of SU monotherapy (glimepiride) to 16 weeks of combination therapy with glimepiride and the dipeptidyl peptidase-4 inhibitor (DPP4i) linagliptin (with 4-week washout) on the mean amplitude of glycemic excursions (MAGE) measured by CGM28. The combination therapy did not have an impact on MAGE over that of SU alone, but the mean (95% CI) HbA1c showed a significant decrease by −0.5% (−0.9 to −0.2, P = 0.0048) between SU and the combination therapy, included as a secondary endpoint.
There were only three studies where HNF4A data were reported separately21,23,34 (Table 5, Supplemental Table 7). Globa et al., report on two individuals with HNF4A-diabetes showing good response to SU, with >1% decrease in HbA1c (one was treatment-naive, the other switched from metformin)34. Another study included seven children with HNF4A-diabetes. Only two were on SU alone (mean HbA1c 7.0%, mean follow-up 8.3 years), while three were treated with insulin, two by choice and one due to inadequate control after switching from insulin to SU. Two others were not on pharmacologic treatment21.
What is the optimal glucose-lowering therapy in the two commonest subtypes of syndromic diabetes due to pathogenic variants in HNF1B and mitochondrial diabetes
After duplicate removal and abstract screening of 1716 articles, 135 articles remained for full-text review. Data was extracted from 18 articles for HNF1B-diabetes, 42 for MD, and 2 for both (Supplemental Fig. 2). We manually added an article not identified in the search46. There were no controlled trials, the overall level of evidence was low, and the risk of bias was high. Tables 6 and 7 show a summary of the data for the 13 articles on HNF1B-diabetes46,47,48,49,50,51,52,53,54,55,56,57,58 and 10 articles on MD46,59,60,61,62,63,64,65,66,67 that reported any treatment response even in single cases, and the prevalent treatment modalities in patient cohorts. The articles represent altogether 293 cases with HNF1B-diabetes and 242 with m.3243 A > G MD. Most articles only stated the current medication, which precludes drawing conclusions on the efficacy, but we note that at least one-fifth of the patients in both groups had no insulin treatment.
There were no randomized studies or trials of treatment in HNF1B-diabetes. Of the 168 individuals with HNF1B-diabetes, for whom treatment data after the genetic diagnosis was available, 132 (79%) used insulin, but it is not known if other medications had been tested (Table 6). One French study systematically evaluated the use of SU or repaglinide after the genetic diagnosis at a median [IQR] duration of 0.75 [0–5.25] years and reported that 29 of 51 (57%) patients displayed an HbA1c decrease. However, the duration of SU or repaglinide treatment in those who did respond was 5 [3–9] years, and at a mean of 12 years follow-up, 79% of the cohort were on insulin47. They also tried replacing insulin with SU for 10 patients, which was successful in three (no details given). In an uncontrolled Irish study, none of five patients on insulin could successfully switch to sulfonylureas49.
There were no randomized studies or trials of treatment in m.3243 MD. Of the 233 individuals with MD, for whom treatment data after the genetic diagnosis was available, 167 (72%) used insulin, but again it is not known if other medications had been tested (Table 7). No studies are reported trying to discontinue insulin by treating with OHAs.
Evidence against the use of metformin was limited to three case reports66,68,69 (Table 7). In two of them, metformin use was associated with elevated lactate levels (2.5–3.7 mmol/L68, and up to 5.9 mmol/L66), and the first was reported to have lactic acidosis. However, pH was not given for either case, and an improved lactate level (2.4 mmol/L) after discontinuation of metformin was only given for the latter case.
Are there alternatives to insulin therapy in 6q24 transient neonatal diabetes (6q24-TND)?
The literature search identified 1489 studies related to 6q24-TND (Supplemental Fig. 3). After duplicate removal, abstract screening, and full-text review, 19 studies met eligibility criteria, including five case series reporting on 16 cases, 14 reports of single cases, for a total of 30 6q24-TND cases for whom data was available regarding treatment with non-insulin therapies (Supplemental Tables 8 and 9).
There were no randomized trials for therapeutic response in 6q24-TND. For 16 cases with relevant data on the initial neonatal phase of diabetes70,71,72,73,74,75,76,77,78,79(Supplemental Table 8), SU (glyburide or glibenclamide in nearly all cases) was the only class of medication used other than insulin. Efficacy of SU during the neonatal phase was inconsistent, with studies reporting no effect or failure of SU to improve diabetes management in seven cases72,74,75,76,78,79, while for nine cases SU treatment was reported to allow insulin to be discontinued70,71,73,77,78,79. Of note, in most cases, diabetes remitted within days to weeks after insulin was discontinued, but three cases were reported to remain insulin-treated as old as 41- 60 months of age at the time of the reports72,74,78. None of these three cases with a possibly more permanent neonatal diabetes phenotype exhibited a response to SU treatment.
For case reports with relevant data on the use of non-insulin therapies during the later relapse phase of diabetes79,80,81,82,83,84,85,86,87,88 (Supplemental Table 9), the apparent efficacy of such treatment was more consistent, with 13 of 14 cases being either able to discontinue insulin at least temporarily or were never started on insulin (three cases)79,80,81,82,83,84,85,86,88. There was a wide range of follow-up time after the initiation of non-insulin therapies, but in most cases, it was at least 6 months and in a few cases many years. Measures of glycemia were reported inconsistently. The most common class of medications utilized was SU (most commonly glyburide or glibenclamide), with some reporting the use of metformin (either as monotherapy or as an adjunctive agent), and a few cases utilized DPP4i (either alone or with SU). For the one patient who was not able to discontinue insulin, metformin was the only additional agent utilized87. The only adverse events reported were rare mild gastrointestinal symptoms and mild hypoglycemia, but these may have been underreported.
Does thiamine supplementation improve glycemia in SLC19A2-diabetes, also known as Thiamine-Responsive Megaloblastic Anemia Syndrome (TRMA)?
The literature search yielded 166 studies (Supplemental Fig. 4). After duplicate removal, abstract screening, and full-text review, 32 studies were included in the review with three larger case series and 29 case reports. Varying data on the effect of thiamine therapy on SLC19A2-diabetes were available for 95 patients (at the individual level, n = 72 patients; at group level n = 23 in one case series) (Table 8, Supplemental Table 10, and Supplementary Data 1). There were no randomized trials for therapeutic response in SLC19A2-diabetes, and the scope of the studies was mainly on the overall description of the phenotype.
