Abstract
Background
Particulate matter with an aerodynamic diameter of ≤2.5 µm (PM2.5) is a heterogeneous mixture, and specific substances that affect cardiovascular events remain unknown. We aimed to examine the association of short-term exposure to PM2.5 and its components with hospital admissions for acute myocardial infarction (AMI).
Methods
The concentrations of total PM2.5 and its individual components were continuously measured using Aerosol Chemical Speciation Analysers. From a national-scale administrative database collected from 828 facilities in 47 prefectures across Japan from April 2017 to December 2019, we extracted AMI data for seven prefectures where these aerosol analysers were installed. The primary outcome was the relationship of PM2.5 and its components with AMI hospitalisation. A time-stratified case-crossover analysis was conducted, and the approximate risk of AMI by pollutant concentrations was estimated using a conditional logistic regression model. In total, 44,232 patients with AMI aged 40–104 years (74.9% male) were examined.
Results
The estimated effect of an increase in the total PM2.5 concentration is significantly associated with AMI-related hospitalisation. Upon further examination of the components of PM2.5, black carbon has a more substantial influence on AMI development than water-soluble organic compounds, nitrate, and sulphate ions.
Conclusions
Short-term exposure to PM2.5 is associated with an increased incidence of AMI. Future research prioritises the components of PM2.5, with particular focus on whether a decrease in black carbon concentrations can mitigate the risk of future air quality-related AMI.
Plain language summary
This study examined whether short-term exposure to fine air particles, known as PM2.5, increases the risk of heart attacks. PM2.5 consists of tiny substances that can enter the lungs and bloodstream. Researchers analysed over 44,000 heart attack cases in seven regions of Japan between 2017 and 2019. They found that higher PM2.5 levels shortly before each case were linked to more hospital visits for heart problems. Among the components of PM2.5, black carbon—produced by car exhaust, factories, and smoke—showed the strongest link to heart attacks. The findings suggest that reducing black carbon in the air may help lower the risk of heart attacks. Cleaner air could lead to better heart health and benefit public well-being.
Similar content being viewed by others
Introduction
The World Health Organization (WHO) data indicate that almost the entire global population (99%) is exposed to air containing high levels of pollutants, exceeding the limits set in the WHO guidelines. This widespread exposure significantly contributes to the global disease burden, posing substantial health challenges worldwide1. Exposure to air pollution is estimated to result in millions of deaths and lead to significant years of healthy life lost annually, with air pollution ranking as the fourth leading risk factor for death in both men and women2.
Air pollution increases the incidence of cardiovascular and respiratory diseases, as well as neurological diseases such as stroke, dementia, Alzheimer’s disease, and Parkinson’s disease. Air pollution is now recognised as the greatest environmental threat to human health3,4. The concentration of particulate matter (PM) with an aerodynamic diameter of ≤2.5 µm (PM2.5) has been associated with the risk of developing myocardial infarction5,6. PM2.5 exposure has been hypothesised to lead to the development of cardiovascular disease through various potential mechanisms, including the generation of inflammation and oxidative stress within the lungs, influx of particulate matter into the circulation, alterations in the composition of the gut microbiota resulting from oral intake, and activation of neurohormonal pathways, which may lead to adrenal and autonomic imbalances7. However, the underlying pathogenesis of cardiovascular disease remains unknown due to the diversity and complexity of PM2.5 components, making it challenging to determine their exact effects and mechanisms. It is probable that not all components of PM2.5 are equally toxic to humans5,6. Black carbon, which is one of the components of PM2.5, represents a significant risk factor for cardiovascular disease and is strongly associated with mortality8. Therefore, we hypothesised that the identification of black carbon from PM2.5 as a risk factor for cardiovascular disease can play a crucial role in the development of future regulations for black carbon.
This study confirms the association between short-term exposure to PM2.5 and its components and hospitalisation due to acute myocardial infarction (AMI). Furthermore, we explore the individual components of PM2.5 and to demonstrate that black carbon can serve as an additional indicator of air quality management for AMI risk.
