Abstract
Metabolic dysfunction-associated steatotic liver disease (MASLD) is often regarded in society as a disease caused by personal lifestyle and dietary choices. Healthcare providers who have empathy and are able to explain the disease trajectory can better engage with people with MASLD and actively work with them to improve their metabolic health on a sustainable basis. Non-invasive tests can assist in this process, but healthcare providers must ensure they explain their advantages and limitations. Discussing and setting lifestyle goals are priorities before initiating specific pharmacological treatment, since living a healthy lifestyle will remain the backbone of the multimodal management of MASLD. In this review, we discuss challenges and opportunities to actively engage with people living with MASLD in a multimodal treatment framework as a healthcare provider.
Similar content being viewed by others
Introduction
Non-communicable diseases (NCDs) account for 74% of global mortality, resulting in 41 million deaths annually. Cardiovascular diseases, cancers, respiratory diseases, and diabetes mellitus have been traditionally regarded as the ‘big 4’ NCDs since these conditions are responsible for the highest numbers of deaths1.
Metabolic dysfunction-associated steatotic liver disease (MASLD) (formerly known as non-alcoholic fatty liver disease (NAFLD)) affects approximately one-third of the adult population2 and is at the interface between multiple NCDs, including type 2 diabetes mellitus (T2DM), obesity, and cardiovascular disease3. A nationwide cohort study including 10,568 biopsy-confirmed patients with MASLD showed that patients with MASLD had an increased overall mortality rate compared to controls (28.6 versus 16.9/1000 person-years) caused by extra-hepatic cancers, cirrhosis, cardiovascular disease, and hepatocellular carcinoma (HCC)4 with an average life-expectancy that is 2.8 years shorter5. As a result, MASLD also poses challenges to financial health care systems6. In addition, 9% of patients with MASLD experience stigmatization because of their liver condition, resulting in impairment of health-related quality of life7 and potentially also healthcare avoidance8. Therefore, MASLD should feature prominently on the public health agenda9,10.
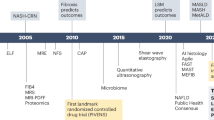
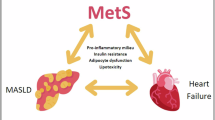
MASLD encompasses a spectrum of disease stages ranging from isolated liver steatosis to progressive metabolic dysfunction-associated steatohepatitis (MASH), fibrosis, cirrhosis, and HCC, with only a minority of patients with non-cirrhotic MASLD experiencing more severe liver-related outcomes11. MASLD progression is driven by environmental and genetic factors and is part of the metabolic syndrome in which obesity and T2DM are prevalent and important disease modifiers12,13. Societal factors contribute to the increasing prevalence of MASLD by promoting the development of these metabolic disorders through a sedentary lifestyle and encouraging ultra-processed food consumption (Fig. 1)2,14. Policy makers and the food industry hence have important roles in controlling MASLD in the population, although these are often affected by financial incentives. The most readily modifiable factors, depending on the stage of MASLD and comorbidities, involve personal behavior, including having a healthy diet and regular physical activity, and avoiding tobacco and alcohol use15,16,17. Since MASLD is a chronic and silent liver disease, there is limited knowledge about it in the general population which complicates effective patient communication and engagement18. In addition, patients with MASLD have limited readiness to adopt lifestyle changes, especially regarding exercise19, while liver disease also often receives less attention compared to many other diseases20. This practical guide for the hepatologist and allied healthcare workers aims to provide concrete tips to encourage patients with non-cirrhotic MASLD to adopt lifestyle changes and improve their liver condition and overall metabolic health. Now the first drug to treat MASH, Resmetirom, has received approval by the Food and Drug Administration (FDA)21,22, we also emphasize strategies to promote adoption of a healthy lifestyle on a sustainable basis whilst using MASH-specific drugs.
Navigating in a relationship of trust
Empathetic listening and building mutual trust
Because obesity might be the only visible indicator of MASLD, it can be challenging to present a strong message to patients, even more to those who are lean23. However, creating a safe environment and providing statements such as ‘we will make a plan together to improve your liver condition, which will put you on track for a healthy life’ can be motivational and give a sense of a provider-patient partnership striving for a better quality of life and reduced risk of both liver- and non-liver-related outcomes (Fig. 2). People with MASLD should therefore be seen as persons with lived experience rather than patients waiting for treatment. Listening to each story requires in depth attention and empathy but can yield mutual advantages through advancing insights into personal behavior. Patients can experience a feeling of importance and dignity, and this can be used as a solid foundation for making plans together using shared decision making24,25. Family or friends accompanying the patient can be involved in this process to assist in a supportive network on a daily basis26.
Patients suffering from MASLD may either experience difficulties in communicating about their liver disease because of societal stigmatization or have a lack of motivation to actively work on their metabolic health. Creating a safe environment in which up-to-date terminology is used with attention for psychological and societal inequalities seems key for effective communication in MASLD to attain sustained lifestyle changes. A multidisciplinary team with continuous evaluation should therefore be composed based on individual needs. MASLD metabolic dysfunction-associated steatotic liver disease.
Establishing mutual strategies and identifying personal factors that hinder change to engage the patient to collaborate with other experts
Once a feeling of mutual trust and common goals is accomplished, personal factors that hinder adoption of lifestyle changes should be identified, for example through motivational interviewing27,28. Ideally, this process results in collaboration with other experts such as dietitians, cardiologists, endocrinologists, pharmacists, physiotherapists, nurses, psychologists, and/or social workers, as needed, leading to multimodal treatment of MASLD and associated diseases29. From a practical point of view, the central persons coordinating these partnerships would be the hepatologist and nurse in cases of more advanced disease, and the primary care provider for patients with early stage MASLD. Monthly or quarterly multidisciplinary meetings could be organized to evaluate the goals and needs of the individual patients.
Constructive approaches to address failures to adopt lifestyle changes
During the process of changing lifestyle, failure should be seen as an opportunity to identify points to improve and as a step in the right direction as this is more motivating than viewing it as personal malfunctioning30,31. Since nearly all patients will encounter relapses when changing their lifestyle and moments of uncertainty, it can be explained that these are non-linear achievements of the goal and integral elements of their process, and that changing lifestyle requires learning and reflecting on what can be improved (Fig. 3). Numbers related to lifestyle and weight loss that appeal to the imagination are available for MASLD and sharing these can assist in this process. Patients able to lose 10% or more of their body weight through lifestyle modifications show reductions in the NAFLD activity score after 1 year, while 90% show resolution of MASH and 45% even have regression of hepatic fibrosis32. It must however be noted that among people living with overweight, only approximately 20% can achieve and maintain a 10% intentional weight loss for at least 1 year33,34. Maintaining weight loss is therefore perhaps the most challenging part of adopting lifestyle changes in patients with MASLD and some of them might never return to a non-overweight/obese state. For these individuals, it is essential to affirm that any weight loss still adds significant benefits to their health, and that gradual weight reductions can sometimes be easier to adhere to than drastic changes in lifestyle35,36. In addition, discussing the differences between body weight and body composition37 is crucial for patients who are improving their health through physical exercise without achieving weight loss.