Diabetes was diagnosed at the median age of 1.15 (range, 0.2–5.4, n = 44) years in case reports89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117 (Supplemental Table 10 and Supplementary Data 1) and between median ages of 1.4 and 2.2 (0.1–12, n = 51) years in the three case series118,119,120 (Table 8). Insulin was the most common initial therapy for diabetes (n = 89/95) with one patient also using SU (glimepiride), and 6 not receiving any antidiabetic therapy prior to thiamine therapy. Thiamine therapy was initiated after a median duration of diabetes of 4 months (0–17.9 years, n = 53/95 with available data), two additional patients were already on thiamine therapy at the time of diabetes onset.
The median thiamine treatment duration at the time of follow-up was 0.9 years (2 days–25 years, n = 39) in case reports, 4.7 years (2–17.5 years, n = 15) in one case series, and not available in the remaining patients (n = 41). The initial and maximum median thiamine doses were 100 (25–200) and 100 (25–600) mg/day, respectively (n = 41), adjusted according to the response to anemia.
The effect of thiamine treatment on glycemic control was inconsistently reported using different outcome measures. Regarding the 72 patients with individual data, 8% (n = 6) remained insulin-independent from diabetes onset until the median follow-up time of 1 year (3 days–3 years) after initiation of thiamine. Among those on insulin, the commencement of thiamine treatment was associated with: achievement of insulin-independency in 24% (n = 17), a decrease in daily insulin requirement in 38% (n = 27), improvement in glycemic control without specification in 4% (n = 3), no response defined as unchanged insulin requirement or glycemic control in 26% (n = 19). Three patients required insulin treatment again at puberty. Only 11 of all 95 patients had reached adulthood (aged >18 years) –all of these were on insulin therapy.
Glycemic control both prior to and after the initiation of thiamine treatment was reported for 29 of 95 (31%) patients with a median pre-HbA1c of 8.7% (5.9–21.0%) and median post-HbA1c 6.7% (5.2–9.1%)90,94,95,101,102,103,104,106,110,113,114,115,116,118. Notably, the reported pre-HbA1c was in most cases measured at diabetes onset, therefore a decrease in HbA1c does not alone reflect the thiamine effect on glycemic control, but could also reflect the effect of the standard treatment of diabetes. Insulin dose at both timepoints was available for 16 of 89 (18%) insulin-treated patients, with the median dose of 0.68 (0.42–1.8) IU/kg/day prior to thiamine treatment and 0.4 (0.0–0.8) IU/kg/day while on thiamine treatment.
The initial and maximum median thiamine doses were 100 (25–200) mg/day and 100 (25–600), respectively, mg/day (n = 41), adjusted according to the response to anemia.
Discussion
To the best of our knowledge, this is the first systematic review to look at the evidence for precision treatment of beta-cell monogenic diabetes outside of KATP neonatal diabetes, in which SU was previously shown to be an effective treatment with the success conditioned by differences in pharmacogenetics, age, pharmacokinetics, compliance, and maximal dose used5. Monogenic diabetes is an excellent candidate for precision medicine as the genetic etiology identified by genetic testing defines a subtype of diabetes with a specific pathophysiology enhancing the likelihood for a specific therapy to be most effective. For several forms of beta-cell monogenic diabetes, the underlying pathoetiology provides a rationale for precision treatment, but it is important to assess to what extent these rationales are supported by published evidence.
We sought to assess the evidence base for current treatment recommendations for several more common forms of beta-cell monogenic diabetes. Overall we found that there is limited, and mostly poor-quality evidence, mainly consisting of descriptive case reports and case series. There were limited randomized studies providing stronger evidence, some with considerable effect sizes, but limited by small numbers of participants and short durations resulting in inadequate power to detect or exclude differences. The majority of included cases are of European descent. Future efforts in this field should include racial and ethnic groups that have been underrepresented in the study of monogenic diabetes. The strongest evidence for a precision approach was, in order, HNF1A-diabetes, GCK-related hyperglycemia, relapse of 6q24-TND, and SLC19A2-diabetes. The evidence for insulin or non-insulin therapies in MD and HNF1B-diabetes was not clear and there was almost no evidence for HNF4A-diabetes treatment. Each subtype is discussed below.
GCK-related hyperglycemia
The aggregate of data suggests that glucose-lowering treatment should not be given in GCK-related hyperglycemia. HbA1c stays stable in target range without initiating or after cessation of medical treatment and there is no evidence to support treatment for lowering glycemia. However, diagnostic testing for GCK is often guided by recruiting individuals with mild hyperglycemia and this may introduce bias to the phenotype seen. Against this, in non-selected sequencing in the general population121 and type 2 diabetes122, a mild glycemic phenotype was seen in subjects with GCK variants.
There was no evidence to support dietary recommendations specific to GCK-related hyperglycemia. Further work is needed on whether a dietary approach needs to be any different from the general population.
Other important topics for assessment in GCK-related hyperglycemia in the future were identified. While GCK-related hyperglycemia occurring in isolation does not need pharmacologic treatment, it is important to develop clinical recommendations for diagnosing and treating type 2 diabetes co-occurring with GCK-related hyperglycemia. Additionally, the long-term cardiovascular effects of mild hyperglycemia in GCK-related hyperglycemia need to be established in an older population.
A limitation of this systematic review is that we did not address the evidence for a precision medicine approach in pregnancies affected by maternal beta-cell monogenic diabetes. This is an important area, particularly in GCK-related hyperglycemia, where treatment may be required for pregnancies in women with GCK-related hyperglycemia carrying non-affected fetuses123. Management guidelines exist but are debated. Additionally, the potential impact of cell-free fetal DNA to provide early genotype information to guide maternal insulin therapy will need to be studied both in terms of treatment impact and cost-effectiveness.
Recommendation: No medical treatment should be given in GCK-related hyperglycemia (grade C evidence). No recommendation can be given regarding dietary treatment in GCK-related hyperglycemia.
HNF1A-diabetes and HNF4A-diabetes
With the available data, we can only provide assessment of the evidence in HNF1A-diabetes although combined cohorts suggest results may be similar in HNF4A-diabetes. Clearly, more treatment studies are required in HNF4A-diabetes.