Methods
This retrospective observational study with a time-stratified case-crossover design included multiple centres in Japan and was conducted using the Japanese Registry of all Cardiac and Vascular Diseases (JROAD)−Diagnostic Procedure Combination (DPC) database. As of 2019, the JROAD-DPC is a national survey of cardiovascular care in which a total of 828 Japanese Cardiovascular Society (JCS)-accredited hospitals participated. This database is a comprehensive resource for improving the quality of cardiovascular care9. The DPC system calculates the cost of inpatient care for acute care hospitals in Japan according to the International Classification of Diseases, 10th Revision diagnoses and medical treatment. The validity of the primary diagnosis, procedure records, and surgical records in the DPC database has been confirmed10,11. This study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. This study was approved by the Ethics Committees at the National Institute for Environmental Studies (No. 2022-003), Toho University (No. A22035), and Sakurajyuji Hospital (No. 2022-01). As all data were anonymised by the participating institutions before collection by the data holder, i.e., the JCS, the requirement for individual informed consent was waived.
Air pollutants and environmental data
In April 2017, the Ministry of the Environment, Japan, initiated the deployment of the Aerosol Chemical Speciation Analyser (ACSA-14, Kimoto Electric Co., Ltd., Osaka, Japan) at 10 monitoring sites across Japan, aiming to enhance the PM2.5 monitoring system12. This device performs continuous automatic measurement of the chemical and mass components of atmospheric aerosols separately for fine and coarse particles, enabling more stable measurements13. The concentrations of PM with an aerodynamic diameter of >2.5 and ≤10 µm (PM10−2.5), along with PM2.5 concentrations and PM2.5 composition, were continuously measured at 1-h intervals. The PM2.5 components included optically measured black carbon (OBC), water-soluble organic compounds (WSOC), nitrate ion (NO3–), and sulphate ion (SO42–), all of which can contribute to PM2.5 countermeasures by enabling a detailed and rapid understanding of domestic sources and the adverse effects of transboundary pollution from the continent12. The mass concentration of PM was determined using the beta-ray absorption method. The OBC mass concentration was measured optically using the near-infra-red spectroscopy method. The mass concentrations of WSOC and NO3– were determined using the ultraviolet photometric absorption method. The mass concentration of SO42– was measured using the BaSO4-based turbidimetric method with the addition of BaCl2 dissolved in polyvinylpyrrolidone solution13. For the days that had ≥20 h or more measurements, we calculated daily mean concentrations. From the JROAD-DPC database collected from 828 facilities in 47 prefectures across Japan from April 2017 to December 2019, we extracted AMI data for seven prefectures (Hokkaido, Niigata, Tokyo, Aichi, Osaka, Hyogo, and Fukuoka) where ACSA was installed. These sites were used to measure the concentrations of urban background total PM10−2.5, total PM2.5, and individual PM2.5 components. The remaining three sites situated in three distinct prefectures (Miyagi, Shimane, and Nagasaki) were not included in the present analysis. This was because each ACSA in these three sites is situated in a rural area, the mountains, or an island where people do not reside (ACSA was initially installed with the primary objective of monitoring transboundary pollution) (Supplementary Fig. 1).
We assigned prefecture-based concentrations to each patient because there was one monitoring site in each prefecture. Although exposure misclassification was inevitable, we used concentrations measured in populated areas where many patients with AMI resided. In our previous study, the daily mean concentrations of total PM2.5 measured at individual sites were highly correlated with those measured at other sites within the same prefecture14. For co-pollutants, including nitrogen dioxide (NO2), sulphur dioxide (SO2), and ozone (O3), we obtained data on hourly concentrations that were measured at ambient air pollution monitoring stations located in each prefectural capital, separately from the ACSA measurements, from the atmospheric environment database of the National Institute for Environmental Studies in Japan. From hourly measurements, we estimated the daily mean concentrations of NO2 and SO2 and the daily maximum 8 h mean concentrations of O3. Data published by the Japan Meteorological Agency were used to evaluate the daily mean temperature and relative humidity levels. Influenza incidence was referenced from the National Institute of Infectious Diseases database in Japan. An influenza epidemic period was defined as a week in which the number of recorded cases exceeded the 90th percentile of the distribution during the study period.
