Abstract
Background
Growth is the holy grail of tissue implants in pediatrics. No vascular graft currently in use for surgical repairs of congenital heart defects has somatic growth capacity.
Methods
Biologically-engineered grafts (6 mm) grown from donor ovine fibroblasts in a sacrificial fibrin gel were implanted into the left pulmonary branch of 3-month old lambs for 3, 6, and 18 months. A control group of Propaten® PTFE grafts was implanted for 6 months.
Results
The engineered grafts exhibit extensive site-appropriate recellularization after only 3 months and near-normal increase of diameter from the preimplant value of 6 mm to 12.9 mm and also a doubling of length from 6.0 mm to 13.0 mm at 6 months (n = 3) associated with apparent somatic graft growth (collagen content increase of 265% over 18-month, n = 2), along with excellent hemodynamics and no calcification, in contrast to the Propaten® grafts. The left-right flow distribution is nearly 50–50 for the engineered grafts at 6 months (n = 3) compared to about 20–80 for the Propaten® grafts (n = 3), which have less than one-half the diameter, a 6-fold higher pressure gradient, and stunted vascular development downstream of the graft. The engineered grafts exhibit a stable diameter over months 12–18 when the lambs become adult sheep (n = 2).
Conclusions
This study supports the use of these regenerative grafts with somatic growth capacity for clinical trial in patients born with a unilateral absent pulmonary artery branch, and it shows their potential for improving development of the downstream pulmonary vasculature.
Plain Language Summary
Blood vessel implants that are currently used to repair heart defects at birth do not grow with the child. This means that children need to have multiple open heart surgeries to replace implants with larger implants as they grow. We grew implants from a donor sheep’s skin cells, and then completely removed the cells from the graft. We then implanted the grafts in 3-month old lambs. The lambs’ cells repopulated the implants and the implants increased in size as the lambs grew. Further experiments are required first, but our preliminary findings suggest that using a similar implant in children could improve the quality of life of children with heart defects by avoiding the need for them to have multiple surgeries to replace implants as the child grows.
Similar content being viewed by others
Introduction
Growth is the holy grail of tissue implants in pediatrics. In the absence of implant growth, the patient must undergo consecutive surgeries to upsize the implant to match their somatic growth. In the case of implants for congenital heart disease, this growth limitation necessitates repeated open heart surgeries and/or interventional catheterization procedures that represent repeated trauma to the patient, which can have adverse psychological and physiological consequences. These are compelling factors for developing implants with growth capacity even disregarding the survival risks of repeated sternotomies1,2. Beyond reduction of patient morbidity and mortality risk of multiple open heart surgeries, grafts that grow would present a substantial cost savings to the healthcare system.
We have developed a novel biologically-engineered tube of cell-produced collagenous matrix that has demonstrated growth potential for repair of congenital cardiovascular defects. We have shown it becomes a living artery-like vessel populated by the recipient’s cells and that it shows comparable increase in size to the adjacent native artery when implanted into the main pulmonary artery (PA) of a lamb as it grows to adult size over 10 months3. Moreover, there was no evidence of macro-calcification or overt systemic immune response. This study was the first report of somatic growth of an acellular material. A tissue tube is grown from allogeneic donor dermal fibroblasts entrapped in a sacrificial fibrin hydrogel tube that is then decellularized using sequential detergent treatments and is storable in cold sterile saline solution. The resulting cell-produced matrix tube is thus non-immunogenic and “off-the-shelf.” It possesses physiological strength, compliance, and circumferential fiber alignment3.
While that study demonstrated growth potential of this novel tube graft, placement of a conduit in the main PA (MPA) is not directly related to repair of a congenital heart defect. However, discontinuity of the left or right PA branches is a complicating feature of several congenital heart malformations, most commonly associated with tetralogy of Fallot, but also occurring as an isolated vascular malformation4: In patients with discontinuous pulmonary arteries, the left or right branch PA is in continuity with the MPA while the proximal portion of the contralateral branch PA is absent or atretic. When there is branch PA discontinuity, one of the branch PAs is initially fed by an ipsilateral patent ductus arteriosus, which typically closes or becomes very stenotic after birth. In other congenital defects, a single or multiple aortopulmonary collateral arteries supplies the distal pulmonary arterial bed(s). In addition, in the malformation termed hemitruncus, either the left, or more commonly, the right branch PA originates from the ascending aorta while the contralateral PA branch is in continuity with the MPA.
In these vascular malformations, collectively termed absent branch pulmonary artery, surgical interventions include an end-to-end interposition of a conduit between the MPA and the distal intra-parenchymal pulmonary arteries to establish continuity. According to the 2015–2019 STS Congenital Heart Surgery Database, there are (at least) 55 cases per year in the U.S. of isolated discontinuous pulmonary arteries or hemitruncus. In addition, there are nearly 750 cases of main pulmonary artery or central branch pulmonary artery reconstructions that are primarily managed with patch angioplasty, but a subset requires a tubular conduit to relieve stenoses. The current standard-of-care is to use small caliber ( ~ 6 mm diameter) homograft vessels, if available, or synthetic tubes (e.g. Propaten®); however, neither of these can grow, and there is the inevitable development of outflow resistance as the patient grows. This results in maldistribution of pulmonary blood flow and potentially right heart pressure overload resulting in impaired ventilatory efficiency, exertional dyspnea, and functional limitation5,6, with the requirement for one or more surgical reoperations to up-size these conduits.
FDA allowance of a clinical trial requires a preclinical study implanting the graft to be used clinically in the target anatomical site. For repair of absent pulmonary artery branch in an infant, this requires implantation of a 6 mm diameter tube into the left PA (LPA) or right PA (RPA) in the same growing lamb model as we previously used for the implantation of a 16 mm tube into the MPA3. (The LPA was chosen for surgical convenience in this study.) However, this introduces a new variable besides graft diameter, namely, the influence of a time lag associated with graft regeneration following implantation into the LPA as compared to zero time lag for the native RPA. Specifically, the LPA-RPA flow distribution could be altered from normal, with more flow diverted into the RPA that immediately grows and thus presents less resistance to flow due to its larger diameter. If hemodynamic conditions affect (or even control) regeneration of a growing vascular graft, the growth of the LPA graft could then be negatively affected leading to its diminished growth.
