Abstract
Background
Bifidobacterium colonization of the intestine is believed to have beneficial effects on our health from infancy throughout life. However, how particular members of the genus Bifidobacterium colonize the neonatal intestine and whether early-life bifidobacterial colonization affects establishment of Bifidobacterium-rich microbiota in later life remain unanswered. α-Defensin secreted from small intestinal Paneth cells elicits selective bactericidal activities that efficiently kill pathogens while hardly affecting commensals including Bifidobacterium in vitro, thus contributing to intestinal microbiota regulation.
Methods
One hundred forty-eight fecal samples were serially obtained from 33 children from postnatal 3–5 days to 3 years old, conducting a longitudinal cohort study of mothers and children living in Iwamizawa city, Hokkaido, Japan (SMILE Iwamizawa study). Microbiota composition and secretory level of α-defensin, human defensin 5 (HD5), were assessed to investigate the relationship between HD5 and Bifidobacterium colonization.
Results
We show that HD5 is associated with colonization of Bifidobacterium in early life from pre-weaning to weaning periods. Furthermore, high relative abundance of Bifidobacterium in the weaning period, which positively correlates with HD5 secretion, is associated with the establishment of Bifidobacterium-rich microbiota at 3 years old, when the intestinal microbiota matures.
Conclusions
This study suggests the importance of the weaning period in establishing long-lasting homeostasis interwoven with the host innate immunity and Bifidobacterium in the intestinal microbiota.
Plain language summary
Bifidobacterium is a beneficial bacterium that lives in the intestine and supports our health from infancy to adulthood. However, it is not fully understood how these bacteria first colonize the babies’ intestine, or whether this early colonization affects the intestinal environment in later life. We followed 33 children from 3–5 days to 3 years old and collected fecal samples to study their intestinal bacteria. We also measured an antimicrobial peptide called HD5, which is known to control the intestinal bacteria. We found that HD5 modulates the colonization of Bifidobacterium, and that a high level of these bacteria during weaning may help establish a healthy intestinal environment that lasts. These findings suggest that supporting intestinal health during weaning benefit long-term well-being.
Similar content being viewed by others
Introduction
The concept of Developmental Origins of Health and Disease (DOHaD) that environmental factors during the first 1000 days of life, from embryonic period to the first 2 years of life, affect future risks of various diseases such as obesity, diabetes, cardiovascular diseases, and allergy has been widely recognized1,2,3. The intestinal microbiota is a complex ecosystem that influences a wide range of host physiology4,5, and early-life intestinal microbiota is considered as a factor that influences health and disease in later life. The immune and nervous systems rapidly develop from neonatal to early childhood, and the intestinal microbiota plays an important role6,7. It has been known that exposure to environmental factors that disrupt the intestinal microbiota such as cesarean section, malnutrition, and antibiotics administration in early life is associated with future increased risks of diseases such as allergic diseases, obesity, and developmental disorders8,9,10,11,12,13, suggesting that the establishment of a healthy intestinal microbiota in early life is crucial to promote lifelong health.
Several members of Bifidobacterium increase rapidly in the human intestinal tract from the first few days after birth and become one of the dominant bacteria in the early-life intestine14. It has been reported that Bifidobacterium in early life promotes maturation of host immune system15,16,17 and tissue development, such as the brain and intestine18,19. Furthermore, the establishment of Bifidobacterium-rich intestinal microbiota in early life is associated with the reduced risk of various diseases such as atopy, asthma, obesity, and developmental disorders in later life20,21,22,23, suggesting that Bifidobacterium in early life contribute to future health by promoting appropriate physiological development of host. Although decreasing upon weaning, Bifidobacterium is also one of the major genera in the adult intestine24. Furthermore, several epidemiologic studies suggest that the establishment of a Bifidobacterium-rich microbiota not limited in early life contributes to health from adolescence to old age24,25,26,27. The intestinal microbiota of children develops rapidly from immediately after birth and matures with forming the structure similar to that of adults by ~3 years old28,29. In addition, some strains of Bifidobacterium detected in feces of children with in the first year of life are also detected in their childhood (6–11 years old), showing long-term colonization of Bifidobacterium to the intestine30,31. Thus, it is supposed that higher relative abundance of Bifidobacterium in the early-life microbiota supports the establishment of Bifidobacterium-rich microbiota in later life via inheriting its population, and further contributes to lifelong health. However, whether Bifidobacterium in the early-life microbiota affects the relative abundance of Bifidobacterium in the intestinal microbiota at maturity remains unknown because there have been no longitudinal comprehensive analyses for the effect of Bifidobacterium from the neonatal to infancy on the future intestinal microbiota.
Paneth cells, a lineage of small intestinal epithelial cells, contribute to the regulation of the intestinal microbiota32,33,34 by secreting antimicrobial peptides, α-defensins termed cryptdin (Crp)s in mice and human defensin (HD) 5 and 6 in humans35,36,37,38,39, into the intestinal lumen in response to bacteria40,41 and food components42. Recently, it has been reported that abnormal Crp secretion is involved in the pathological progression of mouse models of several diseases, including Crohn’s disease and depression via inducing imbalance of the intestinal microbiota, dysbiosis43,44,45,46. Decrease of α-defensin expression in Paneth cells is also reported in patients with Crohn’s disease47. Furthermore, decreased levels of HD5 secretion relate to disturbance of the intestinal microbiota by aging48 and short sleep49 in humans. Expression of α-defensins in Paneth cells is already detected in the fetal small intestine of both mice and humans50,51. In mice, Paneth cell α-defensin is also known to increase rapidly during birth to weaning (21 days after birth)52. Infants suffered with necrotizing enterocolitis (NEC) are reported to have fewer number of Paneth cells53. In addition, Crps possess selective bactericidal activities in vitro, which strongly kill both pathogenic and opportunistic bacteria whereas shows no or minimal bactericidal activities against commensal bacteria, including Bifidobacterium bifidum (B. bifidum) and Bifidobacterium breve (B. breve)54. Therefore, it is suggested that maturation of α-defensin secretion in early life plays an important role in the establishment of the intestinal microbiota in later life via promoting the colonization of Bifidobacterium in the early-life intestine. However, the maturation process of α-defensin secretion in early life and the effects of α-defensin on Bifidobacterium in the human intestinal microbiota remain unknown.