Study participants and participants’ selection
Data on patients admitted with AMI at 828 JCS-accredited hospitals between April 1, 2017, and December 31, 2019 (prior to the onset of the coronavirus pandemic), were extracted from the JROAD-DPC database9. During the study period between April 2017 and December 2019, we extracted the data of 102,233 patients with AMI along with the International Classification of Disease, Tenth Revision (ICD-10) codes for AMI (I21·0, I21·1, I21·2, I21·3, I21·4, and I21·9) in the fields for the main diagnosis, admission-precipitating diagnosis, and/or most resource-consuming diagnosis from the JROAD–DPC database. We selected 101,124 patients aged ≥40 years and identified 52,157 patients admitted to hospitals located in seven prefectures (Hokkaido, Niigata, Tokyo, Aichi, Osaka, Hyogo, and Fukuoka) where Aerosol Chemical Speciation Analysers were installed. After excluding 3,166 patients without emergency hospitalisation, 4488 patients who had not undergone coronary angiography (procedure code [claim code]; D206-2 [170020710]) and/or re-vascularisation (procedure code [claim code]; K5461 [150374910], K5462 [150375010], K5463 [150375110], K547 [150260350], K5481 [150284310], K5482 [150359310], K5491 [150375210], K5492 [150375310], K5493 [150375410], K550 [160107550], K550-2 [150318310]), and 271 patients who were not assigned concentration of PM2.5 total mass and/or its components, a total of 44,232 patients with AMI (33,143 men and 11,089 women) were included in the present analysis (Supplementary Fig. 2).
Statistics and Reproducibility
Patient characteristics and variables are summarised using descriptive statistics, such as mean (standard deviation [SD]), median, quartiles, and interquartile range (IQR) for continuous variables and as frequencies and percentages for categorical variables. A time-stratified case-crossover design was employed to estimate the association between short-term exposure to PM2.5 and admission of patients with AMI. The odds ratio (OR) and 95% confidence interval (CI) for IQR increase in PM2.5 concentrations were calculated using a conditional logistic regression model. The results were presented as %increase in AMI incidence, calculated as follows: [(OR − 1) × 100]. The ‘case’ day was defined as the day of admission, and three or four ‘control’ days were selected from the same days of the week within the same year and month as the ‘case’ day. Time-variant factors, including ambient temperature at lag0-20, relative humidity at lag0-1, and influenza epidemics, were included in the model. For temperature, we modelled using a cross-basis function with a quadratic b-spline with internal knots at the 10, 75, and 90th percentiles of temperature distributions for the exposure–response relationship between temperature and AMI, and with a natural cubic spline for lag with 3 degrees of freedom for its lag–response relationship over the lag of 21 days15,16,17. For humidity, we used a cubic spline function with 3 degrees of freedom. A single-component model was used to evaluate the association between individual PM2.5 components and hospital admission of patients with AMI, which was adjusted for PM10–2.5 concentrations. A multi-pollutant model was constructed, adjusted for PM10−2.5 and/or each co-pollutant one by one (NO2, SO2, and O3). Furthermore, a multi-component model was constructed, mutually adjusted for concentrations of four PM2.5 components, to examine the potential confounding effects of different pollutants and PM2.5 components. For example, when calculating the point estimate for OBC, adjustments were made for WSOC, NO3-, and SO42-.
From the JROAD-DPC database, we extracted information on the date of admission, the prefecture code of residence, age, sex, height, weight, Brinkman index (number of cigarettes per day multiplied by smoking year), and comorbidities (including hypertension, diabetes, and hyperlipidaemia). We categorised the hospitalisation season into warm (May to October) and cold (November to April). Analyses were stratified by age (40 − 64, 65 − 74, or ≥75 years), sex, the season of onset (warm [May to October] or cold [November to April]), body mass index (BMI) ( < 25 or ≥25 kg/m2), smoking history, hypertension, diabetes mellitus, and hyperlipidaemia. Smokers were defined as patients who had a Brinkman index >0. A likelihood ratio test was employed to assess the statistical associations between age, sex, season of onset, BMI, smoking history, hypertension, diabetes mellitus, and hyperlipidaemia with AMI. Pearson’s correlation coefficients were calculated between the daily mean concentrations of total PM2.5 and individual PM2.5 components. All analyses were conducted using STATA version 16.1 (Stata Corporation, College Station, TX, USA), and R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria) with the dlnm package15. All tests were two-sided and performed at a 5% level of statistical significance.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Patient characteristics at baseline
Of the 44,232 patients with AMI (mean [SD]: 69.6 [12.5] years), 14,251 were aged ≥75 years (38.4%). The study population comprised 74.9% male patients. The prevalence of hypertension, diabetes, and hyperlipidaemia was 64.6, 30.8, and 65.8%, respectively. Furthermore, 54.2% of the patients experienced AMI during the warm season, defined as the period between May and October (Table 1).