Considering the role of society
Although multimodal treatment of MASLD is a good approach29, one needs to be cautious with patients who feel stigmatized and guilty about their metabolic condition and are potentially frustrated by a virtual environment portraying a beauty ideal, elite athleticism, sexuality, and misguided appearance of health7,20,38. Today, a sedentary lifestyle is supported by innovation, industrialization, and urbanization, and the impact of this on someone’s daily life can be enough to develop diseases related to metabolic dysfunction (Fig. 1). To that end, a Sustainable Development Goal score has been developed as an advocacy tool for NCDs, including MASLD. The Sustainable Development Goal score for MASLD provides an estimate of the country-level preparedness to manage MASLD from a societal perspective, which can facilitate multisectoral collaborations. Indicators for sustainable development regarding MASLD are child wasting, child overweight, NCD mortality, a universal health coverage service coverage index, health worker density, education attainment, and an urban green space indicator, which is important for physical and mental health. Each of these factors can negatively or positively influence the development of MASLD, highlighting the role of society, education, and upbringing. For example, mortality due to T2DM and cardiovascular disease might not have been a health priority in low- and middle-income countries that were instead focused on communicable diseases. Such factors are captured by the Sustainable Development Goal score, which can subsequently be used to inform policy makers to take action at the population level in an objective manner38.
A holistic approach to communicating the need for lifestyle changes can allow patients to realize that there are forces working against them as they aim for better health outcomes. Highlighting the societal perspective can also make a patient more aware of lifestyle- and food-related signals in daily life. Digital therapeutics including internet applications specifically designed for patients with MASLD can be proposed to assist in this process. Such applications are tailored to the individual’s needs and assess motivation to change and consciousness of their disease. Further, they provide education using interactive slides and can guide physical exercise39,40. However, social support is the greatest facilitator for making lifestyle changes in patients with MASLD41. From a social and societal perspective, another approach to promote lifestyle changes lays in social prescribing. Patients can be ‘prescribed’ non-medical activities to promote their metabolic health utilizing local initiatives based on their personal interest and motivation. These can consist of joining a local sports club, gardening group, or cooking club focused on healthy eating habits. This way, patients can meet other facets of society, join new social networks and discover health-promoting hobbies that match their personality42,43. In addition, it offers an elegant way to circumvent the general sports advice while still empowering physical activity and healthy habits. Patients with MASLD can reside in a microenvironment that promotes the development of MASLD, so sharing these interventions with family members and cohabitants can yield metabolic benefits beyond the individual patient, while also creating a supportive atmosphere44.
Considering psychiatric comorbidity
A pitfall in MASLD patient engagement lays in the fact that depression is a highly prevalent condition among patients with MASLD (prevalence of 18.21% (95% CI 11.12;28.38) for MASLD and 40.68% (95% CI 25.11;58.37) for MASH)45,46, which can impede motivation to work on lifestyle-related factors. Since depression is often treated in primary care, it is important to also involve general practitioners in the interdisciplinary team. Nonetheless, it remains vital to identify details that are suggestive for a clinically relevant or subthreshold depression since it would require involving specific care47. Furthermore, several antidepressants and other drugs used for psychiatric diseases are appetite-promoting and induce weight gain48, which can be problematic. It is the shared responsibility of the prescribing physician and pharmacist to select the least weight-inducing agent for a specific patient, and to inform the patient of this potential side-effect, in particular when the patient is overweight or obese. A potential future avenue consists in the additional prescription of a glucagon-like peptide-1 (GLP-1) receptor agonist to pro-actively address expected weight gain induced by psychiatric drugs49,50.
Apart from depression, there is also a relationship between MASLD and the development of anxiety disorders, in particular in women (hazard ratio for women 1.29 with 95%CI 1.13;1.48—hazard ratio for men 1.15 with 95%CI 0.99;1.34)51, which can further complicate patient communication and engagement. For these patients, emotional support and creating a safe space for sharing experiences and fears are even more important. Interaction with, and obtaining accurate details from, these patients can be achieved by showing curiosity and requesting the patient to correct you if something feels not right or sounds unclear52.
Once compensated advanced chronic liver disease/cirrhosis has developed, one should be attentive for signs of hepatic encephalopathy, a neuropsychiatric disorder characterized by confusion, cognitive impairment, poor concentration, and changes in personality and behavior53. These symptoms can be relatively easily linked to advanced liver disease together with other signs and symptoms, while the true difficulty lies in the recognition of minimal hepatic encephalopathy (MHE), in which only subtle symptoms occur related to vigilance and integrative function54. Although some reports exist on cognitive impairment caused by MASLD55, MHE should not be ignored as it relates to impaired quality of life, frequent road traffic accidents and a poor prognosis. In addition, MHE is present in 35% of patients with cirrhosis, making it a prevalent condition53. Testing for MHE can be undertaken via checks such as the psychometric hepatic encephalopathy score (PHES) if communication is impaired by possible symptoms56. Since the PHES is a time-consuming test, a simplified animal naming test can be used instead in daily clinical practice, although it is less specific57.
Engagement by information
Importance of being aware of changing terminology
Three different acronyms have been used in recent years to describe in essence the same disease entity: NAFLD, metabolic dysfunction-associated fatty liver disease (MAFLD), and MASLD58. This has not only led to confusion in the scientific literature, but also heterogeneity in information sources available to the public. A key element to maintaining credibility in the patient-physician relationship when communicating with patients is consistency in what is being told to them. Since June 202312, professional societies including the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL) and the Latin American Association for the Study of the Liver (ALEH) have undertaken a consensus process among stakeholders and advised that MASLD will be the term to be used in future healthcare activities. This change in terminology should be explained to people with MASLD. For example, newly diagnosed patients with MASLD are likely to encounter patient documentation in which the NAFLD (or MAFLD) nomenclature is used. Vice versa, patients who have previously received a diagnosis of ‘NAFLD’ should be educated on the new MASLD terminology so they can find up to date information about their disease.
The change in MASLD nomenclature constitutes an opportunity for physicians to motivate and inform their patients. In this nomenclature, the potentially stigmatizing terms ‘fatty’ and ‘alcoholic’ were removed, and the role of metabolic dysregulation was highlighted12. Further, it is in line with the preferred communication by the subset of patients with MASLD and obesity, who opt for terms such as ‘weight’ rather than ‘fat’ or ‘obese’23,59.
Education on prognosis
The unawareness in the population regarding liver disease in general and more specifically MASLD also results in a lack of basic knowledge about the disease and more importantly, its long-term consequences. Sharing prognostic details on MASLD can engage patients and encourage the introduction and maintenance of behavioral changes9,60. The most easily explained disease perspective is perhaps the suggested ‘20% rule’ for progression in F3/F4 MASH, stating that 20% of patients with MASH and bridging fibrosis develop cirrhosis in 2 years, while 20% of patients with cirrhosis develop hepatic decompensation in 2 years61. Yet, being aware you have liver fibrosis can potentially already promote a healthier lifestyle, since unawareness about liver fibrosis stage by people with MASLD/MASH has been shown to be associated with poor adherence to lifestyle changes62. Nonetheless, most patients have isolated liver steatosis and might never develop MASH and more advanced liver disease and having a ‘fatty’ or ‘steatotic’ liver is often considered as being a condition without severe hepatic consequences. To these patients, it can be explained that MASLD is an important contributor to T2DM and cardiovascular disease with potential mortality63,64,65,66. In addition, HCC can also develop in patients with MASLD in the absence of cirrhosis67,68, which can be a strong call to action. In light of these complications, it is of importance to link this chance of progression to the ability to change the disease course by decisive action and life-style choices. Therefore, education on the natural history of MASLD and the reversibility of liver steatosis, MASH, and fibrosis through adopting lifestyle changes are key aspects to promoting intrinsic motivation and preventing the terminal complications of MASLD32,69. Educational material from medical associations (such as EASL70) and patient organizations can assist in this process. In line with this, an initiative to inform patients with MASLD is the ‘Global Fatty Liver Day’, which is a public education campaign supported by liver patient organizations and multiple medical societies71.