In this study, we analyzed the transition of the relative abundance of Bifidobacterium and HD5 secretion during the early-life period and further evaluate its effects in the establishment of Bifidobacterium-rich microbiota in later life by conducting the Survey of Mothers, Infants, and Children’s Lives and Environments in Iwamizawa (SMILE Iwamizawa), the ongoing longitudinal cohort study targeting mothers and children living in Iwamizawa city, Hokkaido, Japan55. Here we show that the intestinal microbiota of children shows a mature composition similar to that of their mothers by 3 years old. Additionally, the relative abundance of Bifidobacterium at weaning and at 3 years old shows a positive correlation. Furthermore, the secretory level of HD5 positively correlates with the relative abundance of Bifidobacterium throughout the infancy, especially at the weaning. These results indicate that Bifidobacterium colonization during the weaning period, which is modulated by HD5 secretion, contributes to the development of Bifidobacterium-rich microbiota in later life, indicating the importance of the weaning period in establishing long-term health through the intestinal microbiota.
Methods
Study design and population
This study was conducted as a part of the SMILE Iwamizawa, an ongoing longitudinal cohort study in Iwamizawa, Hokkaido, Japan. The cohort study of mothers and children from pregnancy to the postpartum period began in January 2017 and will continue until 2027 to explore factors that influence children’s growth and development55. Pregnant women living in Iwamizawa are recruited when the municipal government issues the Maternal and Child Health Handbook and are enrolled in the cohort if informed consent is obtained. As a part of the survey, fecal samples are collected from children at 3–5 days old (d), 1 month old (m), 4–5 m, 8–9 m, 1.5 years old (y), 3 y, 5 y, and school-age. Fecal samples are also obtained from mothers at 24 weeks of gestation and when their children reach 3–5 d, 1 m, and 4–5 m. A small part of each fecal sample is collected by brush-type collection kits containing guanidine thiocyanate (Techno Suruga Laboratory Co., Ltd., Shizuoka, Japan) for the intestinal microbiota analysis. The remaining fecal samples are stored at −80 °C until use and subjected to a series of analyses, including HD5 quantification. In addition, anthropological data of the children measured at the time of regular check-ups are provided by the mothers at each time point.
At the time of this study (December 2022), 257 mother-child dyads were enrolled in the cohort, and pairs of data on fecal HD5 and the intestinal microbiota at 3 y were obtained from 33 children, who composed the most advanced group in the cohort. From these 33 children, pairs of HD5 and intestinal microbiota data were obtained from 152 fecal samples in total (n = 22, 21, 21, 27, 28, and 33 at 3–5 d, 1 m, 4–5 m, 8–9 m, 1.5 y, and 3 y, respectively). Also, 28 pairs of data were obtained from their mother at 4–5 m. From these 180 samples, 4 samples were excluded due to insufficient data quality and quantity of bacterial 16S rRNA gene sequencing (all samples were obtained from children at 1 m. Further details can be found in the Methods section under Bacterial 16S rRNA gene-based taxonomic analysis). Finally, 176 samples were analyzed in this study. This study was conducted in accordance with the guidelines of the Declaration of Helsinki and all procedures involving human subjects were approved by the ethics committees of the Graduate School of Medicine at Hokkaido University and the Morinaga Milk Industry Co., Ltd (approval number: 16-039 and 16005-144). Written informed consent was obtained from all mothers, and research consent from children was obtained on the basis of the consent signatures of their mothers.
Bacterial 16S ribosomal RNA gene sequencing
Total genomic DNA was extracted from the fecal samples by bead-beating and purified for intestinal microbiota analysis by using the automated DNA Extraction system Gene Prep Star PI-480α (Kurabo Industries, Ltd, Osaka, Japan)56. The V3-V4 region of the bacterial 16S ribosomal RNA (rRNA) gene was subsequently amplified by using TaKaRa Ex Taq HS Kit (TaKaRa Bio Inc., Shiga, Japan) with the primer sets Tru357F (5′-CGCTCTTCCGATCTCTGTACGGRAGGCAGCAG-3′) and Tru806R (5′-CGCTCTTCCGATCTGACGGACTACHVGGGTWTCTAAT-3′) and sequenced via the pair-end method on a MiSeq instrument (SY-410-1003, Illumina Inc., Hayward, CA)57.
Bacterial 16S rRNA gene-based taxonomic analysis
Paired-end fastq files obtained from MiSeq were demultiplexed and analyzed by the QIIME2 platform (version 2022.8)58. Quality-filtering, denoising, pair-end merging, and chimera removal of sequences were conducted via the qiime dada2 denoise-paired command of the DADA2 plugin59 with the following parameters: --p-trim-left-f 23; --p-trim-left-r 23; --p-trunc-len-f 270; --p-trunc-len-r 210; --p-max-ee-f 2; --p-max-ee-r 2. After this step, samples for which more than 2000 reads could not be obtained were excluded (4 samples obtained from children at 1 m) because sequence data quality and quantity were considered insufficient for the analysis. All remaining reads were rarefied to 2000 per sample. The phylogenic tree was subsequently constructed via FastTree260 after alignment with MAFFT61 using the qiime phylogeny align-to-tree-mafft-fasttree command. The taxonomy of each feature at the phylum and genus level was assigned by the qiime feature-classifier classify-sklearn command using a naïve Bayes classifier trained on the GreenGenes2 2022.10 database62. For the species-level analysis of Bifidobacterium, taxonomic assignments were conducted by aligning each 16S rRNA V3-V4 sequence to the RefSeq database of 16S rRNA gene sequences released by NCBI (https://ftp.ncbi.nlm.nih.gov/refseq/TargetedLoci/Bacteria/; updated at 2023.4.15) using the qiime feature-classifier classify-consensus-blast command with the parameter --p-perc-identity 0.99. The α-diversity (observed features) and β-diversity (weighted UniFrac distance) were calculated by the qiime diversity alpha-rarefaction and qiime diversity core-metrics-phylogenetic commands, respectively. The statistical significance of β-diversity was determined via the permutational multivariant analysis of variance (PERMANOVA) test by using the qiime diversity beta-group-significance command. Additionally, a linear discriminant analysis effect size (LefSe) test63 was conducted by the web-based Galaxy platform (https://huttenhower.sph.harvard.edu/galaxy/).
Calculation of body mass index (BMI) and BMI percentile
BMI and BMI percentile based on the sex-matched Japanese growth curve at 3 y were calculated from weight and height by using a Microsoft Excel-based tool for growth evaluation provided by the Japanese Society for Pediatric Endocrinology (http://jspe.umin.jp/medical/chart_dl.html). The participants were subsequently divided into LowBMI (n = 7; BMI percentile ≤ 33.3rd), MidBMI (n = 11; 33.4–66.7th percentile), and HighBMI (n = 14; >66.7th percentile) groups.