In this study, 6 mm diameter biologically-engineered tubes (grown from an allogeneic donor sheep’s fibroblasts and then decellularized, referred to as “TEVG” for tissue-engineered vascular graft hereafter) of 0.5–0.7 cm length, the maximum length for this experimental surgical model, were implanted as interposition grafts into the LPA of young lambs for 3, 6, and 18 months. These timepoints were motivated by our prior results showing extensive TEVG recellularization and regeneration after 10 months in the MPA and an interest in characterizing their evolution and longer-term graft stability. Propaten® 6 mm diameter vascular grafts (referred to as “control grafts” hereafter) of similar length were implanted in the same lamb model for 6 months as a control group. The grafts were imaged with echocardiography and CT-angiography in-vivo at defined intervals. Catheterization was also performed at euthanasia for pressure and compliance data in addition to the flow data. Explanted grafts were characterized histologically and mechanically.
The TEVG exhibit extensive site-appropriate recellularization after only 3 months and near-normal increase of diameter from the preimplant value of 6 mm to 12.9 mm and a doubling of length associated, along with excellent hemodynamics and no calcification, in contrast to the Propaten® grafts. The left-right flow distribution is nearly 50–50 for the TEVG at 6 months compared to about 20–80 for the Propaten® grafts, which have less than one-half the diameter, a 6-fold higher pressure gradient, and stunted vascular development downstream of the graft. The TEVG exhibit a stable diameter over months 12–18 when the lambs become adult sheep.
Methods
TEVG production
As previously reported7, tubular, cell-seeded fibrin gels were fabricated by mixing aqueous solutions of ovine dermal fibroblasts (Coriell), bovine fibrinogen (Sigma), thrombin (Sigma), and calcium chloride. The final component concentrations were as follows: 1 million cells/mL, 4 mg/mL fibrinogen, 0.38 U/mL thrombin, and 5.0 mM Ca++. The mixed cell suspension was injected into tubular glass molds which had a 6 mm inner diameter mandrel, a 6 mm annulus, and were 15 cm in total length. The final dimensions of each graft were as follows: length, 6 to 8 cm; inner diameter, 6 mm; and thickness, ~0.5 mm.
Following gelation, the tubular cell-entrapped fibrin gels on glass mandrels were cultured in Dulbecco’s Modified Eagle Medium (DMEM) + 10% fetal bovine serum (FBS, Hyclone), 100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B, 2 μg/mL insulin, and 50 μg/mL ascorbic acid. Culture medium was changed three times per week for 2 weeks while allowing the longitudinal shortening of the gels to 6–8 cm. The tissue tubes were then matured on the mandrel for 9 weeks with continuous medium mixing via a rocker.
Following maturation, the biologically engineered tubes were decellularized by immersion in 1% sodium dodecyl sulfate (SDS, Sigma) and 1% Triton X-100 (Sigma) for 6 h and 30 min, respectively, at room temperature with continuous shaking. The tubes were then extensively rinsed in 1X phosphate buffered saline before and after overnight incubation in culture medium plus 2 U/mL deoxyribonuclease (DNAse, Worthington Biochemical). The final decellularized TEVG were stored at 4 oC until implantation, with the first surgery performed within 30 d of storage and last surgery done after 14 months storage. All TEVG used in this study were produced in the same batch, including culture at the same time in the same chamber on the rocker.
TEVG explant preparation
Explanted TEVG were cut axially (longitudinally) cut into 4 axial segments. Three segments were kept for histology. The fourth segment, intentionally chosen to contain any portion of the graft that appeared thin visually, was used for mechanical testing within 5 h of retrieval from the animal. Loose connective tissue covering the abluminal surface of the graft was removed for this segment only prior to the thickness measurement and mechanical testing. It was cut into 3 circumferential strips. Two were used for tensile testing and one was used for suture retention strength testing. To establish the histology was independent of graft location, the fourth segment was also kept for histology for the 3-month explants.
Suture retention strength
The native PA edge of the strip was gripped on one arm of a tensile testing system (Instron Systems). Using a 6-0 MaxonTM suture, a loop was placed around 1–2 mm of the graft’s tissue from the opposite edge and looped around a force transducer on a second arm of the Instron system. That arm was extended at 50 mm/min until failure. The suture retention strength, thus measured in the graft’s axial direction, is reported in the units of gram-force.
Circumferential tensile strength
The width (in middle of the strip) and thickness (near the end of strip) were measured using a caliper. The tissue strip was then mounted between compressive grips of an Instron testing system and pulled at rate of 50 mm/min until failure. Maximum tension is defined as maximum force divided by width of the tissue strip and reported in units of N/m. The ultimate tensile strength is defined as maximum force per unit area and reported in units of MPa.
Collagen content
The collagen mass content was quantified using a hydroxyproline assay8 assuming 7.46 mg of collagen per 1 mg of hydroxyproline. The total protein content was measured using the ninhydrin assay9. The tissue volume calculated using the measured length, width, and thickness of the samples (in units of ml) was then used to calculate the sample mass concentration (mg protein/ml tissue) of collagen and total protein.
Gross pathology and histology
A complete necropsy, including gross evaluation of target and non-target tissues, as well as the explanted graft was performed by a board-certified veterinary pathologist (JSF). The axial segments from the TEVG explant preparation for histology were placed in cassettes and fixed in 10% neutral buffered formalin (NBF), then sent to an independent histology laboratory (Scientific Solutions LLC, Fridley, MN) for paraffin embedding and immunohistochemical staining with Hematoxylin & Eosin (H&E), Masson’s Trichrome (MT), Von Kossa (VK), Von Willebrand’s (vWb), and Verhoeff Van Gieson (VVG) using standard methods. Slides were evaluated using light microscopy by a board-certified veterinary pathologist (JSF) using a semi-quantitative scale for the presence of inflammation, tissue ingrowth, thrombus formation, mineralization, and endothelialization. From each of the axial segments used for histology, which span 3/4 of the graft circumference for the 6-month explants and the entirety for the 3-month explants, at least one 5-µm embedded axial section was stained with each of these stains and evaluated.
Animals
All protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee, conducted in compliance with the Animal Welfare Act, and animals were housed and cared for according to the standards detailed in the Guide for the Care and Use of Laboratory Animals.