Apart from these liver-related perspectives, pregnancy-related and inter-generational factors can also be used to promote behavioral changes for specific individuals. MASLD is independently associated with hypertensive complications (pre-eclampsia, eclampsia, and/or HELLP syndrome (Hemolysis, Elevated Liver enzymes and Low Platelets)) (odds ratio 3.13 with 95%CI 2.61;3.75), postpartum hemorrhage (odds ratio 1.67 with 95%CI 1.28;2.16), and preterm birth (odds ratio 1.60 with 95%CI 1.27;2.02), invigorating the need for pre-conception counseling72. In addition, maternal obesity increases the risk of MASLD in the offspring (odds ratio 3.26 with 95%CI 1.72;6.19)73, which is an additional argument for adopting lifestyle changes in potential future mothers.
Role of non-invasive tests
As patients generally prefer to have direct access to their medical results74, even when these are normal75, non-invasive test (NIT) results that screen for MASLD phenotypes including the fibrosis-4 (FIB-4) score and vibration-controlled transient elastography (VCTE)-based scores, using the controlled attenuation parameter (CAP) and liver stiffness measurement (LSM), can be convenient tools to inform patients about their liver status and initiate discussion (Table 1)29. Nonetheless, as highlighted in Table 1, these test results can be complex to interpret76, which can lead to unfounded worries in patients74. Moreover, small changes in these NITs, including CAP and LSM, do not reflect histological or clinically relevant improvements77,78, indicating the importance of targeting clinically relevant changes. For example, patients with a high Agile3+ score at baseline should attain a decrease in their score of more than 20% to have a considerable reduction in the risk of liver-related events79. In addition, the inaccuracy of CAP in differentiating higher steatosis grades can be discouraging in patients starting from steatosis grade 380. More accurate NITs to quantify hepatic steatosis and fibrosis over time include magnetic resonance imaging-proton density fat fraction (MRI-PDFF)81 and magnetic resonance elastography (MRE)82, respectively. These could be valuable alternatives but their cost-effectiveness for this purpose is unclear83. Therefore, exact results from currently used NITs should be used with caution in patient communication, although highlighting improvements in these tests over time might be further motivational. In addition, their use in patient communication promotes patient participation which can positively influence patient-reported84, and clinical outcomes, including liver- and cardiovascular events85. A potential alternative to sharing NIT data is showing pictures of livers86.
Although NITs specifically designed for liver disease can encourage patient participation and provide information, one should keep in mind that changes in these parameters can lag behind more commonly used laboratory measurements of metabolic health. Therefore, earlier changes in for example blood pressure, cholesterol, triglycerides, and HbA1c can be used for achieving shorter-term goals while also working towards metabolic health in general85,87,88.
Empathizing with different communities
Consideration of socio-economic status and education
Socio-economic status is a risk factor for NCDs, mediated by a low-quality diet, living a sedentary lifestyle and a lack of higher education89, and this also applies to MASLD89,90. A low socio-economic status goes hand in hand with food insecurity, which is a risk factor for MASLD. In this regard, ‘food deserts’, areas with sparse options to acquire nutritious food, and ‘food swamps’, areas with a high concentration of fast food- and junk food-selling restaurants, create an obesogenic climate that promotes the development and worsening of MASLD91. In line with this, low socio-economic status may also limit access to physical activity options and exercise routines because of costly memberships or time constraints92.
Access to healthcare is a prerequisite for patients with a lower socioeconomic status to adopt lifestyle changes, which should not only be available, affordable, and acceptable, but also sustainable93. A silent liver disease does not inspire change in many people94, especially when other issues in life, among which securing food and financial stability, tend to be a higher priority. Dietitians and social workers can play a key role in aiding the transition to eating healthy food, although this may be adopted at the expense of financial burden. Referral to these professionals can therefore be communicated to a patient by emphasizing the goal of a tailor-made nutritional plan, taking into account budgetary constraints and practical feasibility, including transportation. Informing a patient that financial issues have been discussed beforehand with the health professional, can give the patient reassurance to effectively make use of these consultations and advice.
Consideration of ethnicity and cultural background
Ethnical origin is often discussed in relation to genetic predisposition to MASLD, in particular the polymorphism in the patatin-like phospholipase domain-containing protein 3 (PNPLA3), rs73840995, which is associated with a higher frequency of MASLD in Hispanics96. Ethnicity and cultural background can also have indirect effects on MASLD through lifestyle choices and dietary patterns97,98 and so is a consideration when communicating with patients who have a different ethnical/cultural background than their physician. In this regard, assumptions made from the physician’s own perspectives should be avoided to obtain an accurate view of someone’s lifestyle and dietary pattern. Standardized questionnaires about food intake can assist in the process of identifying points of potential improvement99,100, after which culturally tailored adaptations to diet can be made by suggesting foods that patients are familiar with that could allow sustainable behavioral changes101,102,103. Digital devices providing information about body composition and artificial intelligence-guided personalization of digital tools that factor in a person’s own habits and culture can potentially aid in this process104.
Roles of alcohol consumption and smoking
Reducing alcohol consumption
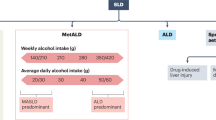
No amount of alcohol consumption improves health105, but much heterogeneity exists in the conclusions of studies on the effects of alcohol consumption on MASLD, with some reporting that modest alcohol consumption protects against MASH106 and some stating that any level of alcohol use should be avoided by patients with MASLD107. Nonetheless, there is consensus that alcohol consumption should be strongly discouraged in patients with MASLD who have F3-F4 fibrosis108. The recent introduction of the SLD nomenclature affirmed the added pathogenic value of alcohol in MASLD by introducing MetALD, in which patients have SLD originating from both metabolic dysregulation and alcohol consumption of 140 to 350 g/week for females and 210 to 420 g/week for males12. This new disease category not only allows better classification of patients, but its use can also motivate patients to limit their alcohol consumption. The first important steps in avoiding or limiting alcohol use consist of depersonalizing drinking habits and explaining potential negative liver- and non-liver related health outcomes. In a second step, barriers can be identified that hinder efforts to stop drinking109. These barriers often include social drinking and family habits, which are generally considered as relatively innocent drinking moments110. Social drinking can be reduced by sincerely expressing concerns and agreeing on a plan to reduce alcohol use111. For family habits, such as daily alcohol consumption during dinner, including other family members in the discussion can be beneficial112.