The strongest evidence in this systematic review for precision therapy was for the use of SU in HNF1A-diabetes. The key evidence came from a randomized cross-over study of SU and metformin therapy in HNF1A-diabetes and matched subjects with type 2 diabetes25 and was additionally supported by cohort and case studies demonstrating the initial efficacy of SU at diabetes diagnosis and the possibility of transitioning from insulin therapy after a genetic diagnosis is made, especially when close to diagnosis and when the HbA1c is well controlled23. While SU are a clear representation of diabetes precision medicine, they are non-efficacious in some individuals and their efficacy may not be durable in others, with diabetes duration and weight gain being two factors associated with reduced SU efficacy. Further studies are needed to elucidate factors that influence the response to SU within HNF1A-diabetes.
While there is support for the effectiveness of glinides in HNF1A-diabetes as an alternative to SU, particularly to address hypoglycemia2631,45, and for the newer diabetes medications as monotherapy (GLP1RA)27,30,43 or augmentative therapy (DPP4i)21,28,32,34,37,39, studies are of short duration and limited in number. There are no studies on drug-naïve newly-diagnosed patients with HNF1A- or HNF4A-diabetes comparing the different treatment options. This would be needed to conclusively suggest first-line (or second-line) treatment of hyperglycemia. The increased cardiovascular risk associated with HNF1A-diabetes provides another reason that carefully designed larger, long-term comparison studies of glycemic and cardiovascular outcomes of these newer classes of diabetes medications, which offer cardiovascular benefit, are needed124,125,126. On the other hand, it is not clear whether SGLT2i can be safely used in HNF1A-diabetes, which features insulin deficiency and already reduced expression of SGLT2 and glucosuria.
Recommendation: SU should be used as first-line therapy for HNF1A-diabetes (grade C evidence). Glinides can be used if frequent hypoglycemia is experienced with SU treatment (grade D evidence). GLP1RA is an option in the treatment of HNF1A-diabetes (grade C evidence). DPP4i are an option in the augmentative treatment of HNF1A-diabetes (grade C evidence). No recommendation can be given for HNF4-diabetes.
HNF1B-diabetes and Mitochondrial diabetes
There is no defined precision treatment approach or even clear first-line medication for MD or HNF1B-diabetes, with the evidence for specific treatment in HNF1B-diabetes and MD being of low quality with no trials and very few studies. The degree of insulin deficiency among individuals with HNF1B-diabetes and MD can range from mild hyperglycemia to absolute insulin deficiency. While insulin has been advocated as the choice of treatment for both HNF1B-diabetes and MD4,127, we found no systematic evidence favoring the use of insulin. There are no RCTs on HNF1B-diabetes or MD and even open treatment studies and cohort or case reports are few. Thus, the results of the systematic search remain descriptive, reflecting the choices of the treating physicians. In the included case reports and case series, which were mainly cross-sectional, most patients diagnosed with HNF1B-diabetes or MD seem to have commenced insulin treatment at some stage. Besides insulin deficiency and secondary failure of other medications, this could be affected by a diagnostic selection bias or a progressive kidney disease precluding many modes of treatment. Similarly, there is no evidence for using or avoiding any of the other diabetes medications. We note that metformin, SU or glinides, DPP4i, SGLT2i, and GLP1RA were used in the case reports and case series, especially close to diagnosis. There is a rationale for studying SGLT2i specifically in HNF1B-diabetes, as it might improve the renal outcome of the associated non-diabetes-related kidney disease. However, the marked insulin deficiency often seen in HNF1B-diabetes could markedly increase the risk of diabetic ketoacidosis. Further large systematic and controlled studies are required in both HNF1B-diabetes and MD.
Lactatemia and/or lactic acidosis are part of the syndrome caused by the MT-TL1 m.3243 A > G variant. Thus, avoidance of metformin has been recommended in MD as it impairs mitochondrial function and may further increase the risk of lactic acidosis4. However, the evidence against its clinical use was low and limited to three case reports with adverse effects, including only one patient with possible lactic acidosis68 and another with an increase of lactate levels possibly associated with metformin use66. MD needs additional preclinical and clinical studies to define the risk of metformin and statin use to guide therapy approaches. Patients with MT-TL1 m.3243 A > G variant and associated neurological disease might be on coenzyme Q10 or other dietary supplements, like arginine, in an attempt to support their mitochondrial function but most studies have not examined the effects of the supplements on glucose control.
Recommendation: There is insufficient data to recommend or refute the preferential use of insulin or any other medication in HNF1B-diabetes or mitochondrial diabetes. However, in case of patients not on insulin therapy, the potential deterioration of insulin secretion should be evaluated if glucose control deteriorates.
6q24 transient neonatal diabetes
The evidence guiding the use of non-insulin therapies for 6q24-related diabetes is of low quality and more information is needed to make firm conclusions. In the initial neonatal phase of 6q24-TND, SU use was not always successful in improving diabetes outcomes or allowing for cessation of insulin. The evidence for the efficacy of non-insulin therapies was stronger for the relapse phase of diabetes later in life, where most cases appeared to benefit from a variety of non-insulin therapies, with most not requiring insulin. There is a pressing need to improve the evidence base for the management of 6q24-TND in both the neonatal and relapse phases and for long-term outcome data.
Recommendation: There is insufficient data to recommend or refute the preferential use of non-insulin therapies for the treatment of the neonatal or relapse phase of 6q24-TND. However, non-insulin therapy seems beneficial in patients where diabetes recurs, but we recommend close follow-up to ensure treatment intensification if patients do not reach or remain stable at their glycemic target (grade D evidence).
SLC19A2-diabetes (TRMA)
The evidence for the use of thiamine improving glycemic control in SLC19A2-diabetes is of low quality because it is limited to case reports and series that provide inconsistent data on outcomes. However, there is a trend across the studies reporting thiamine administration resulting either in a reduction in insulin dose or allowing for discontinuation of insulin therapy, with stable or improved glycemic control, in a majority of the affected individuals. Quantifying an improvement in endogenous insulin secretion in patients treated with insulin is difficult but a common assessment is the daily insulin dose per kg in patients who maintain a similar level of glycemia. Further trials assessing this in the short and long term (especially beyond puberty) are required to firmly document the effect of thiamine on glycemic control. Although most cases not controlled by thiamine treatment alone were treated with insulin, further study could reveal the utility of treatment with other diabetes medications. This special situation and the rarity of the disease make clinical trials difficult to conduct.
