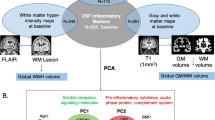
Abstract
Background
Rapamycin has been shown to extend lifespan and acts on pathologies underlying Alzheimer’s disease and related dementias in animal models. However, rapamycin’s clinical application remains underexplored.
Methods
We conducted a single-site open-label phase 1 clinical trial (ClinicalTrials.gov: NCT04200911) to examine the effects of rapamycin in humans. Eligible participants were people 55-85 years old with mild cognitive impairment or early-stage dementia, which was defined as having a Global Clinical Dementia Rating Scale Score of 0.5–1. All participants received rapamycin (1 mg/day) for eight weeks. The primary aim was to evaluate rapamycin’s central nervous system penetrance by assaying drug levels in the cerebrospinal fluid (CSF) before and after treatment. Secondary aims evaluated safety, cognition, Alzheimer’s disease, and inflammatory biomarkers in the CSF and plasma.
Results
In ten participants (mean age 74 ± 4 years, 60% female), we find that rapamycin is not detectable in the CSF before or after treatment. After treatment, we find that twenty, mostly mild adverse events occur, systolic blood pressure and hemoglobin A1c increase, multiple erythrocyte parameters decrease, and there are no significant cognitive changes. Furthermore, we find that CSF phosphorylated tau-181 (mean change (95% confidence interval) pg/ml), 2.64 [0.70–4.59]), glial fibrillary acidic protein (6262.21 [3787.44–9373.84]), and neurofilament light (367.19 [204.28–561.61]) and plasma interferon gamma (4.37 [3.01–5.74]), interleukin 5 (0.33 [0.12–0.64]), vascular endothelial growth factor D (3741.03 [1505.98–5976.07]), soluble fms-like tyrosine kinase-1 (258.88 [89.03-428.74]) and placental growth factor (20.81 [12.38–29.25]) significantly increase (FDR-corrected p-value < 0.05).
Conclusions
Rapamycin is not detectable in the CSF before or after treatment, but several Alzheimer’s disease and inflammatory biomarkers increase after treatment. Our results highlight the need to better understand the biological effects and clinical impact of repurposing rapamycin for Alzheimer’s disease.
Plain language summary
The drug rapamycin has been shown to increase longevity and reverse changes in the brain associated with Alzheimer’s disease and related dementias in animal models. However, rapamycin’s role in the clinical setting is unclear. Here we show data from a phase 1 clinical trial in ten participants with mild cognitive impairment or Alzheimer’s disease who were treated with rapamycin (1 mg/day) for eight weeks. Findings show that rapamycin levels were not detectable in cerebrospinal fluid before or after treatment. All participants knew they were receiving rapamycin and did not experience any serious negative health events due to the treatment. Additionally, several Alzheimer’s disease and inflammatory biomarkers were increased from baseline to post-treatment. These results highlight the need to better understand the impact of rapamycin on Alzheimer’s disease in humans.
Similar content being viewed by others
Introduction
Dementia is among the top five leading causes of mortality in adults aged 65 and older1. Most dementia is due to multiple etiologies, with the two most common underlying pathologies being Alzheimer’s disease (AD); defined by the excessive brain accumulation of amyloid beta (Aβ) and hyperphosphorylated tau proteins, and vascular contributions to cognitive impairment and dementia. A recent landmark development has been the emergence of anti-amyloid monoclonal antibodies that reduce cerebral Aβ accumulation and exert downstream effects on tau2. However, these treatments have shown only modest efficacy in slowing the rate of cognitive decline. This may be due in part to the multiple pathophysiological changes in clinical dementia, highlighting the need to explore pharmacological agents that target broader mechanisms3. Aging is, by far, the strongest risk factor for sporadic AD4. Biological processes underlying aging emerge in clinical dementia, including changes in the cerebral vasculature and metabolism, blood-brain barrier integrity, neuronal and glial senescence, immune activation and inflammation, and mitochondrial and endolysosomal dysfunction. These biological changes, along with synaptic loss and synergistic adverse interactions among multiple proteinopathies (alpha-synuclein, TDP-43, among others)5, have been implicated in the spectrum of AD and related dementias (ADRD). Preclinical research suggests that targeting the biology of aging may offer novel therapeutic approaches to ADRD4.
The FDA-approved immune-modulator, rapamycin (i.e., sirolimus), has potent effects on longevity, increasing lifespan in multiple species even when administered for short durations or beginning at older ages6. Rapamycin is an allosteric inhibitor of the mechanistic target of rapamycin (mTOR) complex I (mTORC1), a serine-threonine kinase that has diffuse effects on cell signaling pathways governing metabolism, growth, and proliferation, autophagy, and protein synthesis7. mTOR complex 2 (mTORC2), which activates cell metabolism and survival pathways, is relatively insensitive to the acute effects of rapamycin, but may be inhibited by prolonged rapamycin use8. In preclinical models, rapamycin attenuates the progression of multiple age-related chronic conditions, including kidney disease, cardiac dysfunction, macular degeneration, and cancer9. Rapamycin also modulates cellular and molecular processes implicated in AD. Through its inhibition of mTORC1, rapamycin increases autophagy, which is critical for the clearance of misfolded Aβ and tau aggregates. In transgenic AD mouse models (3xTg-AD and hAPP (J20)), rapamycin has been shown to upregulate Aβ and tau clearance, attenuate microglial activation, dampen neuroinflammation, enhance nitric oxide-dependent cerebral blood flow, and enrich vascular density10,11,12,13. In humans, postmortem studies have indicated hyperactivation of the phosphoinositide 3-kinase (PI3K)/Akt/mTOR pathway in the cortices and hippocampi of individuals with mild cognitive impairment (MCI) and dementia due to AD, which worsens with disease progression14. A bioinformatics analysis examining repurposed medications that may modulate ADRD-related gene expression identified mTOR inhibitors as a leading candidate15, and rapamycin use has been associated with reduced likelihood of dementia in retrospective analyses of electronic health record data16.
In light of rapamycin’s pleiotropic effects on key processes linked to ADRD pathogenesis, it has been proposed as a potential, yet, untested, translational therapeutic17. A benefit of using repurposed drugs, like rapamycin, is that the safety profile has already been examined, potentially advancing clinical trials for AD by several years18. Rapamycin first received FDA approval in 1999 as a prophylactic against organ rejection following renal transplantation. It is now also approved for the treatment of pancreatic cancer and for the prevention of restenosis after angioplasty7. The most commonly reported side effects are mouth sores, acneiform rash, hypercholesterolemia, and hyperlipidemia, which occur in 5–20% of patients7,19. However, most safety data have been collected from adults with other serious medical conditions, often following solid organ transplantation17. To address this limitation, Kraig et al. conducted an 8–16-week randomized placebo-controlled study of rapamycin (1 mg/day) in generally healthy older adults aged 70–95 years20. The treatment was generally well-tolerated with no clinically significant changes in laboratory values. In addition, a recently completed 18-week randomized placebo-controlled trial of rapamycin (1 mg/m2/day or 2 mg/m2/day) for amyotrophic lateral sclerosis (ALS) also reported an acceptable safety profile21, as have studies with other mTOR inhibitors in generally healthy older adults22,23.