Reducing tobacco use
Smoking has been associated with MASLD in several studies (with an odds ratio for MASLD when smoking of 1.11 with 95%CI 1.03;1.20)113,114,115. Initially, patients can be told of the negative effects and risks of smoking on their metabolic and cardiovascular health116, after which the potential impact on their partner or children can be discussed as well in certain cases. Although such a direct communicative strategy may lead to self-stigmatization, it has been reported that it can result in reductions in smoking117. For patients with T2DM and MASLD it can be further emphasized that the combination with tobacco use adds to the risk of hepatic fibrosis, while it is a potentially readily changeable factor when compared to T2DM (prevalent fibrosis odds ratio for cigarette smoking and T2DM interaction = 3.04 with 95%CI 1.62;5.76; odds ratio for T2DM alone = 2.28 with 95%CI 1.37;3.85)118.
Considering patient’s expectations and preferences
Multidisciplinary healthcare teams have been increasingly proposed in recent years to address the complexity of MASLD and multiple comorbidities29,41. However it is important to aim for patient-centered communication and minimally disruptive care, so the expectations patients have and their preferred level of care should be considered119,120. Taking time to listen to these expectations in the very first consultation can save considerable undesired and therefore ineffective efforts and costs. In addition, patient satisfaction is generally driven by the feeling that their physician provides enough time to listen to understand their situation, which is essential to maintain the patient-physician relationship121,122. Credibility to patients can be strengthened by also communicating their expectations and preferences to the other healthcare professionals involved, so a feeling of immediate common commitment can be attained.
On the other hand, some patients might not have the motivation to set goals and work together towards metabolic health. If patients’ intrinsic engagement is thought to be insufficient to achieve improvements in their liver condition, one should not be afraid to acknowledge the emotional and structural factors that may need additional support. This can enable hindering factors to be identified and made discussable, potentially resulting in improved motivation. Such conversations should be undertaken on a regular basis as personal engagement can change over time and different opportunities arise on different occasions. In line with expectational follow-up, regularly ascertaining satisfaction with the treatment plan and professionals involved will further augment the chances of success123. There exist, for example, diverse needs for behavioral support for successful lifestyle change which could range from peer support to coaching to structured psychology and psychiatry needs both at the inter-individual and intra-individual level over time47,89. For example, a meta-analysis investigating the effect of cognitive behavioral therapy on lifestyle changes found that it improves weight loss (effect size (BMI) −0.63 with 95%CI −1.17; −0.10) and weight maintenance (effect size (BMI) −0.55 with 95%CI −0.90; −0.20)124, which are key to treating MASLD32.
Furthermore, it remains important to underscore that MASLD is a slowly progressing disease and that there is space to take incremental small steps towards a metabolically healthy condition to avoid progression to cirrhosis61, which can be reassuring.
The impact of MASH-specific drugs
The lack of an effective pharmacological treatment for MASH125 and the drastic nature of bariatric surgery126 can engage patients to adopt lifestyle changes. Recently, Resmetirom, a thyroid hormone receptor-beta agonist, was approved by the FDA as the first drug to treat MASH21,127. The availability of this drug will enable liver-related goals to be more easily attained, and this needs to be considered to ensure an optimum patient-physician relationship. Nevertheless, lifestyle changes will remain essential to achieve holistic better metabolic health, including cardiovascular benefits, and avoid sarcopenia128,129, and discussing these issues is a priority before initiating pharmacological treatment (Fig. 4). It can be helpful to also emphasize that Resmetirom was approved as an addition to diet and exercise and not as standalone treatment130.
Adopting and continuously evaluating lifestyle changes is a priority when initiating MASH-specific drugs to attain metabolic health on a sustainable basis. Therefore, multimodal treatment of MASLD remains an important element even when MASH drugs are available. MASH metabolic dysfunction-associated steatohepatitis, MASLD metabolic dysfunction-associated steatotic liver disease.
Improved health-related quality of life related to drug treatment, as observed in a 36-week phase 2 trial with Resmetirom131, can be used as an opportunity to introduce new dietary and physical exercise habits during drug treatment, given that a better quality of life may come with enhanced motivation132,133. In that regard, GLP-1 receptor agonists that are indicated for the treatment of T2DM, also often induce considerable weight loss134,135, which can eliminate obesity-related stigmatization and motivate patients to further work on their metabolic health through lifestyle modifications. In a 72-week randomized phase 2 trial, the GLP-1 receptor agonist Semaglutide induced significantly more MASH resolution (up to 59% in the highest dose (0.4 mg) group, compared to 17% in the placebo group, p < 0.001), but no statistically significant improvement in fibrosis stage, compared with placebo (43% in the 0.4 mg group, compared to 33% in the placebo group, p = 0.48). Nonetheless, GLP-1 receptor agonists (and dual/triple agonists with additional agonism of the glucagon receptor and/or glucose-dependent insulinotropic polypeptide receptor) might pave the way for future combined treatment of MASH and obesity128,135,136 while also potentially having destigmatizing effects with resulting societal benefits. So far, GLP-1 receptor agonists are only regionally registered for the treatment of obesity and there exists much inequality in access to it due to requirements for access and lack of financial coverage137,138.
Conclusions and perspectives
MASLD is an increasingly prevalent health problem139. Lifestyle changes are the most readily available and best treatment currently available for MASLD, but these require a careful communicative strategy (Box 1). Mutual trust in the patient-physician relationship and involved healthcare providers in a multidisciplinary team are key to enable patients to reverse MASLD and prevent the progression to advanced disease. However, MASLD should not only be managed at the individual patient level, but also more comprehensively at the political level9,10. The EASL-Lancet commission10, the ‘Healthy Livers Healthy Lives’ coalition139, and ‘Liver Health is Public Health’ initiative140 have highlighted the need for better liver care and preventive strategies to be on the agenda of policy makers and provide examples of how to develop a public health strategy.
It is often highlighted that there exists unawareness regarding MASLD in the general population, even in patients who have obesity and T2DM18. Yet, this unawareness reaches beyond the general population as the relevance of MASLD and its impact on related comorbidities are also often poorly known in specialty disciplines outside hepatology29. This unawareness in medical care also hampers effective communication of a unified message to patients with MASLD. One of the most important health care professionals who need more knowledge about MASLD are general practitioners, since these physicians are responsible for initial diagnosis of MASLD and referral to specialty care. In recent years, much effort has been made to create efficient and practical referral pathways from primary care to specialty care, by, for example, using the FIB-4 score as a screening modality for advanced hepatic fibrosis in patients suffering from pre-T2DM or T2DM141. A concern when setting up referral pathways is that it may result in large numbers of patients needing specialty care, resulting in long waiting lists to access this care. Nonetheless, relying solely on primary care for patients with MASLD and initiating a holistic treatment plan involving a tailored multidisciplinary team is not feasible due to time constraints and specialized aspects of follow-up. To enable a practical structure in secondary and tertiary care that allows communicating MASLD from a societal, psychological, and socio-economic perspective with patients, along with a resulting treatment plan, governmental reimbursement should be foreseen to finance the management of the team and contributions of healthcare professionals making part of it, including dietitians and psychologists142.
As obesity and MASLD are potentially stigmatizing7, the use of NITs can partly eliminate self-blaming and provide objective targets when adopting lifestyle modifications. However, there is no consensus on which NIT should be used to monitor disease and inform patients over time. Using the FIB-4 score would be a convenient way to allow general practitioners to evaluate disease over time since it only requires assessment of transaminases, platelet count, and age. However, the FIB-4 score remains a screening tool and fluctuating results in the indeterminate range (FIB-4 score 1.3 – 2.67) may be difficult to interpret and lead to unnecessary worry77,78. Given the substantial costs and limited availability of MRI-PDFF and MRE to assess hepatic steatosis and fibrosis, respectively82,143, VCTE-based measurement of CAP and LSM seems to offer a better balance between accuracy, accessibility, and costs80. A VCTE-based follow-up could also be partly implemented in primary care to promote accessible liver-oriented healthcare, patient-centered participation, and consistency with specialty care.