Total PM2.5 and PM2.5 components
The environmental factors during the study period are summarised in Table 2. The mean (SD) daily total PM2.5 and total PM10−2.5 concentrations were 11.5 (6.7) and 12.7 (9.4) μg/m³, respectively. The mean concentrations (SD) of PM2.5 components, including OBC, WSOC, NO3–, and SO42–, were 0.2 (0.3), 0.7 (0.7), 1.0 (1.2), and 2.9 (2.5) μg/m³, respectively. The proportion of daily mean OBC concentrations in the total mass of PM2.5 concentrations was 3.1% (2.4%), with a minimum of 0.0% and a maximum of 27.7%. The remaining PM2.5 components, including WSOC, NO3–, and SO42–, accounted for 6.6% (3.5%) (minimum 0.0%, maximum 35.7%), 8.9% (5.4%) (minimum 0.1%, maximum 49.4%), and 23.7% (10.9%) (minimum 0.1%, maximum 89.9%), respectively.
IQR increase in total PM2.5 and AMI hospitalisation
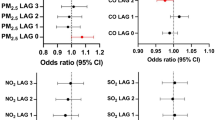
A clear health effect was observed for various lags in this study (Fig. 1). On examining the relationship between total PM2.5 and AMI hospitalisation, we observed that the estimated effects of %increase for an IQR increase in PM2.5 at lag0-1 were associated with a significantly higher incidence of AMI (%increase: 2.44, 95% CI: 0.85−4.07) following adjustment for ambient temperature, relative humidity, and influenza epidemics. The observed significance remained even after additional adjustment for PM10−2.5, with a %increase of 2.22 (95% CI: 0.30−4.19).
Estimated effects of %increase for an IQR increase in PM2.5 at lag0–1 were associated with a significantly higher incidence of AMI following adjustment for ambient temperature, relative humidity, and influenza epidemics. The error bars in the forest plot show the 95% confidence intervals for n = 44,232.
The association between total PM2.5 exposure and AMI hospitalisation was sustained after adjustment for co-pollutants in addition to PM10−2.5 (Supplementary Table 1). Exposure to PM10–2.5 at lag0-1 was not associated with AMI hospitalisation (PM2.5 adjusted %increase for an IQR increase = 0.34; 95% CI: −1.37–2.08).
IQR increase in PM2.5 components and AMI hospitalisation
We examined the relationship between each PM2.5 component and AMI hospitalisation for various lags. Compared with WSOC, NO3-, and SO42-, OBC at lag 0-1 was strongly associated with AMI hospitalisation, even after adjustment for ambient temperature, relative humidity, and influenza epidemics (%increase: 2.75, 95% CI: 0.96−4.57) (Fig. 2). This significant relationship remained unchanged in a multi-component model, with a %increase of 2.54 (95% CI: 0.00−5.14) (Supplementary Table 2).
The relationship between each PM2.5 component (OBC, WSOC, NO3- and SO42-) and AMI hospitalisation for lag 1, lag 2, lag 3, lag 0–1, lag 0–2, and lag 0–3 after adjustment for ambient temperature, relative humidity, and incidence of the influenza epidemic was examined. Compared with WSOC, NO3-, and SO42-, OBC at lag 0–1 was strongly associated with AMI hospitalisation, even after adjustment for ambient temperature, relative humidity, and influenza epidemics. The error bars in the forest plot show the 95% confidence intervals for n = 44,232.
Stratified analysis for the relationship between total PM2.5 and AMI hospitalisation
Supplementary Fig. 3 presents the stratified analysis data on the relationship between total PM2.5 and AMI hospitalisation at lag0-1. There were percentage increases associated with an IQR increase in PM2.5 levels for age ≥75 years (%increase: 3.45%; 95% CI: 0.30 − 6.70%), male sex (%increase: 2.59%; 95% CI: 0.36 − 4.87%), diabetes mellitus (%increase: 4.43%; 95% CI: 0.89 − 8.10%), smoking history (%increase: 2.82%; 95% CI: 0.07 − 5.65%), and warm season (%increase: 2.81%; 95% CI: 0.05 − 5.65%). Nevertheless, although all factors underwent effect modification, the results did not reach statistical significance.