Animal information
Animals utilized in this study were castrated male or female, domestic ovine (polypay or polypay cross breed) lambs ranging from 4 to 7 weeks of age. Animals were sourced through the University of Minnesota Research Animal Resources, which purchased animals from USDA licensed vendors. Prior to entry into the study a licensed veterinarian certified each animal to be healthy. Animals received their implants in the order they arrived at the test facility and the test/ control article to be implanted was selected using simple randomization. Pre-established criteria for animals to be excluded from the study included any remarkable cardiothoracic lesions or anatomic anomaly noticed by the implant surgeon during implant, and animals that did not survive to their full scheduled term. One animal (GLPA-6) in this study did not survive test article implant and one animal (GLPA-3) implanted with a control article died on post-operative day 75 due to suspected peritonitis.
Surgical procedures
After establishing cardiopulmonary bypass (see Supplementary Methods), the MPA and LPA were exposed and isolated from surrounding structures for interposition graft placement in the LPA. The LPA was cross clamped at the distal most portion, as well as, proximal to the bifurcation of the MPA. The LPA was transected between the clamps. The graft length was trimmed to fit and then sutured into place as an interposition graft. The distal anastomosis was performed first. For the first two TEVG implants (18 months group), continuous running stitches using 6-0 MaxonTM sutures were used for the anastomoses. After indication of early scar tissue around the sutures potentially preventing anastomotic growth in early echo examination, the suture technique in the 3 and 6 months groups was altered. Approximately 2/3 of the circumference of the medial portion of each anastomosis was done with running suture, and interrupted sutures (also 6-0 Maxon™) were placed along the remaining lateral portion of each anastomosis for TEVG implants. For control grafts, continuous 7-0 prolene suture was used as PTFE is not known to integrate with the native vessel and was thus done to avoid any adverse late bleeding event that may have incurred with Maxon™ suture resorption. Prior to finishing the final anastomosis, the animal was rewarmed and air was evacuated from the LPA by removing one or both of the cross clamps. Once all air was purged from the site, the anastomosis was completed. When normal sinus rhythm and normothermia were achieved, mechanical ventilation was reinstated and the animal was weaned from bypass. All bypass cannulas were removed. Protamine was administered, IV, at the discretion of the surgeon. For the 3 and 6 months animals, epicardial echo was performed to measure diameter at both anastomoses and mid-graft. The animal was weaned from the ventilator and then transferred to the post-operative care facility.
Anticoagulation therapy
The 18-month survival animals received heparin, 2000 IU, IM, twice daily for 1 year. The 3- and 6-month survival animals that were subsequently implanted in a GLP study aimed at emulating the clinical scenario received enoxaparin, 1 mg/kg, twice daily, for 1 month.
In-life monitoring
Animals were observed post operatively for normal recovery from surgery, appetite, fluid intake, voiding, willingness and ability to ambulate, respiratory rate and effort, heart rate, temperature and survival. Independent veterinary assessment was performed in the presence of atypical clinical appearance. After discharge from postoperative care animals were housed in a natural environment with pasture and appropriate shelter with on-site veterinary technical support. If animals presented with adverse clinical diagnoses, they were returned to ESS for veterinary examination and treatment.
Trans-thoracic ultrasound imaging
Animals were manually restrained in the right decubitus position for ultrasound examination. Trans-thoracic ultrasound was used to evaluate heart function. Any additional information about flow or diameter of the graft that could be obtained was also captured at the time of the exam. The 18-month study animals were imaged 1 weeks, 1 months, 2 months, 3 months (LPA-1 only), 4 months (LPA-1 only), 12 months, and 18 months p.o. The 3-month survival animals were imaged at 1 week and 3 months p.o., and the 6-month survival animals were imaged 1 week, 3 months, and 6 months p.o.
CT-angiography (CTA)
The Computed Tomography (CT) Services of MHealth UMMC East Campus (Fairview University Hospital) performed CTA exams on one 18-month animal (LPA-1) at 3 months, 6 months, 12 months, and 18 months, on the other 18-month animal (LPA-2) at 2 months and 18 months, and on the 6-month animals at 6 months only.
The animals were sedated and anesthetized similar to the implant procedure. The left and right chest was shaved and cleaned for placement of ECG electrodes. Both left and right thighs were cleaned and ECG electrodes secured with vet wrap. The animal was then transported to the M Health Fairview CT suite. Each animal was secured on the CT table head-first in the right lateral position. Mechanical ventilation was initiated at 10–15 breaths per minute. Oxygen was set at 2–4 L/min, and isoflurane set between 1 and 4% as needed to maintain a deep plane of anesthesia. ECG monitoring leads were connected to the animals. A pulse oximeter was secured.
Noncontrast and CTA cardiac scans were performed using standard clinical techniques (Heart: 2 topograms, noncontrast scan, CTA heart scan). Following collection of these images, the animal was disconnected from mechanical ventilation, transported back to the ESS laboratory and recovered. RadiAnt DICOM viewer was used to recreate 3D images from the digital CT exams. LPA graft and native RPA lengths and diameters in multiple planes as well as cross-sectional areas and perimeters were measured from the recreated images. Reported lengths and diameters are the averages of values measured mid-graft in the axial and sagittal planes, and in the lateral and medial planes, respectively.
Terminal study
Six- and 18-month survival animals were sedated and anesthetized similar to the implant procedure. The thoracic cavity was accessed via sternotomy to perform epicardial echos. Heparin was administered, 300IU/ kg, IV, and introducer sheaths were surgically placed in a jugular vein and carotid artery. These access sites were used to introduce catheters with the intention of measuring cardiac output, intra-cardiac blood pressures, pressure gradient across the grafts, and performing angiography of the pulmonary arterial system (under C-arm and phase contrast) as detailed below.
With C-arm fluoroscopic guidance, a 7Fr. Swan-Ganz catheter was introduced through the venous sheath and positioned in the main pulmonary artery. Cardiac output was measured by thermodilution using a Spacelabs Qube patient monitor. Using OsiriX MD® angiographic software, digital angiograms were used to make graft and pulmonary artery measurements for distensibility coefficients. Auto-calibration was used to take millimeter diameter measurements. The markers on the 5-Fr Merit Performa® pigtail catheters used for angiograms were utilized to cross-check calibration (each marker is separated by 10 mm leading edge-leading edge). The graft’s minimal diameter, the distal normal LPA prior to the hilar bifurcation, and the contralateral normal RPA prior to the hilar bifurcation were all measured. Each angiographic measurement was taken at peak systole and again at end-diastole in the same region.