The recent FDA approval of Resmetirom in the United States21 has been of considerable interest to people with MASH/MASLD. As observed with the GLP-1 receptor agonist Semaglutide, which is registered in most countries for the treatment of T2DM and causes weight loss as an additional benefit135, metabolic goals are more easily achieved by patients with concurrent obesity and T2DM. Yet, metabolic health requires a healthy diet and physical exercise, and metabolic targets should always be viewed in this perspective. As a result, one might question whether certain metabolic goals obtained through patient engagement should be achieved, or at least attempted, before specific drug treatment can be initiated. Adopting lifestyle modifications could be used as a justification for subsequent pharmacological treatment. Conversely, specific treatment for MASH could potentially break a cycle of unhealthy dietary patterns.
In conclusion, effective communication to engage patients with MASLD over the long term will only be achievable if there is open communication, genuine trust, and mutual appreciation122 taking into account societal and mental issues as well as the biological causes of the disease38. With the availability of MASLD/MASH-specific drugs21, it will remain of utmost importance to maintain this relationship to be able to stimulate a healthy diet and physical exercise to obtain sustainable metabolic health.
In the next 5 years, we speculate that patient engagement in MASLD will be enabled in many countries through dedicated multimodal treatment plans.
References
World Health Organization. Noncommunicable Diseases (NCD) (WHO, 2019). www.who.int/gho/ncd/mortality_morbidity/en/.
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7, 851–861 (2022).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10, 330–344 (2013).
Simon, T. G., Roelstraete, B., Khalili, H., Hagström, H. & Ludvigsson, J. F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut 70, 1375–1382 (2021).
Shang, Y., Nasr, P., Widman, L. & Hagström, H. Risk of cardiovascular disease and loss in life expectancy in NAFLD. Hepatology 76, 1495–1505 (2022).
O’Hara, J. et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: The GAIN study. JHEP Rep. 2, 100142 (2020).
Younossi, Z. M. et al. The impact of stigma on quality of life and liver disease burden among patients with nonalcoholic fatty liver disease. JHEP Rep. 6, 101066 (2024). This paper provides a clear message about stigmatization in MASLD.
Puhl, R. M. Weight stigma and barriers to effective obesity care. Gastroenterol. Clin. North Am. 52, 417–428 (2023).
Lazarus, J. V. et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 19, 60–78 (2022).
Karlsen, T. H. et al. The EASL–Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 399, 61–116 (2022).
Akbari, C. et al. Long-term major adverse liver outcomes in 1260 patients with non-cirrhotic NAFLD. JHEP Rep. 6, 100915 (2024).
Rinella, M. E. et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023). This paper describes the rationale of the new NAFLD/MASLD nomenclature.
Phelps, N. H. et al. Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 403, 1027–1050 (2024).
Grinshpan, L. S. et al. Ultra-processed food consumption and non-alcoholic fatty liver disease, metabolic syndrome and insulin resistance: a systematic review. JHEP Rep. 6, 100964 (2024).
Budreviciute, A. et al. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health 8, 574111 (2020).
Lazarus, J. V. et al. A global action agenda for turning the tide on fatty liver disease. Hepatology 79, 502–523 (2023).
Kim, D., Vazquez-Montesino, L. M., Li, A. A., Cholankeril, G. & Ahmed, A. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatology 72, 1556–1568 (2020).
Alemany-Pagès, M. et al. Insights from qualitative research on NAFLD awareness with a cohort of T2DM patients: time to go public with insulin resistance? BMC Public Health 20, 1142 (2020).
Centis, E. et al. Stage of change and motivation to healthier lifestyle in non-alcoholic fatty liver disease. J. Hepatol. 58, 771–777 (2013).
Wahlin, S. & Andersson, J. Liver health literacy and social stigma of liver disease: A general population e-survey. Clin. Res Hepatol. Gastroenterol. 45, 101750 (2021).
Madrigal Pharmaceuticals. Madrigal Pharmaceuticals Announces FDA Approval of RezdiffraTM (resmetirom) for the Treatment of Patients with Noncirrhotic Nonalcoholic Steatohepatitis (NASH) with Moderate to Advanced Liver Fibrosis (Madrigal Pharmaceuticals, 2024).
Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390, 497–509 (2024). This paper describes the phase 3 randomized controlled trial of the first approved drug to treat MASH.
Cusi, K. Nonalcoholic steatohepatitis in nonobese patients: Not so different after all. Hepatology 65, 4–7 (2017).
World Health Organization. WHO Framework for Meaningful Engagement of People Living with Noncommunicable Diseases, and Mental Health and Neurological Conditions (WHO, 2023).
Montori, V. M., Ruissen, M. M., Hargraves, I. G., Brito, J. P. & Kunneman, M. Shared decision-making as a method of care. BMJ Evid. Based Med. 28, 213–217 (2023).
Ho, Y. C. L., Mahirah, D., Zhong-Hao Ho, C. & Thumboo, J. The role of the family in health promotion: a scoping review of models and mechanisms. Health Promot. Int. 37, 1–14 (2022).
Stewart, K. E. et al. Readiness for behaviour change in non-alcoholic fatty liver disease: implications for multidisciplinary care models. Liver Int 35, 936–943 (2015).
Resnicow, K. & McMaster, F. Motivational interviewing: moving from why to how with autonomy support. Int J. Behav. Nutr. Phys. Act. 9, 15171788 (2012).
Schattenberg, J. M. et al. A multistakeholder approach to innovations in NAFLD care. Commun. Med. 3, 1 (2023). This paper describes key aspects of the multidisciplinary management of MASLD.
Kasila, K. et al. Individual differences in processes of lifestyle changes among people with obesity: An acceptance and commitment therapy (ACT) intervention in a primary health care setting. Prim. Health Care Res. Dev. 21, e12 0.1017/S146342362000016X (2020).
Guerrini Usubini, A. et al. The ACTyourCHANGE study protocol: promoting a healthy lifestyle in patients with obesity with Acceptance and Commitment Therapy—a randomized controlled trial. Trials 22, 290 (2021).
Vilar-Gomez, E. et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 149, 367–378 (2015).
Mcguire, M. T., Wing, R. R. & Hill, J. O. The prevalence of weight loss maintenance among American adults. Int J. Obes. 23, 1314–1319 (1999).
Wing, R. R. & Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 82, 222S–225S (2005).
Wing, R. R. & Hill, J. O. Successful weight loss maintenance. Annu Rev. Nutr. 21, 323–341 (2001).
Nackers, L. M., Ross, K. M. & Perri, M. G. The association between rate of initial weight loss and long-term success in obesity treatment: Does slow and steady win the race? Int J. Behav. Med. 17, 161–167 (2010).
Ariya, M. et al. Assessment of the association between body composition and risk of non-alcoholic fatty liver. PLoS ONE 16, e0249223 (2021).
Lazarus, J. V. et al. The global fatty liver disease Sustainable Development Goal country score for 195 countries and territories. Hepatology 78, 911–928 (2023). This paper highlights different societal aspects that contribute to MASLD.