Survey data indicate that rapamycin is being used as an off-label treatment for longevity-promotion and AD prevention despite the absence of well-controlled empirical studies in humans24. The popularized use of rapamycin brings urgency to rigorously evaluating its safety and potential efficacy. Given the promising safety data from recent trials for other indications20,21, coupled with the supportive data from preclinical and post-mortem studies10,11,12,13,14, we now conduct an eight-week open-label pilot trial with rapamycin (1 mg/day) in participants with a clinical diagnosis of mild cognitive impairment (MCI) or dementia of the Alzheimer’s type. The 1 mg/day dose of rapamycin is selected based on our prior data in older adults demonstrating acceptable tolerability using this dosing regimen20. The primary aim of the study is to evaluate the central nervous system (CNS) penetrance of the drug by performing mass spectrometry on cerebrospinal fluid (CSF) collected prior to treatment and within 20–60 min of the final study drug dose. We hypothesize that orally administered rapamycin would penetrate the CNS based on previous animal model10 and clinical data from individuals with glioblastoma25. Our secondary endpoints are to examine safety and tolerability, baseline to post-treatment changes in CSF and plasma ADRD and inflammatory biomarkers, and changes in cognition, functional status, and physical functioning. CSF ADRD biomarkers are measured using automated Simoa assays (Quanterix, Lexington, MA). As an exploratory outcome that was not pre-specified in the protocol, ADRD biomarkers in CSF are further interrogated using high-resolution tandem mass spectrometry (HRMS) assays developed at Washington University in St. Louis (WashU)26,27. The HRMS assays quantified distinct Aβ species28,29, tau and phosphorylated tau (p-tau) isoforms26,27, and microtubule-binding region (MTBR) of tau residues30, which can be leveraged to enhance understanding of the intervention’s effect on specific neuropathological changes.
Methods
The study was an open-label single-site phase 1 clinical trial of rapamycin (ClinicalTrials.gov NCT04200911) in older adults with a clinical diagnosis of MCI or mild dementia due to AD conducted at the University of Texas Health at San Antonio. Participants were recruited from the University of Texas Health Science University Department of Neurology, outside clinics, and media postings. The primary outcome was to determine whether the drug penetrated the CNS and was detectable in the CSF. The secondary outcomes were: (1) examine the safety and tolerability of the intervention in this population, (2) investigate baseline to post-treatment changes in ADRD and inflammatory biomarkers in plasma and CSF; and (3) assess changes in cognition, functional status, and physical functioning from baseline to post-treatment. As an exploratory outcome that was not pre-specified in the protocol, CSF ADRD biomarkers were further evaluated using HRMS assays to better understand changes in distinct Aβ species28,29, tau and phosphorylated tau (p-tau) isoforms26,27, and MTBR of tau residues30. The trial was conducted in alignment with the Guideline for Good Clinical Practice, and the protocol was approved by the University of Texas Health at San Antonio Institutional Review Board. All participants provided written informed consent with appropriate legal representation for individuals who lacked the capacity to consent. The San Antonio Older Adults Independence Center Data Safety and Monitoring Board provided oversight on safety throughout the course of the study. An Investigational New Drug application for off-label use of rapamycin for AD was submitted to the FDA and received a letter of exemption (IND 146446). The study started on 06/01/2020 and was completed on 01/13/2022. Participants were enrolled in the study between 10/29/2020 and 12/16/2021.
Participants
Ten participants (mean age 74 ± 4 years, 60% female) completed the trial. A sample size of 10 was established a priori based on feasibility, given the budgetary and timeline constraints of the pilot study. The minimum detectable effect size to achieve 80% power with a paired samples t-test and a two-sided significance level of p < 0.05 is 1.07, a large effect size31.
Inclusion criteria for the study included adults aged 55–85 years with a clinical diagnosis of MCI or mild dementia due to AD based on the National Institute on Aging-Alzheimer’s Association criteria32 and a Global Clinical Dementia Rating (CDR) Scale score of 0.5 or 133. Coexisting vascular pathology was not a contraindication to enrollment in the study, although no individuals with post-stroke vascular cognitive impairment or dementia were enrolled. Anticholinesterase inhibitors and/or memantine use were permitted following at least a three-month stabilization period before baseline assessments. A study partner and/or legally authorized representative (LAR) was required to attend all study visits with the participant. Exclusion criteria included several medical conditions and treatments that may increase the likelihood of AEs associated with rapamycin use. Full inclusion and exclusion criteria are presented in Supplementary Data 1.
Study design
Participants enrolled in the study completed eight study visits over a 12- to 18-week period (Supplementary Fig. 1). Following the informed consent process, study candidates underwent a screening visit consisting of a non-fasting blood draw, vital signs and anthropomorphic measures, medical history and concomitant medication review, physical and neurological examination, and cognitive assessment (CDR, Mini Mental State Examination). Following confirmation of study eligibility, participants completed two baseline assessment visits. The first consisted of a fasting blood draw and lumbar puncture (Baseline Visit 1), and the second included assessments of cognition and functional status (Baseline Visit 2). Within 15 days of Baseline Visit 2, participants began the eight-week treatment regimen of 1 mg of rapamycin (Rapamune™ (sirolimus), Pfizer) per day, administered in the morning after an overnight fast. The dosing regimen was selected based on our prior research in generally healthy older adults demonstrating adequate safety and tolerability and attainment of blood concentrations within the physiologically relevant range20. During the eight-week intervention period, participants and their study partners/LARs completed three safety monitoring visits consisting of fasting blood draws, AE assessment, and compliance review. Participants and their study partners were provided with a calendar to mark the time of day the medication was taken, and pill bottles were returned for medication accounting. As rapamycin has low bioavailability and is rapidly cleared in the periphery34, CSF for drug concentration assays was collected by lumbar puncture within 20–60 min after the final study drug dose was administered onsite in the morning after an overnight fast. The lumbar puncture was followed by a blood draw (within 60–100 min of the final dose; Visit 7). For one participant, a protocol deviation occurred, and the final dose of rapamycin was not administered until after the lumbar puncture and blood draw. Consistent with the prespecified intention-to-treat analysis plan, this participant was retained in the analyses. Within 7–14 days of the final study drug dose, participants completed the CDR and all assessment procedures performed at Baseline Visit 2 (Visit 8).
The protocol was modified during the COVID-19 pandemic to include confirmation of a negative real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) test within 72 h before treatment initiation. COVID-19 symptoms and exposure were monitored before and after each study visit. Participants were encouraged to receive the COVID-19 vaccine (Tozinameran® or mRNA-1273®) as soon as it was available. Participants were advised to discontinue the study drug four days prior to and three days after a COVID-19 vaccination in an attempt to mitigate the possibility of dampened immune responses.
Lumbar punctures and blood draws
Collection of CSF and blood research specimens was conducted in alignment with established procedures35. Briefly, lumbar puncture was performed by a licensed physician using a 24-gauge atraumatic Sprotte spinal needle under gravity flow (Teleflex, Morrisville, NC). CSF was collected into a sterile polypropylene tube (Rose Scientific, Alberta, CA) and then centrifuged at 2000×g at room temperature for 10 min. Blood was collected via venipuncture in a plasma EDTA vacutainer tube (BD, Franklin Lakes, NJ) and inverted 5–10 times prior to being centrifuged at 2000 x g at room temperature for 10 min. All CSF and blood specimens collected for research purposes were aliquoted and stored at −80 °C within 2 h of collection.
Safety
Vital signs, concomitant medications, and adverse event reviews were conducted at all study visits. Complete blood count (CBC) with differentials was performed at Visits 1, 3, 4, 6, and 8. A comprehensive metabolic panel (CMP) with lipids was conducted at all visits except the Baseline Assessment Visit 2. HbA1c was assessed at baseline, prior to study drug initiation (Visit 4), and post-treatment (Visit 8). PT/PTT/INR was performed at visits immediately preceding visits with lumbar punctures (Visits 1 and 6). In a subset of participants, safety labs (glucose, protein, nucleated cells, and red blood cells) were performed on CSF.