Stratified analysis for the relationship between OBC and AMI hospitalisation
Regarding the relationship between OBC concentration and AMI hospitalisation at lag 0-1, similar results were obtained in stratified analysis as those obtained for PM2.5. In particular, male sex (%increase: 3.27%; 95% CI: 0.31 − 6.31%), diabetes mellitus (%increase: 4.70%; 95% CI: 0.10 − 9.51%), smoking status (%increase: 3.76%; 95% CI: 0.10 − 7.56%), and warm season (%increase: 5.33%; 95% CI: 1.24 − 9.58%) were associated with an increased risk of AMI hospitalisation. However, no significant effect modification was observed (Supplementary Fig. 4).
Correlations between daily total PM2.5 and PM2.5 components
The correlations between the daily total PM2.5 mass and each component were examined. Positive correlations were evident; however, OBC exhibited the weakest correlation (r = 0.58) compared with WSOC (r = 0.72), SO42− (r = 0.71), and NO3− (r = 0.65) (Supplementary Fig. 5).
Discussion
This study used a time-stratified case-crossover design to assess the association between short-term PM2.5 exposure and AMI admissions. The main findings highlighted a significant association between increased PM2.5 concentrations and increased incidence of AMI, with black carbon identified as a critical component of PM2.5 in assessing air quality-related cardiovascular risks. The present study makes a novel contribution to the field by focusing on the PM2.5 components and their relationship to AMI hospitalisation.
In seven of the 47 prefectures with continuous automatic analysers for measuring the air aerosol chemical composition in Japan, elevated PM2.5 concentrations were significantly associated with an increased risk of AMI hospitalisation. Furthermore, even after adjustment for PM2.5 components, OBC was still associated with AMI hospitalisation.
PM components are unlikely to all be equally involved in disease development or equally important in causing health effects18. Given the variability in the components of PM2.5 across seasons and regions19, these components should be considered when investigating the health effects due to exposure of PM2.5. This study suggests that black carbon, a component of PM2.5, is associated with the risk of developing AMI. Black carbon is generated by incomplete combustion of fossil fuels and other materials and is a measure of sooty carbon in the air that is quantified optically4,20. Combustion-related particles, such as black carbon, are more harmful to human health than PM components that are not produced by combustion21. Previous studies have demonstrated a significant association of elevated concentrations of black carbon with acute coronary syndrome development and cardiovascular mortality, given that black carbon was previously regarded as an independent pollutant, rather than as a component of PM2.522,23. The present study raises the possibility that black carbon is an important factor in the development of AMI due to PM2.5. To date, only few studies have examined the potential future consequences of exposure to black carbon in PM2.524.
This epidemiological study revealed that identification of the causal relationship of specific PM with increased risk of developing AMI is challenging. Nevertheless, the results demonstrated a significant association of PM2.5 and black carbon emissions with the incidence of AMI. Consequently, reducing these emissions may help in decreasing the future incidence of AMI. Given that black carbon is also related to global warming25,26, regulating emissions may also help in mitigating climate change.
This study has some limitations. First, similar to past epidemiologic environmental studies on the health effects of air pollution, the occurrence of exposure misclassification was inevitable, given that the results were based on pollutant data measured at a single monitoring station in each prefecture. It was difficult to estimate the bias effect of a combination of the Berkson error and classical error on our estimates of the association between PM2.5 and AMI risk27. Second, as in all epidemiologic studies, the data integrity, validity, and ascertainment bias in this study were potential limitations. However, the uniform data collection, large sample size, and implementation of a population-based design that covered all known AMI in Japan may have minimised the effects of these potential sources of bias28,29. Third, our previous investigation revealed a strong correlation between the daily average concentrations of total PM2.5 measured at monitoring stations in the same prefecture14. However, this assertion may not be applicable to PM2.5 components. Fourth, there are considerable interrelationships among atmospheric particle composition, especially among composition originating from the same source8. In addition to black carbon, PM2.5 contains various other known components, including aluminium, iron, potassium, sodium, Ni, sulphur, silicon, vanadium, and zinc30. It is also possible that PM2.5 contains many unknown chemical compositions. However, the WHO regional office for Europe has indicated that black carbon represents a significant risk factor for cardiovascular disease and is strongly associated with mortality8. Although black carbon is not typically considered a direct cause of AMI, it is often regarded as an indicator of a wide variety of combustion-derived chemical components.