Recommendation: Whereas thiamine treatment is essential for all patients with anemia in SLC19A2-diabetes,
the evidence supporting a specific and sustained effect of thiamine on glycemic control in SLC19A2-diabetes is
weak. We recommend thiamine be started as soon as a diagnosis of SLC19A2-diabetes is made. Additionally, we recommend close follow-up of patients with SLC19A2-diabetes after the initiation of thiamine treatment to adjust the diabetes treatment if required (grade D evidence).
Concluding remarks
Beta-cell monogenic diabetes has been considered the strongest example of a precision medicine approach to diabetes treatment. However, this systematic review demonstrates that there is limited trial evidence to support precision treatment practices and many recommendations rely on case series and case reports. Importantly the strongest available evidence supports the existing practice consensus guidelines4 regarding GCK-related hyperglycemia and HNF1A-diabetes.
Randomized trials with long-term follow-up offer the strongest evidence but the low numbers of cases of each individual subtype make these very difficult to perform. We urge the medical community to publish the follow-up of the complete case series in all subtypes of beta-cell monogenic diabetes to establish the response to therapy, fill the identified gaps in precision treatment, and explore the precision treatment approaches to prevent complications. Future studies of precision treatment in beta-cell monogenic diabetes must account for the perspectives of people with monogenic diabetes, including the acceptability of the different medications in terms of route, patient-facing costs, and potential adverse effects. Cost-effectiveness analyses of the newer diabetes medications also need to be carried out. Finally, there must be purposeful efforts toward equity in achieving optimal diabetes outcomes for individuals living with diabetes. This includes screening approaches and “high-throughput pipelines that can be disseminated in every country worldwide”128. There must be special attention to groups of people and countries underrepresented in studies of monogenic diabetes to inform on potential differences in phenotype, treatment response, and precision medicine approaches.
Data availability
All data used in this systematic review are publically available in the published scientific literature. Search strategies used for each monogenic diabetes subtype are detailed in Supplemental Tables 1–5. PRISMA figures were generated in Covidence. Excel files of the included studies corresponding to each PRISMA diagram are available as supplemental data (Supplementary Data 2).
References
Semple, R. K., Patel, K. A., Auh, S., ADA/EASD PMDI & Brown, R. J. Genotype-stratified treatment for monogenic insulin resistance: a systematic review. Commun. Med. 3, 134 (2023).
Riddle, M. C. et al. Monogenic diabetes: from genetic insights to population-based precision in care. Reflections from a Diabetes Care editors’ expert forum. Diab. Care 43, 3117–3128 (2020).
Chung, W. K. et al. Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diab. Care 43, 1617–1635 (2020).
Greeley, S. A. W. et al. ISPAD Clinical Practice Consensus Guidelines 2022: the diagnosis and management of monogenic diabetes in children and adolescents. Pediatr. Diab. 23, 1188–1211 (2022).
Garcin, L. et al. Neonatal diabetes due to potassium channel mutation: Response to sulfonylurea according to the genotype. Pediatr. Diab. 21, 932–941 (2020).
de Gouveia Buff Passone, C. et al. Sulfonylurea for improving neurological features in neonatal diabetes: A systematic review and meta-analyses. Pediatr. Diab. 23, 675–692 (2022).
Tobias, D. K. et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. 29, 2438–2457 (2023).
Nolan, J. J. et al. ADA/EASD precision medicine in diabetes initiative: an international perspective and future vision for precision medicine in diabetes. Diab. Care 45, 261–266 (2022).
Murphy, R. et al. The use of precision diagnostics for monogenic diabetes: a systematic review and expert opinion. Commun. Med. 3, 136 (2023).
Naylor, R. N. et al. Systematic review of monogenic diabetes prognostics. medRxiv. https://doi.org/10.1101/2023.05.19.23290220 (2023).
Page, M. J. et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372, n160 (2021).
Sherifali, D. et al. Methods. Can. J. Diab. 42, S6–S9 (2018).
Ebrahim, M. S., Lawson, M. L. & Geraghty, M. T. A novel heterozygous mutation in the glucokinase gene conferring exercise-induced symptomatic hyperglycaemia responsive to sulfonylurea. Diab. Metab. 40, 310–313 (2014).
Klupa, T. et al. The influence of dietary carbohydrate content on glycaemia in patients with glucokinase maturity-onset diabetes of the young. J. Int. Med. Res. 39, 2296–2301 (2011).
Almeida, C. et al. A novel genetic mutation in a Portuguese family with GCK-MODY. J. Pediatr. Endocrinol. Metab. 27, 129–133 (2014).
Carmody, D., Lindauer, K. L. & Naylor, R. N. Adolescent non-adherence reveals a genetic cause for diabetes. Diabet. Med. 32, e20–e23 (2015).
DellaManna, T. et al. Clinical follow-up of two Brazilian subjects with glucokinase-MODY (MODY2) with description of a novel mutation. Arq. Bras. Endocrinol. Metab. 56, 490–495 (2012).
Loomba-Albrecht, L. A., Jame, M. & Bremer, A. A. A novel glucokinase gene mutation and its effect on glycemic/C-peptide fluctuations in a patient with maturity-onset diabetes of the young type 2. Diab. Res. Clin. Pr. 87, e23–e25 (2010).
Papadimitriou, D. T., Willems, P. J., Bothou, C., Karpathios, T. & Papadimitriou, A. A novel heterozygous mutation in the glucokinase gene is responsible for an early-onset mild form of maturity-onset diabetes of the young, type 2. Diab. Metab. 41, 342–343 (2015).
Talapatra, I., Kalavalapalli, S., Robinson, J., Ellard, S. & Tymms, D. Successful discontinuation of insulin treatment after gestational diabetes is shown to be a case of MODY due to a glucokinase mutation. Open Med. 3, 225–228 (2008).
Carlsson, A. et al. Absence of islet autoantibodies and modestly raised glucose values at diabetes diagnosis should lead to testing for MODY: lessons from a 5-year Pediatric Swedish National Cohort Study. Diab. Care 43, 82–89 (2020).
Delvecchio, M. et al. Monogenic diabetes accounts for 6.3% of cases referred to 15 Italian Pediatric Diabetes Centers during 2007 to 2012. J. Clin. Endocrinol. Metab. 102, 1826–1834 (2017).