Mazzotti, A. et al. An internet-based approach for lifestyle changes in patients with NAFLD: Two-year effects on weight loss and surrogate markers. J. Hepatol. 69, 1155–1163 (2018).
Pfirrmann, D. et al. Web-based exercise as an effective complementary treatment for patients with nonalcoholic fatty liver disease: intervention study. J. Med. Internet Res. 21, 211250 (2019).
Tincopa, M. A., Wong, J., Fetters, M. & Lok, A. S. Patient disease knowledge, attitudes and behaviours related to non-alcoholic fatty liver disease: a qualitative study. BMJ Open Gastroenterol. 8, e000634 (2021).
Ivancovsky-Wajcman, D. et al. Integrating social nutrition principles into the treatment of steatotic liver disease. Commun. Med. 3, 165 (2023).
Morse, D. F. et al. Global developments in social prescribing. BMJ Glob. Health 7, e008524 (2022).
Siddiqui, M. S. et al. Prevalence and severity of nonalcoholic fatty liver disease among caregivers of patients with nonalcoholic fatty liver disease cirrhosis. Clin. Gastroenterol. Hepatol. 17, 2132–2133 (2019).
Gu, Y., Zhang, W., Hu, Y., Chen, Y. & Shi, J. Association between nonalcoholic fatty liver disease and depression: A systematic review and meta-analysis of observational studies. J. Affect Disord. 301, 8–13 (2022).
Xiao, J. et al. Is fatty liver associated with depression? a meta-analysis and systematic review on the prevalence, risk factors, and outcomes of depression and non-alcoholic fatty liver disease. Front. Med. 8, 691696 (2021).
Uphoff, E. et al. Behavioural activation therapy for depression in adults with non-communicable diseases. Cochrane Database Syst. Rev. 8, CD013461 (2020).
Hasnain, M. & Vieweg, W. V. R. Weight considerations in psychotropic drug prescribing and switching. Postgrad. Med. 125, 117–129 (2013).
Gonzalez, C. L., Azim, S. & Miedlich, S. U. GLP-1 analogs are superior in mediating weight loss but not glycemic control in diabetic patients on antidepressant medications: a retrospective cohort study. Prim. Care Companion CNS Disord. 23, 20m02868 (2021).
Bak, M. et al. Glucagon-like peptide agonists for weight management in antipsychotic-induced weight gain: a systematic review and meta-analysis. Acta Psychiatr Scand. In press (2024).
Labenz, C. et al. Nonalcoholic fatty liver disease increases the risk of anxiety and depression. Hepatol. Commun. 4, 1293–1301 (2020).
Stubbe, D. E. Alleviating anxiety: optimizing communication with the anxious patient. Focus 15, 182–184 (2017).
Gairing, S. J. et al. Prevalence of minimal hepatic encephalopathy in patients with liver cirrhosis: a multicenter study. Am. J. Gastroenterol. 118, 2191–2200 (2023).
Stinton, L. M., Jayakumar, S. & Frcpc, M. D. Minimal hepatic encephalopathy. Can. J. Gastroenterol. 27, 572–574 (2013).
Kjærgaard, K. et al. Cognitive dysfunction in non-alcoholic fatty liver disease—current knowledge, mechanisms and perspectives. J. Clin. Med. 10, 1–20 (2021).
Amodio, P. et al. Detection of minimal hepatic encephalopathy: normalization and optimization of the psychometric hepatic encephalopathy score. a neuropsychological and quantified EEG study. J. Hepatol. 49, 346–353 (2008).
Campagna, F. et al. The animal naming test: An easy tool for the assessment of hepatic encephalopathy. Hepatology 66, 198–208 (2017).
Newsome, P., Rinella, M. E., Lazarus, J. V. & Terrault, N. Reply: NAFLD, MAFLD, or MASLD? Cut the Gordian knot with ‘Ludwig disease’. Hepatology 79, E5–E6 (2024).
Auckburally, S., Davies, E. & Logue, J. The use of effective language and communication in the management of obesity: the challenge for healthcare professionals. Curr. Obes. Rep. 10, 274–281 (2021).
Krist, A. H., Tong, S. T., Aycock, R. A. & Longo, D. R. Engaging patients in decision-making and behavior change to promote prevention. Stud. Health Technol. Inf. 240, 284–302 (2017).
Loomba, R. & Adams, L. A. The 20% rule of NASH progression: the natural history of advanced fibrosis and cirrhosis caused by NASH. Hepatology 70, 1885–1888 (2019).
Carrieri, P. et al. Knowledge of liver fibrosis stage among adults with NAFLD/NASH improves adherence to lifestyle changes. Liver Int. 42, 984–994 (2022).
Boeckmans, J., Sandrin, L., Knackstedt, C. & Schattenberg, J. M. Liver stiffness as a cornerstone in heart disease risk assessment. Liver Int. 44, 344–356 (2023).
Mantovani, A. et al. Non-alcoholic fatty liver disease and risk of new-onset heart failure: an updated meta-analysis of about 11 million individuals. Gut 72, 372–380 (2022).
Mantovani, A., Byrne, C. D., Bonora, E. & Targher, G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diab. Care 41, 372–382 (2018).
Boeckmans, J. et al. Inflammation in liver fibrosis and atrial fibrillation: a prospective population-based proteomic study. JHEP Rep. 6, 101171 (2024).
Mittal, S. et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 14, 124–131.e1 (2016).
Kanwal, F. et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 155, 1828–1837.e2 (2018).
Hagström, H., Shang, Y., Hegmar, H. & Nasr, P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol. Hepatol. 9, 944–956 (2024).
Francque, S. M. et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP Rep. 3, 100322 (2021).
www.globalfattyliverday.com. Consulted June 2024.
Sarkar, M. et al. Non-alcoholic fatty liver disease in pregnancy is associated with adverse maternal and perinatal outcomes. J. Hepatol. 73, 516–522 (2020).
Hagström, H. Maternal obesity increases the risk and severity of NAFLD in offspring. J. Hepatol. 75, 1042–1048 (2021).
Steitz, B. D. et al. Perspectives of patients about immediate access to test results through an online patient portal. JAMA Netw. Open 6, e233572 (2023).
Bensing, J. M. et al. How to make the medical consultation more successful from a patient’s perspective? Tips for doctors and patients from lay people in the United Kingdom, Italy, Belgium and the Netherlands. Patient Educ. Couns. 84, 287–293 (2011).
Vali, Y. et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol. Hepatol. 8, 714–725 (2023). This paper describes the performance of different non-invasive tests for advanced MASLD.
Mózes, F. E. et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol. Hepatol. 8, 704–713 (2023).
Sanyal, A. J., Castera, L. & Wong, V. W. S. Noninvasive assessment of liver fibrosis in NAFLD. Clin. Gastroenterol. Hepatol. 21, 2026–2039 (2023).
Lin, H. et al. Vibration-controlled transient elastography scores to predict liver-related events in steatotic liver disease. JAMA 331, 1287–1297 (2024).
Siddiqui, M. S. et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 17, 156–163.e2 (2019).
Caussy, C., Reeder, S. B., Sirlin, C. B. & Loomba, R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology 68, 763–772 (2018).