Invasive pressures were obtained during the cardiac catheterization using a 6 or 7-Fr balloon wedge catheter. Phasic and mean pressures obtained in main and branch pulmonary arteries (at and beyond the graft) were used to calculate systolic-diastolic pressure swings (peak systolic pressure-end diastolic pressure) and peak gradients across the graft. Graft cross-sectional compliance (CC) was calculated as the ratio of the graft cross-sectional area (ΔA) and the pressure swing (ΔP), i.e., ΔA/ΔP (expressed in mm2/mmHg)10. In each animal, CC was calculated for the graft, TEVG or control, and for the contralateral native RPA.
Epicardial echo was performed to measure cardiac output, and blood flow through the RPA and LPA. Cardiac output and volumetric blood flow of the LPA and RPA were calculated by using 2-dimensional imaging to measure diameters of the right ventricular outflow tract (RVOT), native RPA, and LPA graft; and pulsed-wave spectral Doppler to measure heart rate and blood flow velocity at each site.
The animals were finally heparinized (300IU/ kg) and euthanized following procedures consistent with the AVMA Guidelines for the Euthanasia of Animals. Animals were immediately evaluated by gross necropsy. 3-month survival animals did not have any term procedures.
3D modeling/printing and shape analysis
Materialise Mimics (Materialise, Leuven, Belgium) was used to render 3D models of the RVOT from the CTA scans for the two experimental groups and an age-matched naïve group. Within this software, a mask was created with a threshold that captured most of the blood volume that made up the heart and surrounding blood vessels. Manual editing was then used to remove regions that were not of interest and leave behind the RVOT. The 3D models were printed by a Stratasys J850 printer in Vero Magenta Vivid (TEVG) and Vero Ultra White (control graft).
The Vascular Modeling Toolkit11 was used to extract the (minimum) radius values along the length of the LPA, and they were processed using a custom MATLAB script and converted to (minimum) diameter values. First, the vertices of the 3D model were used to generate a 3D Voronoi network. The Voronoi node closest to the center of the inlet was assigned as a source node, then Voronoi nodes found at the centroid of the branches along the LPA were assigned as target nodes. The shortest path between the target to the source nodes were the centrelines and calculated using the Voronoi graph and Dijkstra’s algorithm12. The radius reported is the radius of the osculating sphere that fits the vessel at each point along the centreline. This “minimum radius” is relevant to hemodynamic resistance.
Statistics and reproducibility
Group sizes are summarized in Table 1. In general, P-values were calculated based on the mean and standard deviation of reported results of TEVG compared to control grafts for n\(\ge\)3 grafts using the t-test (2 tailed, 2 sample, equal variance) function on Microsoft Excel or GraphPad Prism software to assess differences. For the 3D RVOT models specifically, IBM SPSS Statistics was used to conduct One-Way ANOVA tests on the mean (minimum) radius and its variation with distance along the PA branch (i.e. slope) of all three groups across the three indicated regions, followed by Tukey’s test for differences among groups. Use of ‘difference’ below implies P < 0.05.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
TEVG pre-implant characterization
All TEVG used in this study were produced in one manufacturing batch. The mechanical properties of the TEVG (Fig. 1a) were as follows (n = 2 grafts, so ± defines the range): thickness 0.57 ± 0.07 mm, lumen burst pressure 3298 ± 96 mmHg, and suture retention strength of 99 ± 6 gram force. The dry weight was 10 ± 3 mg/cm (of conduit length) and collagen density was 91 ± 6 mg/cm3.
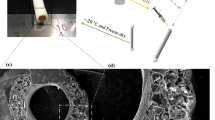
a Pre-implant, b Operating field at implantation into the LPA (control graft) and explanted (c) Control graft, 6 months, d TEVG, 3 months, e TEVG, 6 months, f TEVG, 18 months; luminal surface of (g) Control graft, 6 months, h TEVG, 6 months, i TEVG, 18 months. White arrows indicate locations of graft anastomoses.
Animal Growth
The study design is summarized in Table 1. All lambs that underwent successful implantation into the LPA (Fig. 1b) at age ~5 weeks old exhibited normal weight gain, which was independent of graft type: 13.6 ± 1.8 kg at implantation (n = 9), and weight gains of 24.2 ± 0.6 kg at 3 months (TEVG, n = 3), 42.0 ± 5.3 kg at 6 months (TEVG, n = 3), 42.5 ± 2.2 kg at 6 months (control, n = 3) post-implantation. There were no instances of abnormal right heart function in any of the study animals at any timepoint based on transthoracic echocardiography. There was no difference in cardiac output of the control graft animals (4.9 ± 0.9 L/min) and the TEVG animals (6.1 ± 0.1 L/min) at 6 months based on thermodilution. All lambs were asymptomatic until term, except for one animal implanted with a control graft that died at day 75 due to apparent peritonitis.
Graft Explant Appearance
Images of representative explanted grafts are shown in Fig. 1c–f. All grafts appeared well-healed, properly placed, and covered on the abluminal surface by a variably thick layer of adipose and fibrous appearing connective tissue, consistent with an expected healing response to previous surgical trauma. TEVG grafts, at all timepoints, were highly integrated with the adjacent tissue, resulting in difficulty differentiating the graft from the native tissue when viewing from the abluminal surface (Fig. 1d–f). In contrast, control grafts were readily identified by the prolene sutures, darker coloration, and firm structure (Fig. 1c). On cut section, TEVG were slightly more discernable from the native tissue, identifiable by faint lines at the anastomosis sites, minor variability in thickness, and a minimally-heterogenous surface appearance. Thinner areas of the graft appeared tannish/mauve and thicker areas appeared white and shiny. The thinner areas, when present, were consistently located on the dorsal surface of the graft, along its inner wall downstream of the MPA bifurcation. Differentiation between native tissue and the graft became increasingly difficult with the in vivo duration of the implants, consistent with continued remodeling and maturation of the implanted TEVG. No stenosis was observed at the anastomosis sites or along the length of the TEVG. In contrast, control grafts grossly appeared smaller in diameter than both the proximal and distal native artery, resulting in a slightly pinched and/or cinched appearance. Similar to the TEVG, the luminal surface of the control grafts was slightly variable in thickness and had a mottled appearance, but control grafts also often had multifocal deposits of slightly raised bright red/tan tissue, consistent with thrombus.