Shepherd, M. H. et al. A UK nationwide prospective study of treatment change in MODY: genetic subtype and clinical characteristics predict optimal glycaemic control after discontinuing insulin and metformin. Diabetologia 61, 2520–2527 (2018).
Stride, A. et al. Cross-sectional and longitudinal studies suggest pharmacological treatment used in patients with glucokinase mutations does not alter glycaemia. Diabetologia 57, 54–56 (2014).
Pearson, E. R. et al. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet. 362, 1275–1281 (2003).
Tuomi, T., Honkanen, E. H., Isomaa, B., Sarelin, L. & Groop, L. C. Improved prandial glucose control with lower risk of hypoglycemia with nateglinide than with glibenclamide in patients with maturity-onset diabetes of the young type 3. Diab. Care 29, 189–194 (2006).
Østoft, S. H. et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diab. Care 37, 1797–1805 (2014).
Christensen, A. S. et al. Efficacy and safety of glimepiride with or without linagliptin treatment in patients with HNF1A diabetes (maturity-onset diabetes of the young type 3): a randomized, double-blinded, placebo-controlled, crossover trial (GLIMLINA). Diab. Care 43, 2025–2033 (2020).
Shepherd, M. et al. No deterioration in glycemic control in HNF-1alpha maturity-onset diabetes of the young following transfer from long-term insulin to sulphonylureas. Diab. Care 26, 3191–3192 (2003).
Ahluwalia, R., Perkins, K., Ewins, D. & Goenka, N. Exenatide-a potential role in treatment of HNF1-alpha MODY in obese patients? Diabet. Med 26, 834–835 (2009).
Becker, M., Galler, A. & Raile, K. Meglitinide analogues in adolescent patients with HNF1A-MODY (MODY 3). Pediatrics 133, e775–e779 (2014).
Dashora, U., Golton, R., Combes, A. & Kumar, S. Diabetes in the young but not needing insulin–what type is it? BMJ Case Rep. 10, bcr1120115127 (2012).
Fang, C. et al. A novel nonsense mutation of the HNF1α in maturity-onset diabetes of the young type 3 in Asian population. Diab. Res Clin. Pr. 109, e5–e7 (2015).
Globa, E. et al. MODY in Ukraine: genes, clinical phenotypes and treatment. J. Pediatr. Endocrinol. Metab. 30, 1095–1103 (2017).
Habeb, A. M., George, E. T., Mathew, V. & Hattersley, A. L. Response to oral gliclazide in a pre-pubertal child with hepatic nuclear factor-1 alpha maturity onset diabetes of the young. Ann. Saudi Med. 31, 190–193 (2011).
Jesić, M. D. et al. A case of new mutation in maturity-onset diabetes of the young type 3 (MODY 3) responsive to a low dose of sulphonylurea. Diab. Res. Clin. Pr. 81, e1–e3 (2008).
Katra, B. et al. Dipeptidyl peptidase-IV inhibitors are efficient adjunct therapy in HNF1A maturity-onset diabetes of the young patients–report of two cases. Diab. Technol. Ther. 12, 313–316 (2010).
Khelifa, S. B. et al. Successful switch from insulin to oral sulfonylurea therapy in HNF1A-MODY Tunisian patient with the P291fsinsC mutation. Diab. Res. Clin. Pr. 115, 133–136 (2016).
Lumb, A. N. & Gallen, I. W. Treatment of HNF1-alpha MODY with the DPP-4 inhibitor Sitagliptin(1). Diabet. Med. 26, 189–190 (2009).
Oliveira, R. V., Bernardo, T., Martins, S. & Sequeira, A. Monogenic diabetes: a new pathogenic variant of HNF1A gene. BMJ Case Rep. 14, e231837 (2021).
Pearson, E. R., Liddell, W. G., Shepherd, M., Corrall, R. J. & Hattersley, A. T. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet. Med. 17, 543–545 (2000).
Shepherd, M. Genetic testing clarifies diagnosis and treatment in a family with both HNF1A and type 1 diabetes. Practical Diab. Int. 26, 269–73i (2009).
Urakami, T. et al. Three years of liraglutide treatment offers continuously optimal glycemic control in a pediatric patient with maturity-onset diabetes of the young type 3. J. Pediatr. Endocrinol. Metab. 28, 327–331 (2015).
Kyithar, M. P. et al. Identification of HNF1A-MODY and HNF4A-MODY in Irish families: phenotypic characteristics and therapeutic implications. Diab. Metab. 37, 512–519 (2011).
Raile, K. et al. Treatment of young patients with HNF1A mutations (HNF1A-MODY). Diabet. Med. 32, 526–530 (2015).
Colclough, K., Ellard, S., Hattersley, A. & Patel, K. Syndromic monogenic diabetes genes should be tested in patients with a clinical suspicion of maturity-onset diabetes of the young. Diabetes 71, 530–537 (2022).
Dubois-Laforgue, D. et al. Diabetes, associated clinical spectrum, long-term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) Molecular Defects. Diab. Care 40, 1436–1443 (2017).
Warncke, K. et al. Frequency and characteristics of MODY 1 (HNF4A Mutation) and MODY 5 (HNF1B mutation): analysis from the DPV database. J. Clin. Endocrinol. Metab. 104, 845–855 (2019).
Ng, N. et al. Genotype-phenotype correlations and response to glucose lowering therapy in subjects with HNF1β associated diabetes. Acta Diabetol. 59, 83–93 (2022).
Kettunen, J. L. et al. Biliary anomalies in patients with HNF1B-diabetes. J. Clin. Endocrinol. Metab. 102, 2075–2082 (2017).
Roehlen, N. et al. 17q12 deletion syndrome as a rare cause for diabetes mellitus type MODY5. J. Clin. Endocrinol. Metab. 103, 3601–3610 (2018).
Terakawa, A. et al. Maturity-onset diabetes of the young type 5 treated with the glucagon-like peptide-1 receptor agonist: a case report. Medicine 99, e21939 (2020).
Carrillo, E., Lomas, A., Pinés, P. J. & Lamas, C. Long-lasting response to oral therapy in a young male with monogenic diabetes as part of HNF1B-related disease. Endocrinol. Diab. Metab. Case Rep. 17, 0052 (2017).