Cognitive and functional outcomes
Global cognitive endpoints included the CDR Sum of Boxes (SOB)33 and the Montreal Cognitive Assessment (MoCA36). In addition, participants completed the Hopkins Verbal Learning Test Revised (HVLT-R)—a measure of verbal learning and memory)37, the Hayling Test—a measure of inhibitory control38, and the National Alzheimer’s Coordinating Center (NACC) Uniformed Dataset-3 Neuropsychological Battery39, which includes measures of verbal and visual memory (Craft Story Immediate and Delayed Recall, Benson Figure Delayed Recall), attention/working memory (Number Span Forward and Backwards), processing speed/executive function (Trail Making Test Parts A & B), visuo-construction (Benson Figure Copy), and language (Phonemic and Semantic Fluency, Multilingual Naming Test (MINT)). Neuropsychiatric symptoms were evaluated using the self-reported Geriatric Depression Scale 15-Item (GDS-15)40 and informant-reported Neuropsychiatric Inventory (NPI)41. Functional status was assessed using the Functional Assessment Questionnaire (FAQ)42 and as part of the CDR. Physical functioning was assessed by evaluating grip strength in the dominant and non-dominant hands.
Assays
All assays were conducted by qualified laboratory technicians who were blinded to participant ID and treatment timepoint (baseline vs post-treatment).
Drug concentration quantification
Rapamycin concentrations in baseline and post-treatment specimens were determined using High Performance Liquid Chromatography (HPLC, Shimadzu SIL 20A HT autosampler) with Tandem Mass Spectrometry (AB Sciex API 4000) detection (MS/MS) method (HPLC/MS/MS) at the UT Health San Antonio by the San Antonio Pepper Center Clinical Core, Analytical Pharmacology Lab. Rapamycin, ascomycin (internal standard), and all analytical-grade reagents were purchased from Millipore Sigma (St. Louis, MO, USA). CSF calibrator samples were prepared by spiking blank CSF aliquots with rapamycin at concentrations of 1.56, 3.13, 6.25, 12.5, 25, 50, and 100 ng/ml. Fifty µl of calibrator samples and unknown samples were then mixed with 5 µl of 0.5 µg/ml ascomycin and 20 µl of mobile phase A. The samples were vortexed vigorously, and then 10 µl of each sample was injected into the HPLC system. The ratios of rapamycin peak areas to ascomycin peak areas for each unknown sample were compared against a linear regression of the ratios obtained from the calibrator samples to quantify rapamycin. The rapamycin ion transition from parent ion 931.6 m/z to daughter ion 864.5 m/z was detected and used for quantification in positive mode. The ascomycin internal standard ion transition from parent ion 809.6 m/z to daughter ion 756.6 m/z was also detected in the same mode, always at the same concentration.
ADRD biomarkers
CSF and plasma levels of Aβ40, Aβ42, glial fibrillary acidic protein (GFAP), and neurofilament light (NfL) were assayed using the Simoa Neurology 4-Plex E Advantage kit (Quanterix, Lexington, MA). In the protocol, total tau was also listed as a secondary outcome. However, in 2020, a landmark article was published demonstrating the superiority of plasma phosphorylated tau (p-tau) 181 over even CSF total tau concentrations for AD diagnostic discriminability43. Therefore, we transitioned from total tau to p-tau 181 as a secondary outcome, which was assayed using the Simoa p-Tau 181 Advantage V2 kit (Quanterix, Lexington, MA). The assays were run in duplicate using a Simoa HD-X analyzer (Quanterix, Lexington, MA) as previously described44. In addition, as exploratory outcomes, CSF Aβ, tau, and p-tau proteoforms, and MTBR-tau residues were measured using the WashU HRMS assays26,27,29,30,45. Due to limited remnant sample availability, Aβ HRMS assays were conducted on seven participants, and tau, p-tau, and MTBR-tau HRMS assays were completed on eight participants.
Inflammatory biomarkers
Inflammatory biomarkers in CSF and plasma were assayed using the MesoScale Discovery V-PLEX Neuroinflammation Panel 1 Human Kit (MesoScale Discovery, Rockville, MD). Samples were diluted as recommended by the manufacturer and measured in duplicate using the MESO QuickPlex SQ 120MM instrument. Several of the inflammatory biomarkers were at or below the assay's lower limit of detection, resulting in average coefficients of variance exceeding 20%. These markers were excluded from the analysis.
Statistics and reproducibility
Data were collected in REDCap. Demographic and clinical characteristics of the sample (N = 10) at baseline were assessed using descriptive statistics. Shapiro-Wilk tests were used to evaluate normality. Baseline to post-treatment changes in safety labs, vitals, and body mass index (BMI), biofluid assays, and cognitive and functional assessments were evaluated using paired samples t-tests or Wilcoxon signed rank tests when normality assumptions were violated. Statistical tests were 2-sided with the level for statistical significance set at p < 0.05. FDR-correction for multiple comparisons was applied to cognitive and biomarker outcomes46. FDR-correction was not performed on safety data to be conservative when reporting any potential safety risks associated with treatment. Statistical analyses were performed using SAS version 9.4, and figures were created using GraphPad version 9.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Participants
Thirty-four participants were screened by phone. Of these, 15 did not meet the eligibility criteria (see Supplementary Fig. 2) while the other 19 completed an in-person screening visit. After in-person screening, there were five additional screen failures and four other individuals were withdrawn prior to the start of the intervention [N = 1 suspected transient ischemic attack (TIA), N = 1 recurrent urinary tract infections, N = 1 increasing liver enzymes, N = 1 unsuccessful lumbar puncture], leaving a total of 10 participants (mean age 74 ± 4; 60% female; Supplementary Table 1 (consistent color coding across figures) who were enrolled in the intervention)). All participants identified as non-Hispanic white and had completed some college (10%) or were college graduates or higher (90%). At baseline, 70% of the participants met criteria for MCI and 30% met criteria for mild dementia. Based on medication and drug accountability logs, the average medication adherence rate over the eight-week trial was 92 ± 5%.
Primary outcome—CNS penetrance of rapamycin
Rapamycin was not detectable in study participant CSF at baseline or post-treatment, however, it was measured in the positive control samples where rapamycin was spiked in at various concentrations (see Methods for additional detail).
Secondary outcomes—safety and tolerability
Over the course of the study, 20 Adverse Events (AEs) were recorded (Table 1). The majority of AEs were of mild severity (N = 16) and deemed unlikely to be related to the study. Two AEs (urinary urgency and diarrhea), both rated as mild, were deemed possibly related to the study. All AEs fully resolved, and only one (edema) required a temporary cessation of the study drug. The overall participant retention rate was 100% during the intervention period.
Assessment of hematological studies indicated that all parameters remained within the normal range, although statistically significant reductions in red blood cell distribution width (RDW), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were observed from baseline to post-treatment (Supplementary Data 2). Other erythrocyte parameters, including hemoglobin, hematocrit, and red blood cell count, remained stable. There was a mild, albeit statistically significant, increase in hemoglobin A1c (HbA1c) levels, as well as a trend towards lower albumin from baseline to post-treatment (Supplementary Data 2), but the post-treatment values all remained within the clinically normative range. All other safety labs were stable during the treatment period.
There were no significant changes in BMI (baseline: 24.3 ± 3.4 mg/k2; post-treatment: 24.0 ± 3.5 mg/kg2, t(9) = −1.26, p = 0.24). Systolic blood pressure increased from baseline to post-treatment (baseline: 127.8 ± 19.0 mmHg; post-treatment: 138.2 ± 22.1 mmHg, t(9) = 4.226, p = 0.002). Diastolic blood pressure did not change significantly (baseline: 67.2 ± 10.6 mmHg; post-treatment: 68.9 ± 12.0 mmHg, t(9) = 0.915, p = 0.38).
Secondary outcomes—ADRD and inflammatory biomarkers in plasma and CSF
In the unadjusted analyses of ADRD biomarkers evaluated using the Simoa platform, plasma Aβ42 levels decreased from baseline to post-treatment. However, with multiple comparisons correction, there were no significant changes in plasma ADRD biomarkers (Fig. 1a–e and Supplementary Data 3). In CSF, phosphorylated tau (p-tau) 181 (Fig. 1f), glial fibrillary acidic protein (GFAP) (Fig. 1i), and neurofilament light (NfL) (Fig. 1j) significantly increased from baseline to post-treatment (Supplementary Data 3). These findings persisted with multiple comparisons correction.