Graft Diameter and Length Comparison
CT-angiography images of all TEVG and control grafts at 6-month explantation are presented in Fig. 2. The white arrows point to the location of the grafts implanted into the LPA. In all cases, the TEVG diameter appears largely uniform and of comparable value to the native LPA distal to the graft, and to the native RPA. In contrast, the control graft is much smaller than the native LPA distal to the graft. This is clearly evident upon comparing the 3D models for the two explants shown in Fig. 2d (TEVG) and Fig. 2h (control graft). 3D models for all six 6-month explants and a group of naïve animals are presented in Suppl. Figure 1. Profiles of the downstream LPA diameter are qualitatively different for the TEVG and control grafts as evident in Suppl. Figure 2a showing the 3D models of the LPA for all six animals, with the distal LPA of control graft animals, but not the TEVG animals, being smaller in diameter than in age-matched naïve animals (Suppl. Figure 2b).
The visual differences in graft diameters in Fig. 2 are quantified in Fig. 3a revealing the control graft has not changed from its initial diameter (as expected given PTFE is not a regenerative material), which was the appropriate size at implantation. In contrast, the TEVG has more than doubled in diameter to 12.9 ± 2.0 mm and is not different from the RPA diameter. The TEVG graft length was also greater measured as 13.0 ± 2.0 mm vs. 6.0 ± 0.9 mm at implantation, again indicative of TEVG growth. In comparison, at 6 months the length was 7.1 ± 1.1 mm for the control graft.
a Averaged Diameter (from CT-angiography) (b) Graft Peak Pressure Drop (proximal to distal ends of graft from pressure probe), c LPA/RPA Flow Ratio (from epicardial echo), d Graft Cross-Sectional Compliance (from pressure probe/angiography). Error bars indicate standard deviations (n = 3 all graft groups, n = 6 for RPA diameter of all grafted animals, n = 3 for RPA compliance of TEVG animals as explained in the text). Paired symbols indicate statistical difference at P < 0.05; a *=0.004, ^=0.00008, b *=0.025, c *=0.0006, d *=0.033, ^=0.038.
Graft Function Comparison
Figure 3b presents the peak pressure drop across the same 6-month grafts, which was 6-fold greater for the control graft relative to the TEVG, consistent with the differences in their diameters. The LPA/RPA flow ratio was 0.94 ± 0.07 for the TEVG animals versus 0.22 ± 0.11 for the control graft animals, shown in Fig. 3c, meaning almost an even distribution of blood flow to the PA branches for the TEVG animals and a substantial reduction of blood flow to the LPA for the control graft animals. These flow ratios correspond to LPA-RPA flow distributions of 48–52% and 18–82%, respectively. This difference is also consistent with the difference in diameters, specifically, an increased flow resistance for the smaller diameter control graft. Equivalently, there was a decreased flow area for the control graft (0.32 ± 0.02 mm2) vs. the TEVG (1.26 ± 0.35 mm2). The cross-sectional compliance of the grafts and their comparison to the RPA are plotted in Fig. 3d. The TEVG value of 14.4 ± 7.4 mm2/mmHg is not different from the RPA value of 44.7 ± 39.7 mm2/mmHg, and over 100 times greater than for the control value of 0.10 ± 0.06 mm2/mmHg. (Only the RPA values for the TEVG animals were used here since the abnormal LPA/RPA flow ratio for the control graft animals could more adversely affect the LPA growth).
Graft Histology
Figure 4a shows an H&E stained section of the TEVG pre-implantation and Fig. 4b–f shows the histology of the explanted TEVG at 3 months. Upon histopathology examination, excellent biocompatibility was concluded, with no inflammation, mineralization, fibrin/thrombus, hemorrhage, or necrosis associated with the TEVG. A minor foreign body response was noted surrounding suture material in the anastomosis sites, but not within the TEVG itself. The TEVG showed a high degree of host cell infiltration, including fibroblasts and smooth muscle cells (Fig. 4b, c) intermixed with elastin fibers (Fig. 4d). No mineralization was present within the TEVG (Fig. 4e), with only scant mineralized foci associated with the resorbable sutures along the anastomoses, as expected. Excellent regeneration was also concluded, with almost complete endothelialization of the TEVG luminal surface (Fig. 4f). Grafts were surrounded by variable amounts of mature-appearing fibrovascular tissue admixed with variable amounts of adipose tissue, representative of a mature healing response post-operatively. Figure 5 shows the histology of the explanted TEVG and control grafts at 6 months. For the TEVG explants (Fig. 5a, d–f), the same conclusions were made at 6 months as at 3 months, and it generally proved difficult to distinguish the adjacent native vessel from the TEVG (Fig. 5a, b). In contrast, the control grafts exhibited regions of luminal thrombus, foreign body response, and calcification (Fig. 5c).
a Pre-implant TEVG showing the absence of cellular components. Hematoxylin and Eosin (H&E). TEVG explanted at 3 months b–f: b extensive infiltration of mesenchymal cells into the TEVG, formation of neoadventitia, and absence of inflammation. H&E. c Infiltration of smooth muscle cells (red) along with fibroblasts and mature collagen (blue). Masson’s Trichrome (MT). d Elastin fibers (black) are present within the TEVG. Smooth muscle cells (brown) and fibroblasts/collagen (pink) are also evident. Verhoeff Van Gieson (VVG). e Graft is negative for mineralization (no brown staining present). Von Kossa (VK). f Endothelialization is present on the luminal surface of the graft (brown). Von Willebrand’s (vWb). All photos taken at 10× objective. * denotes lumen. Scalebar = 100 µm.
a TEVG - Masson’s trichrome (200x mag, scalebar = 50 micrometer), b Native LPA - Masson’s trichrome (200x mag), c TEVG - VVG (200x mag), d Native LPA - VVG (200× mag), e TEVG - H&E (200× mag), f Native LPA - H&E (200× mag), g TEVG - von Kossa (200x mag), h control graft - Masson’s trichrome (arrow points to thrombus, oval demarcates foreign body response, rectangle demarcates calcification). * denotes lumen. Scalebar = 50 µm (black), 100 µm (white).