Tao, T., Yang, Y. & Hu, Z. A novel HNF1B mutation p.R177Q in autosomal dominant tubulointerstitial kidney disease and maturity-onset diabetes of the young type 5: A pedigree-based case report. Medicine 99, e21438 (2020).
Aydın, C. et al. A case of familial recurrent 17q12 microdeletion syndrome presenting with severe diabetic ketoacidosis. Turk. J. Pediatr. 64, 558–565 (2022).
Ren, B. et al. De novo mutation in HNF-1β gene as a cause for maturity-onset diabetes of the young type 5 with sustained hypomagnesemia. Int J. Diab. Dev. Countries 41, 354–357 (2021).
Thirumalai, A., Holing, E., Brown, Z. & Gilliam, L. K. A case of hepatocyte nuclear factor-1β (TCF2) maturity onset diabetes of the young misdiagnosed as type 1 diabetes and treated unnecessarily with insulin. J. Diab. 5, 462–464 (2013).
Mateus, J. C., Rivera, C., O’Meara, M., Valenzuela, A. & Lizcano, F. Maturity-onset diabetes of the young type 5 a MULTISYSTEMIC disease: a CASE report of a novel mutation in the HNF1B gene and literature review. Clin. Diab. Endocrinol. 6, 16 (2020).
Guillausseau, P. J. et al. Heterogeneity of diabetes phenotype in patients with 3243 bp mutation of mitochondrial DNA (Maternally Inherited Diabetes and Deafness or MIDD). Diab. Metab. 30, 181–186 (2004).
Esterhuizen, K. et al. One mutation, three phenotypes: novel metabolic insights on MELAS, MIDD and myopathy caused by the m.3243A > G mutation. Metabolomics 17, 10 (2021).
Suzuki, S. et al. The effects of coenzyme Q10 treatment on maternally inherited diabetes mellitus and deafness, and mitochondrial DNA 3243 (A to G) mutation. Diabetologia 41, 584–588 (1998).
Lebbar, M., Timsit, J., Luyton, C. & Marchand, L. Glucagon-like peptide-1 receptor agonists (GLP1-RA) in the treatment of mitochondrial diabetes. Acta Diabetol. 58, 1281–1282 (2021).
Cosentino, C., Contento, M., Paganini, M., Mannucci, E. & Cresci, B. Therapeutic options in a patient with MELAS and diabetes mellitus: follow-up after 6 months of treatment. Acta Diabetol. 56, 1231–1233 (2019).
Keidai, Y. et al. Switched” metabolic acidosis in mitochondrial diabetes mellitus. J. Diab. Investig. 10, 1116–1117 (2019).
Ninomiya, H. et al. Treatment of mitochondrial diabetes with a peroxisome proliferator-activated receptor (PPAR)-gamma agonist. Intern. Med. 55, 1143–1147 (2016).
Lin, W. H., Yang, I. H., Cheng, H. E. & Lin, H. F. Case report: late-onset mitochondrial disease uncovered by metformin use in a patient with acute verbal auditory agnosia. Front. Neurol. 13, 863047 (2022).
Yeung, R. O. et al. Management of mitochondrial diabetes in the era of novel therapies. J. Diab. Complicat. 35, 107584 (2021).
Kim, N. H., Siddiqui, M. & Vogel, J. MELAS syndrome and MIDD unmasked by metformin use: a case report. Ann. Intern. Med. 174, 124–125 (2021).
Tong, H. F. et al. Neurological manifestations in m.3243A>G-related disease triggered by metformin. J. Diab. Complicat. 36, 108111 (2022).
Gore, R. H. et al. Duplication 6q24: more than just diabetes. J. Endocr. Soc. 4, bvaa027 (2020).
Neumann, U., Bührer, C., Blankenstein, O., Kühnen, P. & Raile, K. Primary sulphonylurea therapy in a newborn with transient neonatal diabetes attributable to a paternal uniparental disomy 6q24 (UPD6). Diab. Obes. Metab. 20, 474–475 (2018).
Cao, B. Y., Gong, C. X., Wu, D. & Li, X. Q. Permanent neonatal diabetes caused by abnormalities in chromosome 6q24. Diabet. Med. 34, 1800–1804 (2017).
Zhang, M. et al. Sulfonylurea in the treatment of neonatal diabetes mellitus children with heterogeneous genetic backgrounds. J. Pediatr. Endocrinol. Metab. 28, 877–884 (2015).
Yao, B. et al. 6q24 transient neonatal diabetes mellitus: the first case report from China. Chin. Med. J. 127, 3680 (2014).
Hewes, H. A., Dudley, N. C. & Adelgais, K. M. A case of transient neonatal diabetes mellitus. Pediatr. Emerg. Care 26, 930–931 (2010).
Senguttuvan, R., Wheeler, M., Elrokshi, S. & Chin, C. Transient neonatal diabetes mellitus (TNDM) associated with triplication of chromosome 6q23.3-24.3 [Abstract]. In: Endocrine Society’s 97th Annual Meeting and Expo, March 5–8, 2015—San Diego Endocrine Reviews, 36, Issue Supplement, 1, i1–i1599. https://doi.org/10.1093/edrv/36.supp.1 (2015).
Carmody, D. et al. Sulfonylurea treatment before genetic testing in neonatal diabetes: pros and cons. J. Clin. Endocrinol. Metab. 99, E2709–E2714 (2014).
Li, X. et al. Early transition from insulin to sulfonylureas in neonatal diabetes and follow-up: experience from China. Pediatr. Diab. 19, 251–258 (2018).
Garcin, L. et al. Successful off-label sulfonylurea treatment of neonatal diabetes mellitus due to chromosome 6 abnormalities. Pediatr. Diab. 19, 663–669 (2018).
Schimmel, U. Long-standing sulfonylurea therapy after pubertal relapse of neonatal diabetes in a case of uniparental paternal isodisomy of chromosome 6. Diab. Care 32, e9 (2009).
Kontbay, T., Atar, M. & Demirbilek, H. Long-term follow-up of transient neonatal diabetes mellitus due to a novel homozygous c.7734C>T (p.R228C) mutation in ZFP57gene: relapse at prepubertal age. J. Pediatr. Endocrinol. Metab. 35, 695–698 (2022).
Sato, Y. et al. Longitudinal glycaemic profiles during remission in 6q24-related transient neonatal diabetes mellitus. Horm. Res. Paediatr. 94, 229–234 (2021).