Quantification of fecal HD5 by sandwich ELISA
Fecal samples were lyophilized and pulverized to powder using a bead-beating homogenizer (PV1001, Yasui Kikai, Corp., Osaka, Japan). Ten milligrams of fecal powder was suspended in 100 μL of PBS (−), vortexed at 4 °C overnight, and centrifuged at 15,000 × g for 30 min at 4 °C. Then, the supernatants were subjected to the previously developed sandwich ELISA system using in-house produced mouse anti-HD5 monoclonal antibodies (clones 12-1 and 39E-7)48.
Preparation of the HD5 peptide
Chemically synthesized HD5 peptide (Thermo Fisher Scientific, Waltham, MA) was dissolved in water containing 3 mM reduced-form glutathione, 0.3 mM oxidized-form glutathione, and 8 M urea. Then, the solution was adjusted to pH 8.4 by adding 0.25 M NaHCO3 and air-oxidation was conducted by gently mixing at 4 °C overnight. Oxidized-form HD5 with three intramolecular disulfide bonds was purified by reverse-phase high-performance liquid chromatography by a C18 column (06526-21, Nacalai Tesque, Inc., Kyoto, Japan) in 0.1% trifluoroacetic acid with a 0-40% acetonitrile gradient developed over 50 min at a flow rate of 1 mL/min48,54.
Bactericidal assay of HD5
Bifidobacterium breve (B. breve) JCM 1192, Bifidobacterium longum (B. longum) ATCC15707, Lactobacillus casei (L. casei) ATCC393, Escherichia coli (E. coli) ML35 ATCC 43827, Staphylococcus aureus (S. aureus) ATCC27212, and Bacteroides fragilis (B. fragilis) JCM11019 were used for the analysis. Each bacterium was cultured in the following media: B. breve and B. longum, reinforced clostridial medium (CM0149, Oxoid Ltd., Hampshire, UK); L. casei, de Man, Rogosa, Sharpe broth (CM0359, Oxoid Ltd.); E. coli and S. aureus, tryptic soy broth (211825, Becton, Dickinson and Company); B. fragilis, Gifu anaerobic medium (05422, Shimadzu Diagnostics Company, Tokyo, Japan). E. coli and S. aureus were cultured under aerobic condition at 37 °C with shaking at 180 rpm, and other bacteria were cultured under static, anaerobic conditions using the Anaero Pack system (A-02, Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) at 37 °C. Bacterial growth was monitored by measuring the optical density at 600 nm until reaching the exponential phase and then, the bacterial cultures were centrifuged at 3000 × g for 5 min at 4 °C. After the supernatant was removed, the bacteria were resuspended in 0.2 mM PIPES buffer. Ten microliters of bacterial suspension was mixed with an equal volume of oxidized-form HD5 dissolved in 0.2 mM PIPES buffer to the final concentrations of 0, 0.01, 0.03, 0.11, 0.33, and 1 μM, and then reacted for 1 h at 37 °C. After the reaction, the mixtures were spread on agar plates and incubated at 37 °C. The bacterial survival rates at each concentration of oxidized-form HD5 were calculated from the number of surviving colonies relative to peptide-unexposed controls (0 μM).
Statistical analysis
All the statistical analyses were conducted with GraphPad Prism version. 9.0 software (GraphPad Software Inc., San Diego, CA). For all correlation analyses, Spearman’s rank correlation coefficient test was used. For group comparisons, the Mann-Whitney’s U test and one-way analysis of variance (ANOVA) followed by Dunnett’s test or Tukey’s multiple comparison test, were used depending on the situation. For all statistical tests, p < 0.05 was considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
The intestinal microbiota of children matures by 3 y
Among the participants in the SMILE Iwamizawa study, 33 children having the data on both fecal HD5 concentrations and bacterial 16S rRNA gene sequence with sufficient quality and quantity at 3 y were enrolled in this study. Then, total of 176 fecal samples obtained longitudinally from the children at 3–5 d (n = 22), 1 m (n = 17), 4–5 m (n = 21), 8–9 m (n = 27), 1.5 y (n = 28), and 3 y (n = 33) and their mothers at 4–5 m (n = 28) were included in the analysis (Table 1, Supplementary Tables 1 and 2). First, the maturation process of the children’s intestinal microbiota was analyzed. The α-diversity of the children’s microbiota increased along with their growth and reached the same level as that of mothers at 3 y (Fig. 1a). At the phylum level, Firmicutes- and Bacteroidota-dominant microbiota similar to those of their mothers were observed in the children after 1.5 y (Fig. 1b), indicating that the intestinal microbiota of the children matured by 3 y.
a Comparison of an α-diversity index, observed features between mothers at 4–5 m (n = 28) and children at each time point (n = 22 at 3–5 d; n = 17 at 1 m; n = 21 at 4–5 m; n = 27 at 8–9 m; n = 28 at 1.5 y; n = 33 at 3 y). b Stacked bar chart of relative abundance of the intestinal microbiota at the phylum level. The error bars represent the means ± SD. Statistical significance between mothers and children at each age was evaluated by one-way ANOVA followed by Dunnett’s multiple comparison test in (a). p < 0.05 was considered statistically significant.
High relative abundance of Bifidobacterium at the weaning period is associated with the establishment of Bifidobacterium-rich microbiota at 3 y
Next, the relationships between the relative abundance of Bifidobacterium in the intestinal microbiota at 3 y, when mature, and that at each timepoint from newborn to infancy were analyzed (Supplementary Fig. 1). The relative abundance of the Bifidobacterium genus in the children increased from 3–5 d to 4–5 m, then decreased from 8–9 m and reached approximately the same level as that of the mothers at 3 y (Fig. 2a). Furthermore, the relative abundance of the Bifidobacterium genus at 3 y was positively correlated with that at 8–9 m and 1.5 y, whereas no significant correlation was detected before 4–5 m (Fig. 2b). In the species-level analysis, five taxa of Bifidobacterium species with average relative abundance greater than 0.1% were assigned (Fig. 3a, Supplementary Fig. 2). The relative abundance of total Bifidobacterium, the sum of these 5 species, at 3 y was positively correlated with that at 8–9 m and 1.5 y, whereas no significant correlations were detected between the relative abundance of total Bifidobacterium at 3 y and that of individual Bifidobacterium species at each age (Fig. 3b, Supplementary Fig. 3). Given the guidelines from the Ministry of Health, Labor and Welfare in Japan and the World Health Organization, which recommend weaning between 5–6 m to 1.5 y and between 6 m to 2 y, respectively64,65, we integrated the data from children at 8–9 m and 1.5 y as the weaning period which may more appropriately reflect the children’s developmental process and further analysis was conducted. As a result, B. breve at the weaning period was positively correlated with total Bifidobacterium at 3 y (Fig. 3b, Supplementary Fig. 3). In addition, a positive interspecies correlation was observed between B. breve at the weaning period and B. catenulatum at 3 y (Supplementary Fig. 4). These results indicate that a high relative abundance of Bifidobacterium at the weaning period is involved in the establishment of a Bifidobacterium-rich microbiota at 3 y, which represents a future timepoint.