For both 3 and 6 months TEVG explants, variability in the amount of smooth muscle infiltration was correlated with the heterogeneity of the luminal surface noted on gross pathology. In general, fibroblast infiltration and elastin deposition appeared consistent across all sections of all TEVG, but with varying degrees of smooth muscle infiltration. Areas with less smooth muscle infiltration appeared thinner and tan grossly, whereas areas that appeared white and shiny grossly had a greater degree of smooth muscle infiltration. These areas were located on the dorsolateral surface of the graft (equivalent to posterolateral for the human) along the inner wall (medial aspect) of the TEVG in two of the 6-month TEVG (GLPA-7,10) and in two of the 3-month TEVG (GLPA-1,2) to various degrees.
Long-term TEVG dimensions
Figure 6a presents CT-angiography images of two TEVG (LPA-1, LPA-2) that were implanted for 18 months. Unlike the 3-month and 6-month animals, which had interrupted sutures placed along one-third of the anastomosis, the two 18-month animals (the first two animals implanted in this study) were sutured with a running absorbable suture around the entire anastomosis. The TEVG diameter (Fig. 6b), cross-sectional area (Fig. 6c), and length (Fig. 6d) for both animals increased from the earliest measured values. In one animal (LPA-2), they exhibited sustained increase over the 18 months (152% greater diameter, 579% greater cross-sectional area, and 53% greater length at 18 months over 2 mo), and in the other animal (LPA-1) they plateaued at substantially lower values (51% greater diameter, 70% greater cross-sectional area, and 13% greater length at 18 months over 3 mo). There was no change in the two TEVG diameters from 12 months to 18 months within the margin of measurement error.
Long-term TEVG histology
Figure 7 shows the histology of the two explanted TEVG at 18 months. Both TEVG were well-healed and integrated with surrounding native tissue; there was no inflammatory infiltrate, calcification, or clinically concerning thrombus. Both possessed an endothelium. Interestingly, LPA-1, which only grew from 6 mm to ~8 mm diameter, possessed a mixture of mature fibrous connective tissue containing smooth muscle infiltration and elastin fibers, surrounded by fibrovascular and adipose connective tissue (Fig. 7a, b). Compared to the native PA, there were similar amounts of connective tissue, less smooth muscle, less elastin, and greater surrounding vascularization. In contrast, LPA-2, which grew from 6 mm to ~14 mm diameter, mainly possessed elastin fibers and connective tissue, with rare, scattered smooth muscle cells and minimal adventitial neovascularization (Fig. 7c, d). Compared to the native PA, there was a similar amount of elastin, but less smooth muscle and vascularization. Like the 3 and 6 months TEVG explants, LPA-1, which appeared predominantly white and shiny on the luminal surface, exhibited a greater infiltration of smooth muscle cells, as opposed to LPA-2 which grossly appeared tan and contained less smooth muscle cells. Compared to the 3 and 6 months TEVG, these 18 months TEVG generally appeared grossly similar, displaying a high degree of biocompatibility plus integration with, and infiltration of, host tissue. The minor foreign body response to the resorbable suture material noted in the 3 and 6 months animals appeared predominantly resolved at 18 months.
TEVG Explant Testing
Figure 8a shows the strips of explanted TEVG for mechanical testing for the three 6-month animals. Figure 8b shows that the suture retention strength of all three TEVG at 6 months was 3 to 7 times greater than the pre-implant TEVG, with no apparent difference between the 6-month and 18-month values. Figure 8c shows the tensile strength for all three explanted TEVG was comparable to the ovine MPA3, with no apparent difference between the 6-month and 18-month values. Of all three animals, GLPA-10 had the thinnest region (0.29 mm, Table 2), visually seen in the middle of tissue strip #2 in Fig. 8a. Nonetheless, both the maximum tension (Fig. 8c) and UTS (Table 2) for strips #1 and #2 are comparable for GLPA-10. GLPA-5 had the thickest wall due to an extensive neo-adventitia (0.88–1.58 mm) leading to the highest strength in terms of maximum tension (strip #1) but not in terms of UTS (Table 2). The collagen concentration in the two TEVG explanted at 18 months was 136 ± 44 mg/cm3, 37% higher than the implanted matrix (91 ± 6 mg/cm3). The increase in diameter and length of these TEVG (Fig. 6) along with their measured thickness (Table 2) led to total tissue volume increase by 145 ± 1%, leading to a total conduit collagen content increase by 265 ± 34% over the 18 months of implantation (n = 2 grafts, so ± defines the range for these values). Since a graft segment containing any region that appeared thinner visually was used for mechanical testing and thickness measurement, this is likely a conservative estimate of the increase.
a Images of explanted tissue cut into 3 circumferential strips for mechanical testing for the 6-month animals (n = 3), b Suture retention strength for each TEVG (strip #3) for the 6-month (n = 3) and 18-month (n = 2) animals, in comparison to the pre-implant TEVG (“Pre IM”) (n = 3), c maximum tension for each TEVG (strips #1,2) for the 6-month (n = 3) and 18-month (n = 2) animals in comparison to the ovine main PA (n = 3).
Discussion
The optimal solution for congenital vascular repair is a conduit exhibiting somatic growth that maintains normal hemodynamics, where somatic growth implies an increase in graft dimensions and graft tissue mass (e.g. collagen content). This study used a biologically-engineered conduit (“TEVG”) of acellular collagenous matrix produced by donor fibroblasts. The results presented clearly show a hemodynamic benefit of the TEVG in terms of achieving nearly a 50–50 flow distribution between the LPA and RPA at 6 months post-implantation compared to the control graft distribution of approximately 20–80. This was associated with the TEVG diameter more than doubling from its initial value, becoming nearly the value of the distal LPA and the RPA. No other off-the-shelf (acellular) TEVG evaluated in preclinical growth models has realized this magnitude of diameter increase. Moreover, the TEVG diameter was approximately constant from (at least) 12 months to 18 months in the two animals survived to 18 months, which included nearly one year of adulthood, indicating requisite diameter stability of the TEVG as regeneration proceeded.