Fu, J. L., Wan, T. & Xiao, X. H. Relapsed 6q24-related transient neonatal diabetes mellitus successfully treated with sulfonylurea. Chin. Med J. 132, 846–848 (2019).
Yorifuji, T. et al. Relapsing 6q24-related transient neonatal diabetes mellitus successfully treated with a dipeptidyl peptidase-4 inhibitor: a case report. Pediatr. Diab. 15, 606–610 (2014).
von dem Berge, T. & Kordonouri, O. Successful sulfonylurea treatment of transient neonatal diabetes. Diabetologe 17, 672–676 (2021).
Uchida, N. et al. Relapsing 6q24-related transient neonatal diabetes mellitus with insulin resistance: A case report. Clin. Pediatr. Endocrinol. 29, 179–182 (2020).
Søvik, O. et al. Familial occurrence of neonatal diabetes with duplications in chromosome 6q24: treatment with sulfonylurea and 40-yr follow-up. Pediatr. Diab. 13, 155–162 (2012).
Carmody, D. et al. Role of noninsulin therapies alone or in combination in chromosome 6q24-related transient neonatal diabetes: sulfonylurea improves but does not always normalize insulin secretion. Diab. Care 38, e86–e87 (2015).
Kang, P. et al. Case report: genetic and clinical features of maternal uniparental isodisomy-induced thiamine-responsive megaloblastic anemia syndrome. Front. Pediatr. 9, 630329 (2021).
Zhang, S. et al. Identification of novel compound heterozygous variants in SLC19A2 and the genotype-phenotype associations in thiamine-responsive megaloblastic anemia. Clin. Chim. Acta. 516, 157–168 (2021).
Spehar et al. Importance of immediate thiamine therapy in children with suspected thiamine-responsive megaloblastic anemia-report on two patients carrying a novel SLC19A2 gene mutation. J. Pediatr. Genet. 11, 236–239 (2020).
Odaman-Al, I. et al. A novel mutation in the SLC19A2 gene in a Turkish male with thiamine-responsive megaloblastic anemia syndrome. Turk. J. Pediatr. 61, 257–260 (2019).
Li, X. et al. TRMA syndrome with a severe phenotype, cerebral infarction, and novel compound heterozygous SLC19A2 mutation: a case report. BMC Pediatr. 19, 233 (2019).
Katipoğlu, N. et al. Infantile-onset thiamine responsive megaloblastic anemia syndrome with SLC19A2 mutation: a case report. Arch. Argent. Pediatr. 115, e153–e156 (2017).
Potter, K., Wu, J., Lauzon, J. & Ho, J. Beta cell function and clinical course in three siblings with thiamine-responsive megaloblastic anemia (TRMA) treated with thiamine supplementation. J. Pediatr. Endocrinol. Metab. 30, 241–246 (2017).
Pomahačová, R. et al. First 2 cases with thiamine-responsive megaloblastic anemia in the Czech Republic, a rare form of monogenic diabetes mellitus: a novel mutation in the thiamine transporter SLC19A2 gene-intron 1 mutation c.204+2T>G. Pediatr. Diab. 18, 844–847 (2017).
Taberner, P. et al. Clinical and genetic features of Argentinian children with diabetes-onset before 12months of age: Successful transfer from insulin to oral sulfonylurea. Diab. Res Clin. Pr. 117, 104–110 (2016).
Tahir, S. et al. A novel homozygous SLC19A2 mutation in a Portuguese patient with diabetes mellitus and thiamine-responsive megaloblastic anaemia. Int J. Pediatr. Endocrinol. 2015, 6 (2015).
Mikstiene, V. et al. Thiamine responsive megaloblastic anemia syndrome: a novel homozygous SLC19A2 gene mutation identified. Am. J. Med. Genet. A 167, 1605–1609 (2015).
Beshlawi, I. et al. Thiamine responsive megaloblastic anemia: the puzzling phenotype. Pediatr. Blood Cancer 61, 528–531 (2014).
Ghaemi, N., Ghahraman, M., Abbaszadegan, M. R., Baradaran-Heravi, A. & Vakili, R. Novel mutation in the SLC19A2 gene in an Iranian family with thiamine-responsive megaloblastic anemia: a series of three cases. J. Clin. Res. Pediatr. Endocrinol. 5, 199–201 (2013).
Mozzillo, E. et al. Thiamine responsive megaloblastic anemia: a novel SLC19A2 compound heterozygous mutation in two siblings. Pediatr. Diab. 14, 384–387 (2013).
Pichler, H. Thiamine-responsive megaloblastic anemia (TRMA) in an Austrian boy with compound heterozygous SLC19A2 mutations. Eur. J. Pediatr. 171, 1711–1715 (2012).
Yilmaz Agladioglu, S., Aycan, Z., Bas, V. N., Peltek Kendirci, H. N. & Onder, A. Thiamine-responsive megaloblastic anemia syndrome: a novel mutation. Genet Couns. 23, 149–156 (2012).
Akın, L., Kurtoğlu, S., Kendirci, M., Akın, M. A. & Karakükçü, M. Does early treatment prevent deafness in thiamine-responsive megaloblastic anaemia syndrome? J. Clin. Res. Pediatr. Endocrinol. 3, 36–39 (2011).
Aycan, Z. et al. Thiamine-responsive megaloblastic anemia syndrome with atrial standstill: a case report. J. Pediatr. Hematol. Oncol. 33, 144–147 (2011).
Bay, A. et al. Thiamine-responsive megaloblastic anemia syndrome. Int. J. Hematol. 92, 524–526 (2010).
Bouyahia, O., Ouderni, M., Ben Mansour, F., Matoussi, N. & Khaldi, F. Diabetic acido-ketosis revealing thiamine-responsive megaloblastic anemia. Ann. Endocrinol. 70, 477–479 (2009).
Borgna-Pignatti, C., Azzalli, M. & Pedretti, S. Thiamine-responsive megaloblastic anemia syndrome: long term follow-up. J. Pediatr. 155, 295–297 (2009).
Yeşilkaya, E. et al. A novel mutation in the SLC19A2 gene in a Turkish female with thiamine-responsive megaloblastic anemia syndrome. J. Trop. Pediatr. 55, 265–267 (2009).