Liang, J. X. et al. An individual patient data meta-analysis to determine cut-offs for and confounders of NAFLD-fibrosis staging with magnetic resonance elastography. J. Hepatol. 79, 592–604 (2023).
Ajmera, V. & Loomba, R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol. Metab. 50, 101167 (2021).
McKay, A. et al. Patient understanding and experience of non-invasive imaging diagnostic techniques and the liver patient pathway. J. Patient Rep. Outcomes 5, 89 (2021).
Anstee, Q. M. et al. Prognostic utility of Fibrosis-4 Index for risk of subsequent liver and cardiovascular events, and all-cause mortality in individuals with obesity and/or type 2 diabetes: a longitudinal cohort study. Lancet Reg. Health Eur. 36, 100780 (2024).
Houts, P. S., Doak, C. C., Doak, L. G. & Loscalzo, M. J. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ. Couns. 61, 173–190 (2006).
Targher, G., Byrne, C. D. & Tilg, H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut 73, 691–702 (2024).
Boeckmans, J. et al. Clinical utility of the Fibrosis-4 index for predicting mortality in patients with heart failure with or without metabolic dysfunction-associated steatotic liver disease: a prospective cohort study. Lancet Reg. Health Eur. 48, 101153 (2025).
Allen, L. et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob. Health 5, e277–e289 (2017). This paper highlights societal aspects of non-communicable disease.
Vilar-Gomez, E. et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 75, 1491–1506 (2022).
Zelber-Sagi, S. et al. Food inequity and insecurity and MASLD: burden, challenges, and interventions. Nat. Rev. Gastroenterol. Hepatol. 21, 668–686 (2024).
Richard, V. et al. Socioeconomic inequalities in sport participation: pattern per sport and time trends – a repeated cross-sectional study. BMC Public Health 23, 785 (2023).
McMaughan, D. J., Oloruntoba, O. & Smith, M. L. Socioeconomic status and access to healthcare: interrelated drivers for healthy aging. Front. Public Health 8, 231 (2020).
Lazarus, J. V. et al. NAFLD — sounding the alarm on a silent epidemic. Nat. Rev. Gastroenterol. Hepatol. 17, 377–379 (2020).
Boeckmans, J. et al. PNPLA3 I148M and response to treatment for hepatic steatosis: a systematic review. Liver Int 43, 975–988 (2023).
Rich, N. E. et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 16, 198–210.e2 (2018).
Riazi, K., Swain, M. G., Congly, S. E., Kaplan, G. G. & Shaheen, A. A. Race and ethnicity in non-alcoholic fatty liver disease (NAFLD): a narrative review. Nutrients 14, 4556 (2022).
Vijay, A. et al. Development of food group tree-based analysis and its association with non-alcoholic fatty liver disease (NAFLD) and co-morbidities in a South Indian population: a large case-control study. Nutrients 14, 2808 (2022).
Yasutake, K. et al. Dietary habits and behaviors associated with nonalcoholic fatty liver disease. World J. Gastroenterol. 20, 1756–1767 (2014).
Miwa, T. et al. Usefulness of a questionnaire for assessing the relationship between eating behavior and steatotic liver disease among Japanese male young adults. Sci. Rep. 14, 2194 (2024).
Pavithran, N. et al. The effect of a low GI diet on truncal fat mass and glycated hemoglobin in South Indians with type 2 diabetes—a single centre randomized prospective study. Nutrients 12, 179 (2020).
Islam, N. S. et al. A culturally tailored community health worker intervention leads to improvement in patient-centered outcomes for immigrant patients with type 2 diabetes. Clin. Diab. 36, 100–111 (2018).
Farhat, G. Culturally tailored dietary interventions for improving glycaemic control and preventing complications in South Asians with type 2 diabetes: success and future implications. Healthcare 11, https://doi.org/10.3390/healthcare11081123 (2023).
Aggarwal, A., Tam, C. C., Wu, D., Li, X. & Qiao, S. Artificial intelligence–based chatbots for promoting health behavioral changes: systematic review. J. Med. Internet Res. 25, e40789 (2023).
Burton, R. & Sheron, N. No level of alcohol consumption improves health. Lancet 392, 987–988 (2018).
Dunn, W. et al. Modest alcohol consumption is associated with decreased prevalence of steatohepatitis in patients with non-alcoholic fatty liver disease (NAFLD). J. Hepatol. 57, 384–391 (2012).
Jarvis, H. et al. Does moderate alcohol consumption accelerate the progression of liver disease in NAFLD? A systematic review and narrative synthesis. BMJ Open 12, 12 (2022).
Kimura, T. et al. Mild drinking habit is a risk factor for hepatocarcinogenesis in non-alcoholic fatty liver disease with advanced fbrosis. World J. Gastroenterol. 24, 1440–1450 (2018).
Spence, A. D., Khasawneh, M., Allen, P. B. & Addley, J. Communication of alcohol and smoking lifestyle advice to the gastroenterological patient. Best. Pr. Res Clin. Gastroenterol. 31, 597–604 (2017).
Prestwich, A. et al. Does changing social influence engender changes in alcohol intake? A meta-analysis. J. Consult Clin. Psychol. 84, 845–860 (2016).
McCormick, K. A. et al. How primary care providers talk to patients about alcohol. J. Gen. Intern Med. 9, 966–972 (2006).
Sen, B., Goldfarb, S. & Tarver, W. Family structure and risk behaviors: the role of the family meal in assessing likelihood of adolescent risk behaviors. Psychol. Res Behav. Manag. 7, 52–66 (2014).
Rezayat, A. A. et al. Association between smoking and non-alcoholic fatty liver disease: a systematic review and meta-analysis. SAGE Open Med. 6, 2050312117745223 (2018).
Okamoto, M. et al. Cigarette smoking is a risk factor for the onset of fatty liver disease in nondrinkers: A longitudinal cohort study. PLoS ONE 13, e0195147 (2018).
Chen, B. et al. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature 610, 562–568 (2022).
Pirie, K., Peto, R., Reeves, G. K., Green, J. & Beral, V. The 21st century hazards of smoking and benefits of stopping: A prospective study of one million women in the UK. Lancet 381, 133–141 (2013).
Evans-Polce, R. J., Castaldelli-Maia, J. M., Schomerus, G. & Evans-Lacko, S. E. The downside of tobacco control? Smoking and self-stigma: a systematic review. Soc. Sci. Med 145, 26–34 (2015).
Balogun, O. et al. Effect of combined tobacco use and type 2 diabetes mellitus on prevalent fibrosis in patients with MASLD. Hepatol. Commun. 7, e0300 (2023).
Epstein, R. M. et al. Measuring patient-centered communication in patient-physician consultations: theoretical and practical issues. in. Soc. Sci. Med. 61, 1516–1528 (2005).
May, C. R., Montori, V. M. & Mair, F. S. We need minimally disruptive medicine. BMJ 339, b2803 (2009).
Gross, D. A. et al. Patient satisfaction with time spent with their physician. J. Fam. Pr. 47, 133–137 (1998).
Levinson, W. & Pizzo, P. A. Patient-physican communication it’s about time. JAMA. 305, 1802–1803 (2011).
El-Haddad, C., Hegazi, I. & Hu, W. Understanding patient expectations of health care: a qualitative study. J. Patient Exp. 7, 1724–1731 (2020).