Benefit was also shown in terms of decreased graft peak pressure drop and increased graft compliance of the TEVG compared to the control graft. In the case of the conduits implanted in the LPA for 18 months (n = 2), the total collagen content increased by 265% supporting somatic growth associated with collagen production by the cells invading and regenerating the TEVG, as we previously reported for our study of implantation of the TEVG into the MPA of the same growing lamb model where collagen content of the graft increased 465% over the 10-month implantation (n = 3)3. A greater increase in collagen concentration of the graft in that study (111% in the MPA vs. 49% in the LPA) explains the difference. While the increase in elastin content was not quantified in this study as performed in ref. 3, histology shows that elastin deposition again occurred, consistent with the complex process of tissue growth. The pattern of extensive recellularization underlying the graft’s apparent somatic growth was similar in both studies (only “apparent” in this study since the 265% collagen content increase cannot be statistically supported with n = 2). Specifically, nearly complete endothelialization of the luminal surface and recellularization of the graft interior with cells consistent with fibroblasts and smooth muscle cells, without any evidence of a sustained immune response was observed at 6 months post-implantation. This histological finding was essentially the same after just 3 months of implantation. Additionally, the TEVG increased approximately 4-fold in suture retention strength at 6 months from its pre-implant value and its maximum tension during tensile testing was comparable to the ovine MPA value.
Also consistent with the earlier MPA replacement model3, the implanted matrix did not elicit a sustained inflammatory or immune response, or exhibit macroscopic calcification. The allogeneic acellular matrix produced by the donor dermal fibroblasts is predominantly fibrillar collagen, but contains ~30% other proteins (on a mole basis) that are predominantly matricellular proteins7. In previous studies, we have shown that the decellularization method used here reduced the DNA content by 93%, with β-actin (intracellular protein) and β2-microglobulin (cell membrane protein) being undetectable by Western blot13. The low reactivity of this graft, even relative to Propaten® PTFE, can thus be attributed to this cell-produced matrix that is effectively decellularized without apparent protein denaturation and conducive to regeneration upon recellularization.
A curious finding was the occurrence of thinner regions along the inner wall of the TEVG at 3-month and 6-month (2 of 3 animals in both groups) and that these areas correlated with less smooth muscle cell infiltration. As with any arterial bifurcation, the inner wall of the native LPA and RPA experiences the highest shear stress and a developing boundary layer that affects transport of diffusible species between the arterial wall and blood. Assuming the proximal anastomosis and/or TEVG compliance mismatch did not create a flow disturbance that substantively altered these hemodynamics, then the different regeneration of the TEVG at the proximal inner wall could be attributed to different shear stress-mediated responses of infiltrating cells and/or different concentrations of blood-derived stimuli available to the infiltrating cells, as compared to the other regions of the TEVG. Alternatively, or in addition, the different regeneration could be attributed to differences in the development of the neoadventitia, a potential source of the infiltrating cells, at that location. Abutment of the LPA (hence also the TEVG) near the MPA bifurcation with the trachea occurs along its inner wall. Importantly, despite being thinner in some regions in some grafts, the strength of thinner regions was not lower, and there were no occurrences of graft dilatation or related aneurysm.
The somewhat divergent outcomes of the two animals with TEVG explanted at 18 months does not have a clear explanation (i.e. LPA-2 increased ~100% in diameter comparable to the 6-month TEVG explant group, possessing a more mature, stable histological appearance; LPA-1 increased only ~33% in diameter, possessing a more regenerating histological appearance). Both 18-month animals had a continuous resorbable suture line for the entire anastomosis whereas all 3-month and 6-month animals had interrupted sutures for one-third of the anastomosis and continuous suture for the rest. The interrupted suture approach provided a consistent outcome of growth in terms of diameter increase. It is possible that collagen deposition occurs extensively at the anastomoses (“scarring”) prior to suture resorption, limiting diameter increase at the anastomoses in the case of a continuous suture, which can then limit diameter increase of the entire graft (e.g. due to reduced blood flow). Alternatively, this finding could simply reflect a reduced regenerative capacity of the LPA-1 animal. While the impact of such “biological variability” will not be clarified until results from a study of larger numbers of implants are obtained, it is possible that use of interrupted sutures for at least one-third of the anastomosis may be more permissive to TEVG growth for the reason hypothesized. However, the similar values of strength for both LPA-1,2 at explantation indicate this TEVG is mechanically robust along a spectrum of regeneration outcomes.
A key distinction of this study compared to our previous MPA replacement study3 is that the TEVG implanted into the LPA is not assured of experiencing a blood flow rate as for the MPA. Considering the TEVG is initially acellular, by design, it is quite remarkable that it achieved a near normal diameter in just 6 months given the handicap of zero growth rate initially compared to the normal growth rate of the native RPA, and a reduced growth rate until sufficient recellularization of cells capable of producing extracellular matrix has occurred. If a hemodynamic signal (e.g. wall shear stress) was crucial to TEVG growth, then a negative feedback loop for TEVG growth would have led to shunting of blood to the RPA and diminishing TEVG growth. The histology shows the presence of many such cells throughout the graft after just 3 months, which would explain the near-normal TEVG diameter at 6 months. These cells might have derived from a neo-adventitia associated with the noted wound healing response to the implanted graft, as well as from trans-anastomotic migration. Mathematical models of vessel growth14 would need to correctly capture the recellularization rate and pattern for this TEVG as well as tissue growth in response to hemodynamic signals to predict the reported outcomes.
While allogeneic donor ovine fibroblasts were used to produce the TEVG used in this study to ensure no complication of a xenogeneic response to the implanted extracellular matrix, TEVG similarly produced from human fibroblasts have been shown to safely perform for at least 6 months as arterio-venous (AV) grafts for dialysis patients15, indicating the clinical potential of this TEVG for a pediatric clinical trial for children born with absent pulmonary artery branch.