Kurtoglu, S., Hatipoglu, N., Keskin, M., Kendirci, M. & Akcakus, M. Thiamine withdrawal can lead to diabetic ketoacidosis in thiamine responsive megaloblastic anemia: report of two siblings. J. Pediatr. Endocrinol. Metab. 21, 393–397 (2008).
Olsen, B. S., Hahnemann, J. M., Schwartz, M. & Østergaard, E. Thiamine-responsive megaloblastic anaemia: a cause of syndromic diabetes in childhood. Pediatr. Diab. 8, 239–241 (2007).
Alzahrani, A. S., Baitei, E., Zou, M. & Shi, Y. Thiamine transporter mutation: an example of monogenic diabetes mellitus. Eur. J. Endocrinol. 155, 787–792 (2006).
Lagarde, W. H., Underwood, L. E., Moats-Staats, B. M. & Calikoglu, A. S. Novel mutation in the SLC19A2 gene in an African-American female with thiamine-responsive megaloblastic anemia syndrome. Am. J. Med. Genet. A 125A, 299–305 (2004).
Ozdemir, M. A., Akcakus, M., Kurtoglu, S., Gunes, T. & Torun, Y. A. TRMA syndrome (thiamine-responsive megaloblastic anemia): a case report and review of the literature. Pediatr. Diab. 3, 205–209 (2002).
Gritli, S. et al. A novel mutation in the SLC19A2 gene in a Tunisian family with thiamine-responsive megaloblastic anaemia, diabetes and deafness syndrome. Br. J. Haematol. 113, 508–513 (2001).
Scharfe, C. et al. A novel mutation in the thiamine responsive megaloblastic anaemia gene SLC19A2 in a patient with deficiency of respiratory chain complex I. J. Med. Genet. 37, 669–673 (2000).
Habeb, A. M. et al. Pharmacogenomics in diabetes: outcomes of thiamine therapy in TRMA syndrome. Diabetologia 61, 1027–1036 (2018).
Ricketts, C. J. et al. Thiamine-responsive megaloblastic anaemia syndrome: long-term follow-up and mutation analysis of seven families. Acta Paediatr. 95, 99–104 (2006).
Warncke, K. et al. Thiamine-responsive megaloblastic anemia-related diabetes: long-term clinical outcomes in 23 pediatric patients from the DPV and SWEET registries. Can. J. Diab. 45, 539–545 (2021).
Mirshahi, U. L. et al. Reduced penetrance of MODY-associated HNF1A/HNF4A variants but not GCK variants in clinically unselected cohorts. Am. J. Hum. Genet. 109, 2018–2028 (2022).
Gjesing, A. P. et al. 14-fold increased prevalence of rare glucokinase gene variant carriers in unselected Danish patients with newly diagnosed type 2 diabetes. Diab. Res. Clin. Pr. 194, 110159 (2022).
Chakera, A. et al. Antenatal diagnosis of fetal genotype determines if maternal hyperglycemia due to a glucokinase mutation requires treatment. Diab. Care 35, 1832–1834 (2012).
Steele, A. M. et al. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet. Med. 27, 157–161 (2010).
Razavi, M., Wei, Y. Y., Rao, X. Q. & Zhong, J. X. DPP-4 inhibitors and GLP-1RAs: cardiovascular safety and benefits. Mil. Med. Res. 9, 45 (2022).
Wu, H. X. et al. Cardio-cerebrovascular outcomes in MODY, type 1 diabetes, and type 2 diabetes: a prospective cohort study. J. Clin. Endocrinol. Metab. 108, 2970–2980 (2023).
Hattersley, A. T. & Patel, K. A. Precision diabetes: learning from monogenic diabetes. Diabetologia 60, 769–777 (2017).
Bonnefond, A. et al. Monogenic diabetes. Nat. Rev. Dis. Prim. 9, 12 (2023).
Acknowledgements
We acknowledge the contribution of Dr. Rebecca Brown and Dr. Robert Semple in the working group of the Treatment of Monogenic Diabetes of the ADA/EASD Precision Diabetes Medicine Initiative. R.N.N is supported by the following grants: ADA 7-22-ICTSPM-17; R01DK104942; U54DK118612. KAP is supported by Wellcome Trust (219606/Z/19/Z); M.P. is supported by ANR 22-CE17-0025 Neurogli; S.A.W.G is supported by NIH NIDDK R01DK104942 and U54DK118612; T.T. is supported by the Helsinki University Hospital, the Folkhalsan Research Foundation as well as The Academy of Finland (grants no. 336822, 312072 and 336826) and University of Helsinki for the Centre of Excellence of Complex Disease Genetics. The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidence license was funded by Lund University (Sweden) for which technical support was provided by Maria Björklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmö, Sweden), the University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL).
Author information
Authors and Affiliations
Consortia
Contributions
Review design: R.N.N., K.A.P., J.K., J.S., S.A.W.G., T.V., A.T.H., T.T.; Systematic review implementation: R.N.N., K.A.P., J.K., J.M.E.M., J.S., S.A.W.G., T.V., T.T.; Data extraction manuscripts: R.N.N., K.A.P., J.K., J.M.E.M., S.A.W.G.; Manuscript writing: R.N.N., K.A.P., J.K., J.M.E.M., J.S., J.B., M.P., T.V., S.A.W.G., A.T.H., T.T.; Project management: T.V., S.A.W.G., A.T.H., T.T.
Corresponding author
Ethics declarations
Competing interest
J.K. has received lecture fees from NovoNordisk, international conference costs covered by Medtronic, AstraZeneca, and NovoNordisk; M.P. has been the scientific advisor for the AMGLIDIA (glibenclamide suspension) development; TV has served on scientific advisory panels, been part of speaker’s bureaus, and served as a consultant to, and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, GSK, Mundipharma, MSD/Merck, Novo Nordisk, Sanofi, and Sun Pharmaceuticals. All other authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Philippe Froguel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naylor, R.N., Patel, K.A., Kettunen, J.L.T. et al. Precision treatment of beta-cell monogenic diabetes: a systematic review. Commun Med 4, 145 (2024). https://doi.org/10.1038/s43856-024-00556-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00556-1
This article is cited by
-
Polygenic determinants of monogenic diabetes
Nature Metabolism (2025)
-
Genetic and clinical characteristics of children with mody: insights into novel HNF4A variants and genotype–phenotype correlation
Irish Journal of Medical Science (1971 -) (2025)