Baseline and post-treatment plasma (a–e) and cerebrospinal fluid (f–j) (N = 10 participants) biomarker changes were evaluated using paired samples t-tests or Wilcoxon signed rank tests. FDR-corrected p-values and color coding by participant are displayed. Mean difference from baseline to post-treatment (a) pTau-181: −0.22 pg/ml; (b) Aβ40: −8.56 pg/ml; c Aβ42: −0.668 pg/ml; (d) GFAP: −27.70 pg/ml; (e) NfL: 0.84 pg/ml; (f) pTau-181: 2.64 pg/ml; (g) Aβ40: 615.57 pg/ml; (h) Aβ42: 21.51 pg/ml; (i) GFAP: 6,262.21 pg/ml; and (j) NfL: 367.19 pg/ml. ADRD Alzheimer’s disease and related dementias, p-tau phosphorylated tau, Aβ amyloid beta, GFAP glial fibrillary acidic protein, NfL neurofilament light.
Assessment of inflammatory biomarkers in plasma indicated significant baseline to post-treatment increases in interferon gamma (IFN-γ), interleukin (IL)-5, vascular endothelial growth factor D (VEGF-D), soluble fms-like tyrosine kinase 1 (sFlt1), and placental growth factor (PIGF), as well as reductions in eotaxin-3 and basic fibroblast growth factor (bFGF) (Supplementary Data 4). With correction for multiple comparisons, increases in IFN-γ, IL-5, VEGF-D, sFlt1, and PIGF remain significant (Fig. 2a–e).
Baseline and post-treatment plasma biomarker (a–e) (N = 10 participants) changes were evaluated using paired samples t-tests or Wilcoxon signed rank tests. FDR-corrected p-values and color coding by participant are displayed. Mean difference from baseline to post-treatment. a INF-γ: 4.38 pg/ml; (b) IL−5: 0.33 pg/ml; (c) VEGF-D: 3741.0 pg/ml; (d) sFlt1: 258.88 pg/ml; (e) PIGF: 20.81 pg/ml. IFN‐γ interferon gamma, IL interleukin, VEGF vascular endothelial growth factor, sFLT-1 soluble fms-like tyrosine kinase, PIGF placental growth factor.
In unadjusted analyses, there were significant baseline to post-treatment elevations in CSF IL-5, IL-10, IL-15, VEGF-D, and soluble vascular cell adhesion molecule-1 (sVCAM-1) (Supplementary Data 5). None of these results were significant after multiple comparisons correction.
Secondary outcome—cognition, functional status, and physical functioning
There were no significant changes in cognition, functional status, neuropsychiatric symptoms, and physical functioning in either the unadjusted or FDR-corrected analyses (Supplementary Data 6).
Exploratory outcome—ADRD biomarkers derived from HRMS assays
In unadjusted analyses, CSF levels of Aβ40, Aβ42, tau 151-155, tau 181-190, tau 195- 210, tau 212-221, tau 226-230, pS-tau 199, p-tau 181, p-tau 217, p-tau 231, MTBR-tau 212-221, and MTBR-tau 243-254 increased from baseline to post-treatment (Supplementary Data 7). With FDR-correction, post-treatment elevations in CSF Aβ40, Aβ42, tau 151-155, tau 181-190, tau 195-210, tau 212-221, p-tau 181, p-tau 217, p-tau 231, and MTBR-tau 243-254 remained significant (Supplementary Fig. 3).
Discussion
Given the mechanistic overlap between aging and AD, there has been growing interest in targeting the biology of aging for the treatment of AD4,17. Rapamycin is one of the best studied longevity-enhancing drugs in animal models with pleiotropic effects that may modulate multiple processes dysregulated in ADRD, including autophagy, cellular metabolism, inflammation, and cellular senescence47. We herein present the results of an in-human trial of rapamycin for clinical AD. As an open-label pilot study, our primary aim was to evaluate CSF levels and the safety of rapamycin in older adults with ADRD. Our mass spectrometry assays did not detect rapamycin in study participant CSF, which is consistent with the agent’s high lipophilicity48. There were, however, statistically significant changes in ADRD and inflammatory biomarkers over the eight-week study duration. Without a placebo-controlled group, we are unable to clearly determine if the outcomes observed are due to the study drug, natural disease progression, and/or other unexplored factors, highlighting the need for further research.
The primary aim of the study was to evaluate the CNS penetrance of rapamycin. Our study did not detect rapamycin in CSF before or after treatment; which is consistent with prior findings21. Rapamycin is highly lipophilic48, which may aid its permeability through the blood-brain barrier49. However, protein-binding characteristics also impact CNS pharmacokinetics and pharmacodynamics. Drugs with low plasma protein binding, as is characteristic of rapamycin, typically aggregate intracellularly in brain tissue49,50. Therefore, it is possible that rapamycin penetrated the CNS, but unbound drug concentrations in CSF were below the limit of detection. In preclinical studies, rapamycin has been detected in brain tissue following oral administration10. Similarly, in humans, a clinical trial of rapamycin for the treatment of PTEN-deficit glioblastoma identified the drug in resected tumor tissues with concentrations ranging from 2.7 to 27.1 ng/ml25. The investigators also demonstrated downstream inhibition of mTOR activity following treatment, coupled with a reduction in tumor cell proliferation in 50% of the study sample. Therefore, there is evidence in some patient populations that rapamycin can cross the blood-brain barrier and impact physiological processes in the CNS. Alternatively, it is possible that rapamycin may exert CNS effects through peripheral mechanisms. In circulation, 95% of the drug is retained in erythrocytes, with only about 3% sequestered in plasma51. Further assessments of rapamycin’s pharmacokinetics and pharmacodynamics in CSF are necessary to inform trial design. Future studies may benefit from incorporating novel methodology, such as the identification of downstream biomarkers and use of modeling and simulation methods49. In addition, neuroimaging methods such as FDG-PET may serve as valuable proxies to evaluate the central nervous system effects of rapamycin, which is an approach that is currently underway in other trials (NCT04629495, NCT06022068).
A secondary aim of the study was to evaluate the safety and tolerability of the intervention. In general, the treatment was well-tolerated with no premature discontinuation of the study drug. A total of 20 AEs were recorded over the course of the study, and the majority were mild, consisting of ailments fairly common in the older adult population. One serious AE, a suspected TIA, occurred prior to study drug initiation, resulting in withdrawal from the study. The two AEs deemed possibly related to the intervention, urinary urgency, and diarrhea, fully resolved over the course of the study. In our earlier placebo-controlled study of rapamycin in generally healthy older adults who received the same treatment dose, the medication was well-tolerated with three AEs occurring in the active treatment arm, including gastrointestinal distress, stomatitis, and facial rash20. More significant side effects, including poor wound healing, anorexia, hyperglycemia, hypercholesterolemia, anemia, thrombocytopenia, and pneumonitis, have been reported with rapamycin use following liver transplantation and cancer treatment19,20. In addition to the differences in study populations, our trial utilized a low treatment dose with a short intervention period. Lower dosages of rapamycin have been linked to reduced likelihood of AEs51. While the results suggest that individuals with MCI and dementia do not appear more susceptible to adverse events than other older adults, rigorous monitoring will be important to ensure safety in trials using different dosing regimens or longer intervention durations.