a Transition of the relative abundance of the Bifidobacterium genus in children. b Correlation analysis of the relative abundance of the Bifidobacterium genus between children at 3 y and those of each age. The dashed lines in (b) represent the 95% confidence interval range. Statistical significance among children at each age was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test in (a) and Spearman’s rank correlation coefficient test in (b).
a Stacked bar chart of the relative abundance of Bifidobacterium species in children at each age. b Correlation matrix between the relative abundance of total Bifidobacterium in children at 3 y and each Bifidobacterium species in children at 8–9 m, 1.5 y, and the weaning period (combined data set of 8–9 m and 1.5 y). Statistical significance was evaluated by Spearman’s rank correlation coefficient test in (b). In (b), * in each cell indicates statistically significant correlation (p < 0.05).
A high relative abundance of Bifidobacterium at 3 y is associated with a low BMI and the intestinal microbiota formation characterized by low relative abundance of Parabacteroides
Then, the effects of the formation of the Bifidobacterium-rich microbiota on the physical development of children were analyzed. There was no statistically significant correlation between occupancy of Bifidobacterium genus and either weight, height, or head circumference, but there was a tendency (p = 0.107) for a negative correlation with BMI (Fig. 4a). To conduct a more detailed analysis, the participants were divided into LowBMI (n = 7; BMI percentile ≤ 33.3rd), MidBMI (n = 11; 33.4–66.7th percentile), and HighBMI (n = 14; >66.7th percentile) groups (Supplementary Table 3). As a result, the relative abundance of Bifidobacterium in the LowBMI group was significantly higher than that in the HighBMI group (Fig. 4b). Next, to clarify the effect of the high relative abundance of Bifidobacterium on the intestinal microbiota ecosystem, the children were divided into LowBifido (n = 16) and HighBifido (n = 17) groups in ascending order of the relative abundance of Bifidobacterium at 3 y, and the intestinal microbiota was compared between the groups. In the diversity analysis, the intestinal microbiota in the LowBifido and HighBifido groups showed significantly different composition in the β-diversity, whereas no difference was observed in the α-diversity (Supplementary Fig. 5a, b). In the genus-level analysis, a significant difference other than Bifidobacterium was only observed in the relative abundance of Parabacteroides between the groups, and Parabacteroides in the HighBifido group was lower than that in the LowBifido group (Fig. 4d, Supplementary Fig. 5c). These results indicate that the high relative abundance of Bifidobacterium in children at 3 y is associated with low BMI and the formation of the intestinal microbiota characterized by low Parabacteroides.
a Correlation analysis between anthropological data and the relative abundance of Bifidobacterium in children at 3 y. b Comparison of the relative abundance of Bifidobacterium between the LowBMI (n = 7; BMI ≤ 33.3rd percentile in age- and sex-matched Japanese children), MidBMI (n = 11; 33.4–66.7th) and HighBMI (n = 14; >66.7th) groups in children at 3 y. c Comparison of the relative abundance of Bifidobacterium between the LowBifido (n = 16) and HighBifido (n = 17) groups, to which the children are assigned in ascending order of the relative abundance of Bifidobacterium at 3 y. d Differentially abundant genera between the LowBifido and HighBifido groups identified by LefSe analysis. The error bars represent the mean ± SD. Dashed lines in (a) represent the 95% confidence interval range. Statistical significance was evaluated by Spearman’s rank correlation coefficient test in (a), one-way ANOVA followed by Tukey’s multiple comparison test in (b), and the Mann-Whitney’s U test in (c). In (d), taxa with an |LDA score| > 2.0 were considered differentially abundant between the groups.
High HD5 secretion is associated with a high relative abundance of Bifidobacterium in children during the early life, especially in the weaning period
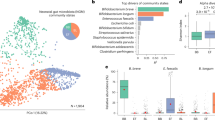
Finally, the effect of HD5 secretion on the colonization of Bifidobacterium during early life was analyzed. HD5 was already detected in feces at 3–5 d, showed higher concentration than their mothers from 3–5 d to 8–9 m, after which it decreased to the same level as that in mothers after 1.5 y (Fig. 5a, Supplementary Table 4). In the correlation analysis between the fecal HD5 concentration and the relative abundance of total Bifidobacterium at each age, a positive correlation was observed at 1.5 y (Fig. 5b). In the analysis of individual Bifidobacterium species, fecal HD5 was positively correlated with B. catenulatum and B. adolescentis at 3–5 d and with B. breve at 1.5 y, and was negatively correlated with B. adolescentis at 3 y (Fig. 5b). In the analysis of each period integrating multiple age, HD5 at the all-time period (all age; all), pre-weaning period (3–5 d, 1 m, and 4–5 m; pre-weaning), and the weaning period (8–9 m and 1.5 y; weaning) were all positively correlated with the relative abundance of total Bifidobacterium (Fig. 5b, Supplementary Fig. 6). Among these time periods, the strongest positive correlation was observed at the weaning period (Spearman’s correlation coefficient rs = 0.357 in all; 0.311 in pre-weaning; 0.484 in weaning), indicating that a high HD5 concentration is associated with a high relative abundance of Bifidobacterium throughout infancy, especially during weaning. No associations were found between mode of delivery and feeding and relative abundance of Bifidobacterium at 3 y (Supplementary Fig. 7). To clarify the effect of HD5 on the colonization of Bifidobacterium directly, we conducted in vitro bactericidal assay of HD5 against B. breve and B. longum focusing on the sub-μM concentration, which is approximately the estimated concentration of HD5 in the colonic lumen (Fig. 6)66,67. HD5 showed significant bactericidal activities against E. coli ML35, pathogenic S. aureus ATCC27217, and opportunistic B. fragilis JCM11019 at concentration higher than 0.01 μM, 0.03 μM, and 0.03 μM, respectively. In contrast, HD5 showed significant bactericidal activity against commensal B. breve JCM1192, B. longum ATCC15707, and L. casei ATCC393 at concentration higher than 0.33 μM. These results indicate that HD5 has weaker bactericidal activity against commensal bacteria including Bifidobacterium compared to non-commensal and opportunistic bacteria, suggesting that HD5 contributes to the colonization of Bifidobacterium in the intestine.
a Transition of fecal HD5 concentration in children along with their development. b Correlation matrix between the relative abundance of each Bifidobacterium species and the fecal HD5 concentration in children at each age, all period (combined data set of all time points), the pre-weaning period (3–5 d, 1 m, and 4–5 m), and the weaning period. Error bars represent the means ± SD in (a). In (b), * indicates statistically significant correlation (p < 0.05). Statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test among children at each age in (a) and Spearman’s rank correlation coefficient test in (b).