Kurnik Mesarič, K., Pajek, J., Logar Zakrajšek, B., Bogataj, Š. & Kodrič, J. Cognitive behavioral therapy for lifestyle changes in patients with obesity and type 2 diabetes: a systematic review and meta-analysis. Sci. Rep. 13, 12793 (2023).
Tilg, H., Byrne, C. D. & Targher, G. NASH drug treatment development: challenges and lessons. Lancet Gastroenterol. Hepatol. 8, 943–954 (2023).
Arterburn, D. E., Telem, D. A., Kushner, R. F. & Courcoulas, A. P. Benefits and risks of bariatric surgery in adults: a review. JAMA 324, 879–887 (2020).
Harrison, S. A. et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 29, 2919–2928 (2023).
Newsome, P. N. & Ambery, P. Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver. J. Hepatol. 79, 1557–1565 (2023).
Polyzos, S. A., Vachliotis, I. D. & Mantzoros, C. S. Sarcopenia, sarcopenic obesity and nonalcoholic fatty liver disease. Metabolism 147, 155676. 0.1016/j.metabol.2023.155676 (2023).
FDA Approves First Treatment for Patients with Liver Scarring Due to Fatty Liver Disease. www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval (2024). Consulted June 2024.
Younossi, Z. M., Stepanova, M., Taub, R. A., Barbone, J. M. & Harrison, S. A. Hepatic fat reduction due to resmetirom in patients with nonalcoholic steatohepatitis is associated with improvement of quality of life. Clin Gastroenterol Hepatol. 6, 1354–1361.e7 (2022). This paper describes the impact of Resmetirom treatment on quality of life in metabolic dysfunction-associated steatohepatitis.
Wrosch, C. & Scheier, M. F. Personality and quality of life: the importance of optimism and goal adjustment. Qual. Life Res. 12, 59–72 (2003).
Younossi, Z. et al. The burden of non-alcoholic steatohepatitis: A systematic review of health-related quality of life and patient-reported outcomes. JHEP Rep. 4, 100525 (2022). This paper describes patient-reported outcomes in MASLD.
Popoviciu, M. S., Păduraru, L., Yahya, G., Metwally, K. & Cavalu, S. Emerging role of GLP-1 agonists in obesity: a comprehensive review of randomised controlled trials. Int J. Mol. Sci. 24, 10449 (2023).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic Steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2021). This paper describes a phase 2 randomized trial of a GLP-1 agonist in MASH.
Bessesen, D. H. & Van Gaal, L. F. Progress and challenges in anti-obesity pharmacotherapy. Lancet Diab. Endocrinol. 6, 237–248 (2018).
Waldrop, S. W., Johnson, V. R. & Stanford, F. C. Inequalities in the provision of GLP-1 receptor agonists for the treatment of obesity. Nat. Med. 30, 22–25 (2024).
Krag, A. et al. Uniting to defeat steatotic liver disease: a global mission to promote healthy livers and healthy lives. J. Hepatol. 79, 1076–1078 (2023).
Global Liver Institute. Liver Health is Public Health initiative (Global Liver Institute, 2024). www.globalliver.org/liver-health-is-public-health.
Rinella, M. E. et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 77, 1797–1835 (2023).
Verma, M. et al. Patient-centered care: Key elements applicable to chronic liver disease. Hepatology 78, 307–318 (2023).
Caussy, C. et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology 67, 1348–1359 (2018).
Bedogni, G. et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 1–7 (2006).
Lonardo, A., Ballestri, S., Bedogni, G., Bellentani, S. & Tiribelli, C. The Fatty liver Index (FLI) 15 years later: a reappraisal. Metab. Target Organ Damage 1, 10 (2021).
Fedchuk, L. et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharm. Ther. 40, 1209–1222 (2014).
Hernaez, R. et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 54, 1082–1090 (2011).
Strauss, S., Gavish, E., Gottlieb, P. & Katsnelson, L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am. J. Roentgenol. 189, W320–W323 (2007).
Cao, Y.-T. et al. Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. EclinicalMedicine 51, 101547 (2022).
Sirli, R. & Sporea, I. Controlled attenuation parameter for quantification of steatosis: Which cut-offs to use? Can. J. Gastroenterol. Hepatol. 2021, 6662760 (2021).
McPherson, S. et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am. J. Gastroenterol. 112, 740–751 (2017).
Blackard, J. T. et al. HIV mono-infection is associated with FIB-4—a noninvasive index of liver fibrosis—in women. Clin. Infect. Dis. 52, 674–680 (2011).
Shah, A. G. et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 7, 1104–1112 (2009).
Angulo, P. et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 45, 846–854 (2007).
Vali, Y. et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: A systematic review and meta-analysis. J. Hepatol. 73, 252–262 (2020).
Lichtinghagen, R. et al. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J. Hepatol. 59, 236–242 (2013).
Wong, V. W. S. et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 107, 1862–1871 (2012).
Gaia, S. et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J. Hepatol. 54, 64–71 (2011).
Petta, S. et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology 65, 1145–1155 (2017).
Acknowledgements
Figures were made using Servier Medical Art (licensed under CC BY 4.0). J.B. receives funding from Onderzoeksraad Vrije Universiteit Brussel and Chair Mireille Aerens for the Development of Alternative Methods. J.B. and J.M.S. take part in the EASL mentorship programme.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
J.B.: conceptualization, visualization, methodology, interpretation, writing—original draft, writing—review and editing. H.H.: interpretation, input of critically important information, writing—review and editing. D.R.C.: interpretation, input of critically important information, writing—review and editing. J.M.S.: conceptualization, supervision, project administration, methodology, interpretation, writing - review and editing.
Corresponding author
Ethics declarations
Competing interests
J.B. reports research funding from Colgate-Palmolive. H.H. reports research funding from AstraZeneca, EchoSens, Gilead, Intercept, MSD, Novo Nordisk and Pfizer. He has served as a consultant for AstraZeneca and Novo Nordisk, and has been or is part of hepatic events adjudication committees for Arrowhead, Boehringer Ingelheim, KOWA and GW Pharma. D.R.C. is an employee of the Global Liver Institute, which convenes the NASH Council, has received grants and sponsorships from several companies in the NASH therapeutic space, and is an advisor to PathAI and Chronwell. J.M.S. reports consulting for Alentis, Alexion, Altimmune, Astra Zeneca, 89Bio, Bionorica, Boehringer Ingelheim, Gilead Sciences, GSK, Ipsen, Inventiva Pharma, Madrigal Pharmaceuticals, Lilly, MSD, Northsea Therapeutics, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, and Siemens Healthineers. speaker honorarium from AbbVie, Academic Medical Education (AME), Boehringer Ingelheim, Echosens, Forum für Medizinische Fortbildung (FOMF), Gilead Sciences, MedicalTribune, MedPublico GmbH, MedScape, Novo Nordisk, Madrigal Pharmaceuticals, Stockholder options: AGED diagnostics, and Hepta Bio.
Peer review
Peer review information
Communications Medicine thanks Michael Betel, James Esteban and Jeff McIntyre for their contribution to the peer review of this work. [Peer review reports are available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boeckmans, J., Hagström, H., Cryer, D.R. et al. The importance of patient engagement in the multimodal treatment of MASLD. Commun Med 5, 148 (2025). https://doi.org/10.1038/s43856-025-00871-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00871-1