In our study, we observed a statistically significant increase in systolic blood pressure from baseline to post-treatment without concomitant changes in diastolic blood pressure. As systolic blood pressure > 160 mmHg was an exclusion criterion for study participation, regression to the mean may be contributing to the observed change. In animal model studies, conflicting reports have emerged with rapamycin both increasing52,53 and decreasing54 blood pressure. In renal transplant recipients taking generally high dosages of rapamycin, hypertension has been reported as an adverse event, occurring in 8-58% of treated individuals55. In our prior placebo-controlled study of rapamycin in healthy older adults, no statistically significant differences in blood pressure were observed20. Therefore, further examination of systolic blood pressure in clinical trials of rapamycin for older adults with MCI and AD are needed. In our study, we did not observe clinically significant changes in safety labs. However, several erythrocyte parameters, including RDW, MCV, and MCH, demonstrated a statistically significant decrease from baseline to post-treatment. Our results are largely consistent with our prior trial in generally healthy older adults, which demonstrated decrements in red blood cell count, hemoglobin, hematocrit, RDW, MCV, and MCH in the active treatment relative to the placebo arm20. Changes in erythrocyte parameters have been observed in association with rapamycin use in transplant recipients, with an estimated 27–57% developing thrombocytopenia and/or anemia56,57. The mechanisms linking rapamycin to hematological changes are not fully known, but have been attributed to possible suppression of bone marrow progenitor cells or functional iron deficiency57. Since rapamycin is FDA-approved as an immunosuppressant, the potential of undesired immune effects when administered for other indications needs to be considered7. In our study, we did not observe changes in white blood cell counts consistent with our prior trial of rapamycin in generally healthy older adults20. Additional evidence against immunosuppressive effects with low-dose treatment is provided by a vaccine trial of an mTOR inhibitor that reported decreased infection rates and better immune function in the active treatment arm relative to placebo22.
We observed a small, albeit statistically significant, increase in HbA1c levels from baseline to post-treatment, with values remaining in the clinically normative range. Hyperglycemia associated with rapamycin use has been documented in some animal models58, as well as in individuals taking higher doses of mTOR inhibitors following transplant or for cancer treatment59. Our prior trial of rapamycin in generally healthy older adults similarly reported larger increases in HbA1c levels from baseline to post-treatment in the active treatment relative to the placebo arm, with values remaining in the clinically normative range. Interestingly, in our prior work, rapamycin did not affect insulin sensitivity as assessed by the HOMA-IR and the Matsuda index. The impact of rapamycin on post-transplant diabetes mellites continues to be debated, and the biological mechanisms remain poorly understood60. Inhibition of mTORC2 with prolonged rapamycin use has been hypothesized to contribute to glucose dysregulation, prompting investigations of safety profiles associated with intermittent rapamycin use61.
To collect preliminary data on efficacy to inform future trials, we evaluated baseline to post-treatment changes in plasma and CSF ADRD biomarkers using Simoa assays as a secondary outcome. We observed statistically significant increases in CSF p-tau 181, NfL, and GFAP over the eight-week study interval, which persisted with multiple comparisons correction. P-tau 181 is a well-validated AD marker that correlates with cerebral Aβ and, to a lesser extent, with cerebral tau burden45. NfL, a marker of neuro-axonal injury, and GFAP, a marker of reactive astrogliosis, change dynamically in response to acute injury and are elevated in numerous neurological conditions62. Higher levels of p-tau 181, NfL, and GFAP have been associated with accelerated cognitive decline, atrophy, and disease progression among individuals with MCI and AD63,64,65,66. In our study, we did not observe statistically significant changes in plasma p-tau 181, NfL, or GFAP levels. Prior studies have demonstrated that plasma GFAP and NFL may have stronger associations with cerebral Aβ levels than CSF measures67,68. However, concentrations of these proteins in plasma are lower than in CSF, and larger sample sizes may be necessary to detect changes in these outcomes. Our study also lacked a placebo-controlled group, which is necessary to clearly distinguish changes in biomarker levels in response to treatment relative to the natural course of ADRD progression. Few studies have longitudinally examined ADRD CSF biomarker changes over short durations as employed by our study. Over a mean of 2.1 years, Lleo et al. examined longitudinal changes in p-tau 181 using the Fujirebio Europe INNOTEST assay and NfL using the Uman Diagnostics assay. When restricting the sample to individuals with AD, CSF p-tau 181 levels remained unchanged, and NfL increased at an annual rate of 4%69. Other studies have similarly reported stability in p-tau 181 in individuals with AD over a 6-month follow-up period70. The findings of higher CSF p-tau 181, NfL, and GFAP from baseline to post-treatment in our study were inconsistent with our hypothesis, as rapamycin has been shown to enhance autophagy, and increase clearance of Aβ and tau in mouse models of AD10,71,72,73,74,75,76. While well-designed preclinical trials are essential for treatment innovations, animal models do not fully recapitulate the neurodegenerative disease process in humans, and findings may fail to translate across species.
As exploratory outcomes, HRMS CSF assays were performed, indicating post-treatment increases in Aβ40, Aβ42, tau 181-190, tau 195-210, tau 212-221, p-tau 181, p-tau 217, p-tau 231, and MTBR-tau 243-254. Lower CSF Aβ40 and Aβ42 levels are observed among individuals with AD relative to controls, which is presumed to reflect less efficient cerebral Aβ clearance among individuals with AD77. Although the Aβ42:40 ratio is known for its high accuracy in distinguishing AD78, the ratio remained unchanged in this study, likely due to the similar increases observed in both Aβ species. Consistent with the findings from the Simoa assays, CSF p-tau 181 levels significantly increased, as did CSF p-tau 217 levels, which have high diagnostic accuracy for AD and correlate with both cerebral amyloid and tau PET retention45. The HRMS assays also enable assessment of changes in tau phosphorylation occupancy rates relative to the total tau protein level, which has been demonstrated to have better discriminability for accelerated tau hyperphosphorylation in AD27. Interestingly, in our study, the ratios of p-tau 181 to tau 181 and p-tau 217 to tau 217, respectively, were not significant, suggesting concomitant increases in both the phosphorylated and total tau protein levels for these isoforms. We also observed increases in multiple tau residues, including tau 151–155, and tau 181–190. Based on previous studies, specific residues in human brain tissue, such as tau 181, 205, and 217 and MTBR-tau 243–254, have been demonstrated to be among the most enriched for phosphorylated residues in insoluble tau30,79, which aggregates into neurofibrillary tangles.
As a secondary outcome, we examined changes in plasma and CSF inflammatory markers, observing significant increases in pro-inflammatory and angiogenic cytokines over the eight-week study course. In plasma, we detected significant baseline to post-treatment increases in plasma IFN-γ, IL-5, VEGF-D, sFLT-1, and PIGF, which survived multiple comparisons correction. IFN-γ is a cytokine that exerts effects on innate and adaptive immunity, apoptosis, and tumor suppression80,81. IFN-γ interacts with the mTOR signaling pathway81 and, in older adults, pharmacological inhibition of mTOR has been shown to upregulate IFN-γ gene expression23,82. In cognitively unimpaired adults, higher plasma IFN-γ has been associated with better cognitive trajectories, which has been attributed to the possibility of enhanced protection from pathogens that may be capable of crossing the blood-brain barrier83. In contrast, among individuals with AD, higher plasma IFN-γ levels have been associated with accelerated cognitive decline84, which may reflect systemic immune dysregulation with disease progression85. VEGF-D and PIGF are angiogenic cytokines, which are integral to blood vessel development, growth, and maintenance86. It is possible that higher VEGF-D and PIGF may reflect increased vessel enrichment and blood flow. However, VEGF is also upregulated in the context of chronic hypoperfusion, and in meta-analysis, elevations in circulating VEGF levels have been reported in individuals with AD relative to age-matched controls87. Unlike our findings, rapamycin was shown to significantly decrease VEGF-D in individuals with lymphangioleiomyomatosis, suggesting that treatment effects may vary based on the underlying disease condition88. PIGF is a soluble protein that has been recognized by the MarkVCID consortium as a leading biomarker for vascular cognitive impairment and dementia89,90. Prior studies have demonstrated that higher circulating levels of PIGF are associated with elevated white matter hyperintensities burden and accelerated cognitive decline90. In our prior clinical trial of rapamycin in generally healthy older adults20, no statistically significant changes in serum cytokine levels were observed. However, TNF-α levels increased in approximately half of the participants in the rapamycin treatment arm. This trend was not observed in the placebo group, suggesting that rapamycin may exert heterogeneous inflammatory effects across individuals. A recent placebo-controlled clinical trial of rapamycin for ALS reported that the active arm displayed lower plasma and mRNA expression of IL-1821, suggesting an anti-inflammatory effect. The divergence in findings across studies provides evidence that rapamycin may elicit a differential impact on peripheral inflammation depending on the study population, treatment dose, and duration20,91. Moreover, different pro-inflammatory mediators are controlled by diverse mechanisms, only some of which are likely to involve mTOR.