In vitro bactericidal activities of HD5 against Escherichia coli (E. coli) ML35, Staphylococcus aureus (S. aureus) ATCC27217, Bacteroides fragilis (B. fragilis) JCM11019, Bifidobacterium breve (B. breve) JCM1192, Bifidobacterium longum (B. longum) ATCC15707, and Lactobacillus casei (L. casei) ATCC393. Five individual experiments were conducted, and the bacterial survival rate at each concentration was calculated relative to 0 μM concentration of each bacterium. The error bars represent the means ± SE. Statistical significance was evaluated by one-way ANOVA followed by Dunnett’s multiple comparison test against 0 μM.
Discussion
The appropriate development of the early-life intestinal microbiota is widely recognized as important for the establishment of healthy microbiota in later life as a part of the DOHaD theory12. Although several studies focused on specific periods or strains regarding later-life colonization of Bifidobacterium that appeared in the early-life intestine have been reported31,68,69, there are no comprehensive and longitudinal studies on the association of Bifidobacterium at each age in early life from neonatal to early childhood at either the community (genus)-level or species-level resolution. In this study, we revealed that the high relative abundance of Bifidobacterium at the weaning period around 1 y relating to HD5 secretion is associated with the establishment of a Bifidobacterium-rich microbiota in later life by using the longitudinal dataset obtained from children at 3–5 d to 3 y. Our results indicate that Bifidobacterium in early life may contribute to long-term health by supporting the establishment of future intestinal microbiota in addition to the well-known pathway by promoting the physiological development of host15,16,17,18,19, providing an expanded perspective on the role of early-life Bifidobacterium in DOHaD theory.
According to the species-level analysis, the relative abundance of B. breve at the weaning period was positively correlated with that of the total Bifidobacterium at 3 y, suggesting that B. breve has a central role in the establishment of Bifidobacterium-rich microbiota in later life. In addition, the positive interspecies correlation between B. breve at the weaning and B. catenulatum at 3 y indicates that individual Bifidobacterium species may indirectly contribute to the establishment of Bifidobacterium-rich microbiota in later life by supporting the growth of other Bifidobacterium species. In general, Bifidobacterium species have a high ability to assimilate glycans derived from the host (e.g., mucin, human milk oligosaccharide: HMO) and food (e.g., starch, maltodextrin) and support each other’s growth through the cross-feeding of mono- and disaccharides produced by glycan degradation70. Although B. breve has limited assimilation activity against HMOs compared with B. bifidum and B. longum subsp. infantis71, it can degrade a wide range of food-derived glycans to ulilize70. In addition, B. breve supports the growth of B. bifidum in the presence of food-derived glycans (starch and xylan)72. Therefore, B. breve at the weaning period may support the colonization of the overall Bifidobacterium community by cross-feeding glycan metabolites derived from food components. Future studies based on our findings are needed to reveal detailed interactions between Bifidobacterium species during the maturation process of the intestinal microbiota.
It has been reported that the copy number of Bifidobacterium spp. in feces is negatively correlated with BMI in children aged 3–11 y73. Because the clinical criteria for overweight and obesity in children based on BMI are ≥85th percentile and ≥95th percentile74, the finding of this study that the relative abundance of Bifidobacterium in the LowBMI group (bottom 1/3 percentiles) was higher than in the HighBMI group (top 1/3 percentiles) at 3 y does not directly indicate association between Bifidobacterium and childhood obesity. In addition, this study does not take into account the potential confounding between BMI and the intestinal microbiota caused by diet. However, given that children who exceed the 50th BMI percentile even once at regular checkups at 2, 3, and 4.5 y have a 4.5 to 8.2-fold higher risk of overweight at 12 y75, a high relative abundance of Bifidobacterium at 3 y may be associated with a reduced risk of obesity in later life. The finding that children with a high relative abundance of Bifidobacterium presented a low relative abundance of Parabacteroides in the intestinal microbiota, may be linked to previous studies reporting positive relationship between a high relative abundance of Parabacteroides and diseases such as atopic dermatitis and developmental disorders in children76,77. Taken together, these finding suggest that a high relative abundance of Bifidobacterium at 3 y contributes to a reduced future disease risk by promoting the appropriate development of children in association with the appropriate intestinal microbiota.
Although it has been reported that α-defensin is already expressed in the fetal human small intestine at embryonic weeks 13–1651, and that, in adults, the secretory amount of HD5 gradually decreases with aging48, the transition of HD5 secretion from the early postnatal period to infancy is completely unknown. In this study, we revealed that α-defensin secretion by human Paneth cells occurs vigorously from the neonatal period and revealed the maturation process of HD5 secretion to early childhood. These findings shed light on the role of innate enteric immunity in regulating the intestinal microbiota fulfilled by Paneth cell α-defensin in the early-life period, with a relatively limited immune system when adaptive immunity, with the exception of IgA, remains immature. Furthermore, although it has been reported that oral administration of HD5 to high-fat diet-fed mice normalizes lipid metabolism and glucose tolerance and increases the relative abundance of Bifidobacterium78, the effect of HD5 on Bifidobacterium in the human intestinal microbiota is not yet understood. We demonstrated that the secretory amount of HD5 was positively correlated with the relative abundance of Bifidobacterium in the early-life intestinal microbiota and that HD5 does not shows in vitro bactericidal activities against commensal B. breve, B. longum, and L. casei at concentration lower than 0.11 μM. The HD5 concentration in ileostomy fluids was reported to be 7.9 μg/mL (2.2 μM) in Crohn’s disease patients and 10.5 μg/mL (2.9 μM) in non-Crohn’s disease controls66; thus, HD5 concentration in ileal lumen is estimated to be 2–3 μM. In addition, it is reported that the concentration of Crp4, a Crp isoform, in the colonic lumen of ICR mice is ~1/50 of that in the ileal lumen67. Therefore, the HD5 concentration of the colonic lumen where Bifidobacterium colonizes is estimated to be ~0.05 μM. Taken together, these findings suggest that HD5 eliminates potentially harmful bacteria to the host whereas shows no bactericidal activities against Bifidobacterium at the physiological concentrations in the colon, suggesting that HD5 contributes to the colonization of Bifidobacterium in the human intestine and the establishment of healthy intestinal microbiota in early life. In contrast, in the correlation analysis between HD5 and individual Bifidobacterium species at each period, a positive correlation was observed with only B. longum and B. bifidum before weaning and with only B. breve in the weaning period. These results indicate that although HD5 is associated with Bifidobacterium colonization in the intestine, the relationship between HD5 and individual Bifidobacterium species flexibly changes with the developmental phase of the host, and is likely related to the intestinal environment with complex interactions among other intestinal bacteria, host immune factors, and environmental factors such as diet. Thus, further understanding of the regulatory mechanism of symbiosis with humans and Bifidobacterium, with a focus on HD5, remains a challenge to be addressed in future studies.