Potential limitations of this study include the length of the TEVG implant (0.5–0.7 cm), the n-value2 for the longest timepoint (18 mo), and the use of enoxaparin for the first 30 days post operatively. Regarding the TEVG implant length, it was dictated by the available length of the left main pulmonary artery before its first branches arise. The TEVG are routinely produced at longer lengths (8 cm in this study and 15 cm in ref. 14), and the surgical repair of absent pulmonary artery branch will only require a 2–4 cm length in an infant. The 18-month timepoint with n = 2 reflects the usual constraints of time and resources. While the diameters and histology of the TEVG explanted from these two animals substantially differed without obvious explanation, the stable TEVG diameter observed during at least the final 6 months of sheep adulthood (months 12–18) without calcification or an immune response suggest that the TEVG grafts will be stable. For a vascular graft that is intended for life-long implantation, an 18-month longest timepoint may seem short, especially given the uncertain timeline for graft regeneration to reach homeostasis, but these findings portend TEVG longevity. Use of enoxaparin for the first 30 days is not current clinical practice but was chosen to minimize early occlusive thrombosis as a failure mode. A similar 6 mm diameter TEVG made from human fibroblasts was reported to be non-thrombogenic in the AV graft clinical study with clopidogrel administered through week 8 post-operatively combined with chronic low-dose aspirin; only one of five grafts occluded during the cannulation period starting at week 9 through 6 months post-implant14. The initial flow rate through the AV grafts (480–1200 mL/min) in that study was comparable to the expected values in the LPA/RPA of infants (650 mL/min for healthy infants). Thus, it is likely that the anticoagulation regimen of dual anti-platelet therapy now commonly used in pediatric practice will be efficacious for this conduit at these blood flow rates.
Data availability
All data associated with this study are presented in the manuscript and available at The University of Minnesota Digital Conservancy, https://hdl.handle.net/11299/264059, repository name “Comms Med Pressure-Flow-Diameter-Length data”. Source data for Figs. 3, 6b–d can be found in this repository. Other data are available upon request. A representative sample of the material for research use only will be considered upon request from non-profit research institutions at cost under a material transfer agreement.
Code availability
The code used to determine the vessel radius along the vessel centerline is available at zenodo, repository name: get-centerline-in-vmtk, https://doi.org/10.5281/zenodo.1262841016.
References
Yin, C. H. et al. Effect analysis of repeat sternotomy in pediatric cardiac operations. J. Cardiothorac. Surg. 10, 179 (2015).
Jacobs, J. P. et al. Reoperations for pediatric and congenital heart disease: an analysis of the Society of Thoracic Surgeons (STS) congenital heart surgery database. Semin Thorac. Cardiovasc Surg. Pediatr. Card. Surg. Annu 17, 2–8 (2014).
Syedain, Z. et al. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat. Commun. 7, 12951 (2016).
Stamm, C. et al. Outcome after reconstruction of discontinuous pulmonary arteries. J. Thorac. Cardiovasc. Surg. 123, 246–257 (2002).
Hiremath, G. et al. Balloon angioplasty and stenting for unilateral branch pulmonary artery stenosis improve exertional performance. JACC Cardiovas. Interventions 12, 289–297 (2019).
Rhodes, J. F. Jr. & Jackson, L. Tipping point: when does unilateral branch pulmonary artery stenosis impede exertional performance? JACC Cardiovasc. Interventions 12, 298–300 (2019).
Syedain, Z. H. et al., Pediatric tri-tube valved conduits made from fibroblast-produced extracellular matrix evaluated over 52 weeks in growing lambs. Sci. Transl. Med. 13, eabb7225 (2021).
Stegemann, H. & Stalder, K. Determination of hydroxyproline. Clin. Chim. Acta 18, 267–273 (1967).
Robinson, P. S., Johnson, S. L., Evans, M. C., Barocas, V. H. & Tranquillo, R. T. Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng. Part A 14, 83–95 (2008).
Tozzi, P., Corno, A. & Hayoz, D. Definition of arterial compliance. Re: Hardt et al., “Aortic pressure-diameter relationship assessed by intravascular ultrasound: experimental validation in dogs.”. Am. J. Physiol. Heart Circ. Physiol. 278, H1407 (2000).
Izzo, R., Steinman, D., Manini, S. & Antiga, L. The vascular modeling toolkit: a Python library for the analysis of tubular structures in medical images. J. Open Source Softw. 3, 745 (2018).
Dijkstra, E. W. in Edsger Wybe Dijkstra: His Life, Work, and Legacy. (2022), pp. 287–290.
Syedain, Z. H., Meier, L. A., Lahti, M. T., Johnson, S. L. & Tranquillo, R. T. Implantation of completely biological engineered grafts following decellularization into the sheep femoral artery. Tissue Eng. Part A 20, 1726–1734 (2014).
Blum, K. M. et al. Tissue engineered vascular grafts transform into autologous neovessels capable of native function and growth. Commun. Med (Lond.) 2, 3 (2022).
Ebner, A. et al., First-in-human evaluation of a biological regenerative vascular conduit for hemodialysis access. J. Vasc. Access, 25(4), 1271–1278 (2023).
Wiputra, H., Shi, Z. https://doi.org/10.5281/zenodo.12628410 (zenodo, 2024).
Acknowledgements
Quality assurance provided by Gwen Kocher and Beverly Norris. Funding from DoD W81XWH-22-1-0489 (to RTT) and the Frank & Eleanor Maslowski Charitable Trust (to RTT).
Author information
Authors and Affiliations
Contributions
Conceptualization: J.E.M., R.M., R.T. Methodology: Z.S., M.L., G.H., J.B., J.P.C., J.S.F., T.S., A.R., H.W., R.S. Investigation: R.T., Z.S., R.B. Funding acquisition: R.T. Project administration: R.T. Supervision: R.T., R.B. Writing – original draft: R.T., Z.S., J.E.M. Writing – review & editing: R.T., Z.S., J.E.M., M.L., G.H., J.P.C., J.S.F., R.M., J.B., T.S., A.R., H.W., R.S.
Corresponding author
Ethics declarations
Competing interests
Z.S., R.T.: financial interest in Vascudyne, Inc., licensee of related University of Minnesota patents; Z.S.: employee of Vascudyne, Inc. Authors J.E.M., R.B., M.L., G.H., J.P.C., J.S.F., R.M., J.B., T.S., A.R., H.W., R.S. declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Francesco Nappi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Syedain, Z.H., Lahti, M., Hiremath, G. et al. Evaluation of an engineered vascular graft exhibiting somatic growth in lambs to model repair of absent pulmonary artery branch. Commun Med 4, 201 (2024). https://doi.org/10.1038/s43856-024-00614-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-024-00614-8