In CSF, pro-inflammatory cytokines, including IL-5, IL-10, IL-15, VEGF-D, and sVCAM-1 increased from baseline to post-treatment, but did not survive multiple comparisons correction. Rapamycin has been shown to dampen neuroinflammation in animal models92. However, rapamycin in cell culture has been demonstrated to induce complex and at times, opposing effects on neuroinflammation93. In a study of lipopolysaccharide (LPS)-exposed BV2 microglial cells, rapamycin downregulated nitric oxide synthase 2 (NOS2) and IL-6, but increased IFN-γ, IL-12, and tumor necrosis factor alpha (TNF-α) mRNA expression94. In human microglial cells, rapamycin has similarly been shown to activate both pro- and anti-inflammatory pathways93, suggesting that the molecular responses to rapamycin are complex and may vary due to the cells examined, the inflammatory stimulus, and the basal state of the inflammatory pathways.
Regarding the secondary outcomes of cognition, functional status, and physical functioning, there were no statistically significant changes from baseline to post-treatment over the 8-week intervention. Due to the gradually progressive disease course and heterogeneity in rates of decline and underlying neuropathologies across individuals, large sample sizes and follow-up durations of at least 18-months have been recommended for detecting clinically meaningful cognitive change in trials for AD95. Therefore, future work is necessary to further evaluate the effect of rapamycin on cognition among individuals with ADRD. In our prior clinical trial of rapamycin in generally healthy older adults, there were no significant differences in cognition between the active treatment and placebo-controlled groups20. In transplant recipients, mTOR antagonists have been associated with improved cognitive performance and reduced likelihood of dementia16,96. However, rapamycin’s impact on multiple physiological processes has been shown to substantially vary and even exert opposing effects across different study populations. Therefore, the findings observed in other studies may not generalize to individuals with AD.
Conclusion
In summary, rapamycin was not detected in CSF before or after treatment. These results highlight avenues for modifications in future studies to better assess the CNS penetrance of rapamycin. In our study, short-term low-dose rapamycin treatment was generally well-tolerated in older adults with MCI or mild dementia due to a clinical diagnosis of AD. However, there were statistically significant changes in systolic blood pressure, HbA1c, and erythrocyte parameters that warrant monitoring in future placebo-controlled trials. There were also statistically significant baseline to post-treatment elevations in multiple ADRD in CSF and inflammatory biomarkers in plasma. Our pilot study was not designed to evaluate efficacy, and the interpretations of the findings are limited by several factors, including the small sample size, lack of a placebo control group, and the short follow-up duration. In addition, participants were enrolled based on a clinical diagnosis of MCI or mild dementia due to AD, which may have enabled inclusion of persons with various mixed dementias. The CSF ADRD biomarker assays employed were not conducted in a certified clinical laboratory and lack established diagnostic cut-points, so additional pathologies may contribute to heterogeneity in results. A randomized placebo-controlled study of rapamycin for MCI and AD is currently underway (NCT04629495) that incorporates broader assessments, including Aβ and FDG PET imaging. It is also worth noting that several geroscience-guided interventions are testing intermittent treatment paradigms, which are currently being evaluated in a clinical trial of rapamycin for AD (NCT06022068)44,97. In addition, rapamycin may exert differential effects depending on the mixed pathologies present and whether it is initiated in the early preclinical stage vs the active symptomatic period, as previously suggested by some preclinical studies10,98. Our findings provide data from a clinical trial in the context of ADRD and highlight the need for well-controlled studies to better understand the safety, biological effects, and clinical impact of repurposing rapamycin for ADRD.
Data availability
The data that underlie the results reported in this article are reported within the manuscript and in Supplementary Data Files 2–7. The source data for Fig. 1 is in Supplementary Data 3. The source data for Fig. 2 is in Supplementary Data 4. The source data for Supplementary Fig. 3 is in Supplementary Data 7. The full protocol, informed consent form, statistical analysis plan, and deidentified individual participant data, will be made available immediately following publication upon a direct request to the corresponding author with an appropriate research proposal. After consideration and the approval of such a proposal, data will be shared through a secure online platform.
References
Alzheimer’s Association Report. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 19, 1598–1695 (2023).
Cummings, J. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer’s disease therapeutics. Drugs 83, 569–576 (2023).
Salehipour, A., Bagheri, M., Sabahi, M., Dolatshahi, M. & Boche, D. Combination therapy in Alzheimer’s disease: Is it time? J. Alzheimer’s Dis. 87, 1433–1449 (2022).
Hara, Y., McKeehan, N. & Fillit, H. M. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology 92, 84–93 (2019).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Wilkinson, J. E. et al. Rapamycin slows aging in mice. Aging Cell 11, 675–682 (2012).
Selvarani, R., Mohammed, S. & Richardson, A. Effect of rapamycin on aging and age-related diseases—past and future. GeroScience 43, 1135–1158 (2021).
Schreiber, K. H. et al. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell 14, 265–273 (2015).
Zhang, Y., Zhang, J. & Wang, S. The role of Rapamycin in healthspan extension via the delay of organ aging. Ageing Res. Rev. 70, 101376 (2021).
Lin, A. L. et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 33, 1412–1421 (2013).
Van Skike, C. E. et al. mTOR attenuation with rapamycin reverses neurovascular uncoupling and memory deficits in mice modeling Alzheimer’s disease. J. Neurosci. 41, 4305–4320 (2021).
Spilman, P. et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5, e9979 (2010).
Caccamo, A., Majumder, S., Richardson, A., Strong, R. & Oddo, S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J. Biol. Chem. 285, 13107–13120 (2010).
Perluigi, M., Di Domenico, F., Barone, E. & Butterfield, D. A. mTOR in Alzheimer disease and its earlier stages: links to oxidative damage in the progression of this dementing disorder. Free Radic. Biol. Med. 169, 382–396 (2021).
Fiscon, G., Sibilio, P., Funari, A., Conte, F. & Paci, P. Identification of potential repurposable drugs in Alzheimer’s disease exploiting a bioinformatics analysis. J. Pers. Med. https://doi.org/10.3390/jpm12101731 (2022).
Silva, J. D., Taglialatela, G. & Jupiter, D. C. Reduced prevalence of dementia in patients prescribed tacrolimus, sirolimus, or cyclosporine. J. Alzheimers Dis. 95, 585–597 (2023).
Hou, S. J., Zhang, S. X., Li, Y., & Xu, S. Y. Rapamycin responds to Alzheimer’s disease: a potential translational therapy. Clin. Inter. Aging 18, 1629–1639 (2023).
Shoaib, M., Kamal, M. A. & Rizvi, S. M. D. Repurposed drugs as potential therapeutic candidates for the management of Alzheimer’s disease. Curr. Drug Metab. 18, 842–852 (2017).
Bee, J., Fuller, S., Miller, S. & Johnson, S. R. Lung function response and side effects to rapamycin for lymphangioleiomyomatosis: a prospective national cohort study. Thorax 73, 369–375 (2018).