Recent studies revealed that supplementation with Bifidobacterium during the newborn to pre-weaning period contributes to children’s health by promoting the development of the immune system, bowel function, and weight gain17,79 and further reduces the risk of NEC80, suggesting the benefit of the administration of Bifidobacterium as probiotics during the pre-weaning period. Furthermore, the relative abundance of Bifidobacterium in adults inversely correlates with serum markers of chronic inflammation (CRP and IL-6)25, and adult patients with several diseases, such as type 2 diabetes and type B hepatitis, have lower Bifidobacterium abundance24. Additionally, high Bifidobacterium abundance is related to healthy aging and longevity26,27. Thus, the establishment of Bifidobacterium-rich microbiota not only in early life but also in adulthood is thought to be important for promoting lifelong health. However, a study on the oral administration of B. longum AH1206 to healthy adults revealed that AH1206 becomes undetectable in more than 70% of the participants within the first month after administration, and the colonization of AH1206 in the intestine after 6 months is found in fewer than 30% of the participants81, indicating that colonization of orally administered live bacteria as probiotics in adulthood is transient in most cases. In this study, we revealed that the colonization of Bifidobacterium in the weaning period correlates with the establishment of a Bifidobacterium-rich microbiota in later life, and implied that HD5 promotes the colonization of Bifidobacterium in this period and after. This study highlights the importance of the weaning period in promoting the establishment of healthy intestinal microbiota throughout life, indicating that the weaning period is a window of opportunity for intervention in the intestinal environment. Recent studies revealed that butyrate and leucine directly induce granule secretion from Paneth cells42 and several food factors, such as rutin, inulin, and arginine increase the number of Paneth cells in vivo and ex vivo82,83,84. Our findings further contribute to the development of an integrated strategy for improving the early-life intestinal environment that combines conventional pre/probiotics with certain dietary factors that activates Paneth cell function.
It should be noted that there are several limitations in this study, including a relatively small sample size and an observation period of 3 years in the longitudinal cohort. In addition, this study could not directly verify the effects of HD5 and Bifidobacterium in the weaning period on the future microbiota due to the basic nature of the observational study. Because the SMILE Iwamizama is an ongoing study, these limitations are expected to be addressed through validation studies using larger and longer-term datasets obtained from future follow-up and interventional trials targeting the weaning period. Despite these limitations, this study provides valuable insights into the mechanism for the establishment of Bifidobacterium-rich microbiota in humans relating to Paneth cell α-defensin and contributes to a better understanding of the interaction between humans and the intestinal microbiota in pursuit of life-course health from the early life to elderly.
Data availability
The 16S rRNA gene sequencing data associated with this study are publicly available in the NCBI BioProject PRJNA1265424. All numerical source data underlying the figures, tables, and supplementary items are also provided in the Supplementary Data 1. Metadata that could compromise the privacy of study participants are not publicly available except upon reasonable request to the corresponding author Kiminori N.
Code availability
The microbiota bioinformatics was implemented in Qiime2 (ver. 2022.8), which is publicly available (https://qiime2.org). All the statistical analyses were conducted by using the commercially available GraphPad Prism version 9.0 software (GraphPad Software Inc., San Diego, CA). BMI and BMI percentile were calculated by using a Microsoft Excel-based tool for growth evaluation provided by the Japanese Society for Pediatric Endocrinology (http://jspe.umin.jp/medical/chart_dl.html). The codes used for the microbiota analysis that support the findings of this study are available upon reasonable request to the corresponding author Kiminori N.
References
Barker, D. J. P., Osmond, C., Winter, P. D., Margetts, B. & Simmonds, S. J. Weight in infancy and death from ischaemic heart disease. Lancet 334, 577–580 (1989).
United Nations Children’s Fund (UNICEF). Improving Child Nutrition: The achievable imperative for global progress. https://www.unicef.or.jp/library/pdf/Nutrition_Report_20130416.pdf (2013).
Hoffman, D. J., Powell, T. L., Barrett, E. S. & Hardy, D. B. Developmental origins of metabolic diseases. Physiol. Rev. 101, 739–795 (2021).
de Vos, W. M., Tilg, H., Van Hul, M. V. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Chen, X. et al. Reactive granulopoiesis depends on T-cell production of IL-17A and neutropenia-associated alteration of gut microbiota. Proc. Natl. Acad. Sci. USA 119, e2211230119 (2022).
Jain, N. The early life education of the immune system: moms, microbes and (missed) opportunities. Gut Microbes 12, 1824564 (2020).
Sharon, G., Sampson, T. R., Geschwind, D. H. & Mazmanian, S. K. The central nervous system and the gut microbiome. Cell 167, 915–932 (2016).
Bager, P., Wohlfahrt, J. & Westergaard, T. Caesarean delivery and risk of atopy and allergic disesase: meta‐analyses. Clin. Exp. Allergy 38, 634–642 (2008).
Zven, S. E., Susi, A., Mitre, E. & Nylund, C. M. Association between use of multiple classes of antibiotic in infancy and allergic disease in childhood. JAMA Pediatr. 174, 199–200 (2020).
Azad, M. B., Bridgman, S. L., Becker, A. B. & Kozyrskyj, A. L. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int. J. Obes. 38, 1290–1298 (2014).
Slob, E. M. A. et al. Early-life antibiotic use and risk of attention-deficit hyperactivity disorder and autism spectrum disorder: results of a discordant twin study. Int. J. Epidemiol. 50, 475–484 (2020).
Sarkar, A., Yoo, J. Y., Dutra, S. V. O., Morgan, K. H. & Groer, M. The association between early-life gut microbiota and long-term health and diseases. J. Clin. Med. 10, 459 (2021).
Subramanian, S. et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 510, 417–421 (2014).
Mitsuoka, T. Establishment of intestinal bacteriology. Biosci. Microbiota Food Health 33, 99–116 (2014).
Izumi, H. et al. The combination of Bifidobacterium breve and three prebiotic oligosaccharides modifies gut immune and endocrine functions in neonatal mice. J. Nutr. 149, 344–353 (2019).
Henrick, B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11 (2021).
Hiraku, A. et al. Early probiotic supplementation of healthy term infants with Bifidobacterium longum subsp. infantis M-63 is safe and leads to the development of Bifidobacterium-predominant gut microbiota: a double-blind, placebo-controlled trial. Nutrients 15, 1402 (2023).
Luck, B. et al. Bifidobacteria shape host neural circuits during postnatal development by promoting synapse formation and microglial function. Sci. Rep. 10, 7737 (2020).
Pang, J. et al. Bifidobacterium animalis promotes the growth of weaning piglets by improving intestinal development, enhancing antioxidant capacity, and modulating gut microbiota. Appl. Environ. Microbiol. 88, e01296–22 (2022).
Fujimura, K. E. et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 22, 1187–1191 (2016).
Ruohtula, T. et al. Maturation of gut microbiota and circulating regulatory T cells and development of IgE sensitization in early life. Front. Immunol. 10, 2494 (2019).
Korpela, K. et al. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 5, 26 (2017).
Pärtty, A., Kalliomäki, M., Wacklin, P., Salminen, S. & Isolauri, E. A possible link between early probiotic intervention and the risk of neuropsychiatric disorders later in childhood: a randomized trial. Pediatr. Res. 77, 823–828 (2015).
Arboleya, S., Watkins, C., Stanton, C. & Ross, R. P. Gut bifidobacteria populations in human health and aging. Front. Microbiol. 7, 1204 (2016).
Munckhof, I. C. L. et al. Role of gut microbiota in chronic low‐grade inflammation as potential driver for atherosclerotic cardiovascular disease: a systematic review of human studies. Obes. Rev. 19, 1719–1734 (2018).
Biagi, E. et al. Gut microbiota and extreme longevity. Curr. Biol. 26, 1480–1485 (2016).
Ghosh, T. S., Shanahan, F. & O’Toole, P. W. The gut microbiome as a modulator of healthy ageing. Nat. Rev. Gastroenterol. Hepatol. 19, 565–584 (2022).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486, 222–227 (2012).
Odamaki, T. et al. Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16, 1–12 (2016).
Chaplin, A. V. et al. Intraspecies genomic diversity and long-term persistence of Bifidobacterium longum. PLoS ONE 10, e0135658 (2015).
Oki, K. et al. Long-term colonization exceeding six years from early infancy of Bifidobacterium longum subsp. longum in human gut. BMC Microbiol. 18, 209 (2018).
Salzman, N. H. et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat. Immunol. 11, 76–83 (2010).
Ehmann, D. et al. Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments. Proc. Natl. Acad. Sci. USA 116, 3746–3751 (2019).
Nakamura, K., Sakuragi, N., Takakuwa, A. & Ayabe, T. Paneth cell α-defensins and enteric microbiota in health and disease. Biosci. Microbiota Food Health 35, 57–67 (2016).
Ouellette, A. J. et al. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J. Cell Biol. 108, 1687–1695 (1989).
Jones, D. E. & Bevins, C. L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J. Biol. Chem. 267, 23216–23225 (1992).
Jones, D. E. & Bevins, C. L. Defensin‐6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 315, 187–192 (1993).
Nakamura, K. et al. Expression and localization of Paneth cells and their α-defensins in the small intestine of adult mouse. Front. Immunol. 11, 570296 (2020).
Selsted, M. E. & Ouellette, A. J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557 (2005).
Ayabe, T. et al. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1, 113–118 (2000).
Yokoi, Y. et al. Paneth cell granule dynamics on secretory responses to bacterial stimuli in enteroids. Sci. Rep. 9, 2710 (2019).
Takakuwa, A. et al. Butyric acid and leucine induce α-defensin secretion from small intestinal paneth cells. Nutrients 11, 2817 (2019).
Hayase, E. et al. R-spondin1 expands Paneth cells and prevents dysbiosis induced by graft-versus-host disease. J. Exp. Med. 214, 3507–3518 (2017).
Shimizu, Y. et al. Paneth cell α-defensin misfolding correlates with dysbiosis and ileitis in Crohn’s disease model mice. Life Sci. Alliance 3, e201900592 (2020).
Suzuki, K. et al. Decrease of α-defensin impairs intestinal metabolite homeostasis via dysbiosis in mouse chronic social defeat stress model. Sci. Rep. 11, 9915 (2021).
Nakamura, S. et al. Decreased Paneth cell α-defensins promote fibrosis in a choline-deficient L-amino acid-defined high-fat diet-induced mouse model of nonalcoholic steatohepatitis via disrupting intestinal microbiota. Sci. Rep. 13, 3953 (2023).
Wehkamp, J. & Stange, E. F. An update review on the Paneth cell as key to ileal Crohn’s disease. Front. Immunol. 11, 646 (2020).
Shimizu, Y. et al. Lower human defensin 5 in elderly people compared to middle-aged is associated with differences in the intestinal microbiota composition: the DOSANCO Health Study. Geroscience 44, 997–1009 (2022).
Shimizu, Y. et al. Shorter sleep time relates to lower human defensin 5 secretion and compositional disturbance of the intestinal microbiota accompanied by decreased short-chain fatty acid production. Gut Microbes 15, 2190306 (2023).
Bry, L. et al. Paneth cell differentiation in the developing intestine of normal and transgenic mice. Proc. Natl. Acad. Sci. USA 91, 10335–10339 (1994).
Heida, F. H. et al. Paneth cells in the developing gut: when do they arise and when are they immune competent? Pediatr. Res. 80, 306–310 (2016).
Inoue, R. et al. Postnatal changes in the expression of genes for cryptdins 1–6 and the role of luminal bacteria in cryptdin gene expression in mouse small intestine. FEMS Immunol. Med. Microbiol. 52, 407–416 (2008).
McElroy, S. J. et al. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G656–G666 (2011).