Kraig, E. et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: immunological, physical performance, and cognitive effects. Exp. Gerontol. 105, 53–69 (2018).
Mandrioli, J. et al. Randomized, double-blind, placebo-controlled trial of rapamycin in amyotrophic lateral sclerosis. Nat. Commun. 14, 4970 (2023).
Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl. Med 6, 268ra179 (2014).
Mannick, J. B. et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaq1564 (2018).
Kaeberlein, T. L. et al. Evaluation of off-label rapamycin use to promote healthspan in 333 adults. Geroscience 45, 2757–2768 (2023).
Cloughesy, T. F. et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 5, e8 (2008).
Barthélemy, N. R., Mallipeddi, N., Moiseyev, P., Sato, C. & Bateman, R. J. Tau phosphorylation rates measured by mass spectrometry differ in the intracellular brain vs. extracellular cerebrospinal fluid compartments and are differentially affected by Alzheimer’s disease. Front Aging Neurosci. 11, 121 (2019).
Salvadó, G. et al. Disease staging of Alzheimer’s disease using a CSF-based biomarker model. Nat. Aging 4, 694–708 (2024).
Brand, A. L. et al. The performance of plasma amyloid beta measurements in identifying amyloid plaques in Alzheimer’s disease: a literature review. Alzheimers Res. Ther. 14, 195 (2022).
Schindler, S. E. et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, e1647–e1659 (2019).
Horie, K. et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nat. Med. 29, 1954–1963 (2023).
Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 4, 863 (2013).
Jack, C. R. Jr. et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s. Dement. 7, 257–262 (2011).
Morris, J. C. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 9, 173–176 (1997).
Sabo, A. N. et al. Sirolimus pharmacokinetics variability points to the relevance of therapeutic drug monitoring in pediatric oncology. Pharmaceutics 13, 470 (2021).
Wilcock, D. et al. MarkVCID cerebral small vessel consortium: I. Enrollment, clinical, fluid protocols. Alzheimers Dement 17, 704–715 (2021).
Nasreddine, Z. S. et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
Benedict, R. H. B., Schretlen, D., Groninger, L. & Brandt, J. Hopkins verbal learning test–revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 12, 43–55 (1998).
Burgess, P. W. & Shallice, T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia 34, 263–272 (1996).
Weintraub, S. et al. Version 3 of the Alzheimer Disease Centers’ neuropsychological test battery in the uniform data set (UDS). Alzheimer Dis. Assoc. Disord. 32, 10–17 (2018).
Shin, C. et al. Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J. Affect Disord. 259, 370–375 (2019).
Weintraub, S. et al. The Alzheimer’s Disease Centers’ uniform data set (UDS): the neuropsychologic test battery. Alzheimer Dis. Assoc. Disord. 23, 91–101 (2009).
Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H. Jr., Chance, J. M. & Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 37, 323–329 (1982).
Janelidze, S. et al. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 26, 379–386 (2020).
Gonzales, M. M. et al. Senolytic therapy in mild Alzheimer’s disease: a phase 1 feasibility trial. Nat. Med. 29, 2481–2488 (2023).
Janelidze, S. et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 11, 1683 (2020).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Laberge, R. M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Wood, M. A. & Bierer, B. E. Rapamycin: biological and therapeutic effects, binding by immunophilins and molecular targets of action. Perspect. Drug Discov. Des. 2, 163–184 (1994).
Srinivas, N., Maffuid, K. & Kashuba, A. D. M. Clinical pharmacokinetics and pharmacodynamics of drugs in the central nervous system. Clin. Pharmacokinet. 57, 1059–1074 (2018).
Shen, D. D., Artru, A. A. & Adkison, K. K. Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv. Drug Deliv. Rev. 56, 1825–1857 (2004).
Hartford, C. M. & Ratain, M. J. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin. Pharmacol. Ther. 82, 381–388 (2007).
Soesanto, W. et al. Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension 54, 1321–1327 (2009).
Reis, F. et al. Hypertension induced by immunosuppressive drugs: a comparative analysis between sirolimus and cyclosporine. Transplant. Proc. 41, 868–873 (2009).
Kumar, V., Wollner, C., Kurth, T., Bukowy, J. D. & Cowley, A. W. Jr. Inhibition of Mammalian target of rapamycin complex 1 attenuates salt-induced hypertension and kidney injury in Dahl salt-sensitive rats. Hypertension 70, 813–821 (2017).
Zaza, G. et al. Systemic and Nonrenal Adverse Effects Occurring in Renal Transplant Patients Treated with mTOR Inhibitors. Clin. Dev. Immunol. 2013, 403280 (2013).
Augustine, J. J. et al. Comparative effects of sirolimus and mycophenolate mofetil on erythropoiesis in kidney transplant patients. Am. J. Transpl. 4, 2001–2006 (2004).
Sofroniadou, S., Kassimatis, T. & Goldsmith, D. Anaemia, microcytosis and sirolimus—Is iron the missing link? Nephrol. Dial. Transpl. 25, 1667–1675 (2010).
Reifsnyder, P. C., Te, A. & Harrison, D. E. Differential effects of rapamycin on glucose metabolism in nine inbred strains. J. Gerontol. A Biol. Sci. Med. Sci. 75, 50–57 (2020).
Sankhala, K. et al. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 4, 135–142 (2009).
Granata, S. et al. mTOR-inhibitors and post-transplant diabetes mellitus: a link still debated in kidney transplantation. Front Med. 10, 1168967 (2023).
Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Stukas, S. et al. Association of CSF and serum neurofilament light and glial fibrillary acidic protein, injury severity, and outcome in spinal cord injury. Neurology 100, e1221–e1233 (2023).
Olsson, B. et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 15, 673–684 (2016).
Mielke, M. M. et al. Comparison of CSF phosphorylated tau 181 and 217 for cognitive decline. Alzheimers Dement .18, 602–611 (2022).
Li, Z., Fan, Z. & Zhang, Q. The associations of phosphorylated tau 181 and tau 231 levels in plasma and cerebrospinal fluid with cognitive function in Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimer’s. Dis. 98, 13–32 (2024).
Leuzy, A. et al. Comparing the clinical utility and diagnostic performance of CSF P-Tau181, P-Tau217, and P-Tau231 assays. Neurology 97, e1681–e1694 (2021).
Benedet, A. L. et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 78, 1471–1483 (2021).
Mielke, M. M. et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 93, e252–e260 (2019).
Lleó, A. et al. Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimers Dement. 15, 742–753 (2019).
Blennow, K. et al. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci. Lett. 419, 18–22 (2007).
Lin, A. L. et al. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 37, 217–226 (2017).
Spilman, P. et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLoS One 5, e9979 (2010).
Van Skike, C. E. et al. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 314, H693–h703 (2018).
Van Skike, C. E. et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell. https://doi.org/10.1111/acel.13057 (2019).
Halloran, J. et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 223, 102–113 (2012).
Caccamo, A., Majumder, S., Richardson, A., Strong, R. & Oddo, S. Molecular interplay between mTOR, Aβ and tau: effects on cognitive impairments. J. Biol. Chem. 285, 13107–13120 (2010).
Paraskevas, G. P. & Kapaki, E. Cerebrospinal fluid biomarkers for Alzheimer’s disease in the era of disease-modifying treatments. Brain Sci. 11, 1258 (2021).
Hansson, O., Lehmann, S., Otto, M., Zetterberg, H. & Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s disease. Alzheimer’s. Res. Ther. 11, 34 (2019).
Horie, K. et al. Regional correlation of biochemical measures of amyloid and tau phosphorylation in the brain. Acta Neuropathol. Commun. 8, 149 (2020).
Platanias, L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 (2005).
Fang, P., Hwa, V. & Rosenfeld, R. G. Interferon-gamma-induced dephosphorylation of STAT3 and apoptosis are dependent on the mTOR pathway. Exp. Cell Res. 312, 1229–1239 (2006).