Masuda, K., Sakai, N., Nakamura, K., Yoshioka, S. & Ayabe, T. Bactericidal activity of mouse α-defensin cryptdin-4 predominantly affects noncommensal bacteria. J. Innate. Immun. 3, 315–326 (2011).
Komatsu, Y. et al. Associations between maternal diet, human milk macronutrients, and breast-fed infant growth during the first month of life in the SMILE Iwamizawa in Japan. Nutrients 15, 654 (2023).
Hosomi, K. et al. Method for preparing DNA from feces in guanidine thiocyanate solution affects 16S rRNA-based profiling of human microbiota diversity. Sci. Rep. 7, 4339 (2017).
Murakami, R., Hashikura, N., Yoshida, K., Xiao, J. & Odamaki, T. Growth-promoting effect of alginate on Faecalibacterium prausnitzii through cross-feeding with Bacteroides. Food Res. Int. 144, 110326 (2021).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
McDonald, D. et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 42, 715–718 (2024).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Ministry of Health, Labor and Welfare of Japan. Guidelines for Breast-Feeding and Weaning. https://www.mhlw.go.jp/content/11908000/000496257.pdf (2019).
World Health Organization. Guideline: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services. https://iris.who.int/handle/10665/259386 (2017).
Elphick, D., Liddell, S. & Mahida, Y. R. Impaired luminal processing of human defensin-5 in Crohn’s disease persistence in a complex with chymotrypsinogen and trypsin. Am. J. Pathol. 172, 702–713 (2008).
Nakamura, K., Sakuragi, N. & Ayabe, T. A monoclonal antibody-based sandwich enzyme-linked immunosorbent assay for detection of secreted α-defensin. Anal. Biochem. 443, 124–131 (2013).
Bazanella, M. et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am. J. Clin. Nutr. 106, 1274–1286 (2017).
Horigome, A. et al. Colonization of supplemented Bifidobacterium breve M-16V in low birth weight infants and its effects on their gut microbiota weeks post-administration. Front. Microbiol. 12, 610080 (2021).
Turroni, F. et al. Glycan utilization and cross-feeding activities by Bifidobacteria. Trends Microbiol. 26, 339–350 (2018).
Ojima, M. N. et al. Priority effects shape the structure of infant-type Bifidobacterium communities on human milk oligosaccharides. ISME J. 16, 2265–2279 (2022).
Turroni, F. et al. Glycan cross-feeding activities between bifidobacteria under in vitro conditions. Front. Microbiol. 6, 1030 (2015).
Ignacio, A. et al. Correlation between body mass index and faecal microbiota from children. Clin. Microbiol. Infect. 22, 258.e1–258.e8 (2016).
Sahoo, K. et al. Childhood obesity: causes and consequences. J. Fam. Med. Prim. Care 4, 187–192 (2015).
Nader, P. R. et al. Identifying risk for obesity in early childhood. Pediatrics 118, e594–e601 (2006).
Reddel, S. et al. Gut microbiota profile in children affected by atopic dermatitis and evaluation of intestinal persistence of a probiotic mixture. Sci. Rep. 9, 4996 (2019).
Acuña, I. et al. Infant gut microbiota associated with fine motor skills. Nutrients 13, 1673 (2021).
Larsen, I. S. et al. Human Paneth cell α-defensin-5 treatment reverses dyslipidemia and improves glucoregulatory capacity in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metabol. 317, E42–E52 (2019).
Oshiro, T. et al. Bifidobacterium supplementation of colostrum and breast milk enhances weight gain and metabolic responses associated with microbiota establishment in very-preterm infants. Biomed. Hub 4, 502935 (2019).
Patole, S. K. et al. Benefits of Bifidobacterium breve M-16V Supplementation in preterm neonates—a retrospective cohort study. PLoS ONE 11, e0150775 (2016).
Maldonado-Gómez, M. X. et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 20, 515–526 (2016).
Guo, X. et al. Rutin and its combination with inulin attenuate gut dysbiosis, the inflammatory status and endoplasmic reticulum stress in Paneth cells of obese mice induced by high-fat diet. Front. Microbiol. 9, 2651 (2018).
Hou, Q. et al. Regulation of the Paneth cell niche by exogenous L‐arginine couples the intestinal stem cell function. FASEB J. 34, 10299–10315 (2020).
Beisner, J. et al. Prebiotic inulin and sodium butyrate attenuate obesity-induced intestinal barrier dysfunction by induction of antimicrobial peptides. Front. Immunol. 12, 678360 (2021).
Acknowledgements
The authors gratefully acknowledge all volunteers who participated in this study, municipal officers of Iwamizawa city, staff members of the Iwamizawa Lady’s Clinic and Iwamizawa Municipal General Hospital, and other people who cooperated in the SMILE Iwamizawa. The authors also acknowledge the experimental support from Ms. Aiko Kuroishi. This study was supported by the Japan Science and Technology Agency [JPMJCE 1301 to T.A., A.T., and K.N., JPMJPF2108 to A.T. and K.N.]; Japan Society for the Promotion of Science [21H02891 to T.A. and 20H04098 and 22K19120 to K.N.].
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.S., Koshi N., A.T., and Kiminori N. Data curation: Y.S., Y.Y., S.O., H.I.M.I., F.T., T.K., Koshi N., and Kiminori N. Formal analysis: Y.S., H.I., and M.I. Funding acquisition: A.T., T.A., and K.N. Investigation: Y.S., Y.Y., S.O., H.I., M.I., F.T., and Kiminori N. Project administration: A.T. and Kiminori N. Resources: Y.Y., S.O., S.K., Y.T., A.T., and Kiminori N. Supervision: Kiminori N. Validation: Y.S. and Kiminori N. Visualization: Y.S., T.A., and Kiminori N. Writing—original draft: Y.S. Writing—review and editing: A.T., T.A., and Kiminori N. All authors read, revised, and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
Y.S., H.I., S.K., F.T., and Y.T. are employees of Morinaga Milk Industry Co., Ltd. Other authors (Y.Y., S.O., M.I., T.K., Koshi N., A.T., T.A., Kiminori N.) declare no competing interests.
Ethics approval
This study was approved by the ethics committees of the Graduate School of Medicine, Hokkaido University (16-039) and Morinaga Milk Industry Co., Ltd (16005-144).
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimizu, Y., Yokoi, Y., Ohira, S. et al. Modulation of Bifidobacterium by HD5 during weaning is associated with high abundance in later life. Commun Med 5, 250 (2025). https://doi.org/10.1038/s43856-025-00977-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43856-025-00977-6