Mannick, J. B. et al. Targeting the biology of ageing with mTOR inhibitors to improve immune function in older adults: phase 2b and phase 3 randomised trials. Lancet Healthy Longev. 2, e250–e262 (2021).
Yang, H. S. et al. Plasma IL-12/IFN-γ axis predicts cognitive trajectories in cognitively unimpaired older adults. Alzheimers Dement. 18, 645–653 (2022).
Leung, R. et al. Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PloS one 8, e64971 (2013).
Holmes, C. et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 73, 768–774 (2009).
Ceci, C. et al. The VEGFs/VEGFRs system in Alzheimer’s and Parkinson’s diseases: Pathophysiological roles and therapeutic implications. Pharmacol. Res. 201, 107101 (2024).
Zakariaee, S. S., Naderi, N. & Azizi, E. Association of vascular endothelial growth factor levels with risk of Alzheimer’s disease: a systematic review and meta-analysis. J. Preven. Alzheimer’s Dis. https://doi.org/10.14283/jpad.2024.18 (2024).
Taveira-DaSilva, A. M., Jones, A. M., Julien-Williams, P., Stylianou, M. & Moss, J. Long-term effect of sirolimus on serum vascular endothelial growth factor D levels in patients with lymphangioleiomyomatosis. Chest 153, 124–132 (2018).
Foley, K. E. & Wilcock, D. M. Soluble biomarkers of cerebrovascular pathologies. Stroke 55, 801–811 (2024).
Hinman, J. D. et al. Placental growth factor as a sensitive biomarker for vascular cognitive impairment. Alzheimer’s. Dement. 19, 3519–3527 (2023).
Hadley, G. et al. Rapamycin in ischemic stroke: Old drug, new tricks? J. Cereb. Blood Flow. Metab. 39, 20–35 (2019).
Siman, R., Cocca, R. & Dong, Y. The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early-stage Alzheimer-type tauopathy. PLoS One 10, e0142340 (2015).
Cappoli, N. et al. The mTOR kinase inhibitor rapamycin enhances the expression and release of pro-inflammatory cytokine interleukin 6 modulating the activation of human microglial cells. Excli j. 18, 779–798 (2019).
Han, H. E., Kim, T. K., Son, H. J., Park, W. J. & Han, P. L. Activation of autophagy pathway suppresses the expression of iNOS, IL6 and cell death of LPS-stimulated microglia cells. Biomol. Ther. (Seoul.) 21, 21–28 (2013).
Tarawneh, R. & Pankratz, V. S. The search for clarity regarding “clinically meaningful outcomes” in Alzheimer disease clinical trials: CLARITY-AD and beyond. Alzheimer’s. Res. Ther. 16, 37 (2024).
Lang, U. E. et al. Immunosuppression using the mammalian target of rapamycin (mTOR) inhibitor everolimus: pilot study shows significant cognitive and affective improvement. Transpl. Proc. 41, 4285–4288 (2009).
Alfaras, I. et al. Health benefits of late-onset metformin treatment every other week in mice. NPJ Aging Mech. Dis. 3, 16 (2017).
Carosi, J. M. & Sargeant, T. J. Rapamycin and Alzheimer disease: A double-edged sword? Autophagy 15, 1460–1462 (2019).
Acknowledgements
We would like to thank the volunteers, study participants, Doyle’s Pharmacy in Houston, Texas, and the Glenn Biggs Institute for Alzheimer’s and Neurodegenerative Diseases at UT Health San Antonio and the South Texas Alzheimer’s Disease Research Center (ADRC) (P30AG066546 to S.S.) faculty (especially Eduardo Zilli, MD) and research staff who conducted the study recruitment and assessments. This work was made possible by pilot funding from the Institute for Integration of Medicine & Science and the Center for Biomedical Neurosciences at UT Health Science Center in San Antonio (to M.M.G. and D.K.). M.M.G. was supported as an RL5 Scholar in the San Antonio Claude D. Pepper Older Americans Independence Center (P30AG044271) and the National Institute on Aging (R01AG077472 and P30AG066546). V.R.G. was supported by a National Institute on Aging Training Grant on the Biology of Aging (T32AG021890), a National Center for Advancing Translational Sciences NRSA Training Core grant (TR002647), and the South Texas ADRC (P30AG066546). M.L.-C. was supported by the San Antonio Claude D. Pepper Older Americans Independence Center (PG30AG044271) and by the National Institute on Aging (P30AG013319 and U01AG22307). M.E.O. was supported by the US Department of Veterans Affairs (I01BX005717), the National Institute on Aging (R01AG068293), the National Institute of Neurological Disorders and Stroke (R21NS125171), Cure Alzheimer’s Fund, and the Hevolution Foundation/American Federation of Aging Research. N.M. was supported by the National Institute on Aging (P30AG044271, P30AG013319, U54AG07594, R01AG069690, and R01AG075684). S.S. was supported by the National Institute on Aging (R01AG066524, R01AG054076, R01AG033193, and RF1AG059421), the National Institute of Neurological Disorders and Stroke (R01NS017950), and the National Heart, Lung, and Blood Institute (R01HL105756). R.J.B. and C.H. were supported by the Tracy Family SILQ Center, NIA/NINDS RF1AG061900, R01NS095773, NIA R21AG067559, and the Tracy Family SILQ Center established by the Tracy Family, Richard Frimel and Gary Werths, GHR Foundation, David Payne, and the Willman Family brought together by The Foundation for Barnes-Jewish Hospital. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript, or in the review or approval of the manuscript.
Author information
Authors and Affiliations
Contributions
Mitzi M. Gonzales (conceptualization, methodology, investigation, writing—original draft, funding acquisition), Valentina R. Garbarino (investigation and writing—review and editing), Tiffany F. Kautz (investigation and writing—review and editing), Xuemei Song (formal analysis and writing—review and editing), Marisa Lopez-Cruzan (investigation, methodology, and writing—review and editing), Leslie Linehan (investigation and writing—review and editing), Candice E. Van Skike (conceptualization and writing—review and editing), Gabriel A. De Erausquin (investigation and writing—review and editing), Veronica Galvan (conceptualization and writing—review and editing), Miranda E. Orr (writing—review and editing), Nicolas Musi (resources, writing—review and editing), Yingxin He (investigation, methodology, writing—review and editing), Randall J. Bateman (investigation, methodology, and writing—review and editing), Chen-Pin Wang (formal analysis and writing—review and editing), Sudha Seshadri (conceptualization, methodology, investigation, resources, and writing—review and editing), Ellen Kraig (conceptualization, methodology, and writing—review and editing), and Dean Kellogg, Jr. (conceptualization, methodology, investigation, writing—review and editing, and funding acquisition).
Corresponding author
Ethics declarations
Competing interests
Mitzi M. Gonzales reports personal stock in AbbVie. Miranda Orr has a patent, Biosignature and Therapeutic Approach for Neuronal Senescence, pending. Randall J. Bateman reports a relationship with C2N Diagnostics and receives income from serving on the scientific advisory board as a co-founder. Randall J. Bateman has received research funding from Avid Radiopharmaceuticals, Janssen, Roche/Genentech, Eli Lilly, Eisai, Biogen, AbbVie, Bristol Myers Squibb, and Novartis. Washington University has equity ownership interest in C2N Diagnostics and receives royalty income based on technology (Stable Isotope Labeling Kinetics, Blood Plasma Assay, and Methods of Diagnosing AD with Phosphorylation Changes) licensed by Washington University to C2N Diagnostics. Dr. Seshadri has consulted for Eisai and Biogen outside the current work. All other authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gonzales, M.M., Garbarino, V.R., Kautz, T.F. et al. Rapamycin treatment for Alzheimer’s disease and related dementias: a pilot phase 1 clinical trial. Commun Med 5, 189 (2025). https://doi.org/10.1038/s43856-025-00904-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-00904-9