The study utilised a nationwide Japanese cardiovascular disease database to ascertain that black carbon, a component of PM2.5, is a significant factor in AMI hospitalisation and may serve as a valuable indicator for future regulations and measures to reduce cardiovascular risk. Further detailed regional analyses, including differences between urban and rural areas and seasonal effects, could lead to more effective countermeasures in the future. Furthermore, a more comprehensive health risk assessment is warranted to evaluate not only the immediate but also the long-term health effects of exposure. Concrete data on the health effects of air pollution to policymakers can aid in the development of effective environmental policies. For the general public, the dissemination of such data can raise awareness about the health effects of air pollution, thereby increasing individual participation in preventive actions and environmental improvements. This study represents an important step toward understanding the link between air pollution and cardiovascular disease and could have a significant impact on future health measures and environmental policies. In addition to reducing the burning of fossil fuels and biomass, it may be important to implement measures to safeguard public health, such as encouraging the use of masks and discouraging outdoor activities when PM2.5 concentrations are elevated.
In conclusion, short-term exposure to PM2.5 and its component black carbon particles is associated with an increased incidence of AMI. Although other components may be involved in the development of AMI, black carbon is a valuable indicator for assessing the AMI risk associated with air quality.
Data availability
The data that support the findings of this study were used under licence from the Japanese Circulation Society for the current study and are not publicly available. However, the data are available from the authors upon reasonable request and with permission from the Centre for the Japanese Registry of all Cardiac and Vascular Diseases (JROAD) at the Japanese Circulation Society (https://www.j-circ.or.jp/jittai_chosa/). The air pollution data utilised for this study are publicly available from the following websites: https://www.env.go.jp/air/osen/pm_resultmonitoring/post_25.html (the Ministry of the Environment) and https://tenbou.nies.go.jp/download/ (National Institute for Environmental Studies). The air pollution dataset used in the present study can be available upon reasonable request for research purposes to Dr Takehiro Michikawa (takehiro.michikawa@med.toho-u.ac.jp). The numerical data (source data) for Fig. 1 are in Supplementary Data 1. The numerical data (source data) for Fig. 2 are in Supplementary Data 2. The numerical data (source data) for Supplementary Fig. 2 are in Supplementary Data 3. The numerical data (source data) for Supplementary Fig. 3 are in Supplementary Data 4. The numerical data (source data) for Supplementary Fig. 4 are in Supplementary Data 5. The numerical data (source data) for Supplementary Fig. 5 are in Supplementary Data 6.
References
World Health Organization (WHO). Air pollution https://www.who.int/health-topics/air-pollution#tab=tab_1.
GBD. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1223–1249 (2020).
Fu, P., Guo, X., Cheung, F. M. H. & Yung, K. K. L. The association between PM2.5 exposure and neurological disorders: a systematic review and meta-analysis. Sci. Total Environ. 655, 1240–1248 (2019).
WHO European Centre for Environment and Health. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulphur dioxide and carbon monoxide https://www.who.int/publications/i/item/9789240034228/ (WHO, 2021).
Luo, C. et al. Short-term exposure to particulate air pollution and risk of myocardial infarction: a systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 22, 14651–14662 (2015).
Farhadi, Z., Abulghasem Gorgi, H., Shabaninejad, H., Aghajani Delavar, M. & Torani, S. Association between PM2.5 and risk of hospitalization for myocardial infarction: a systematic review and a meta-analysis. BMC Public Health 20, 314 (2020).
Zhang, K., Brook, R. D., Li, Y., Rajagopalan, S. & Kim, J. B. Air pollution, built environment, and early cardiovascular disease. Circ. Res. 132, 1707–1724 (2023).
WHO Regional Office for Europe. Review of Evidence on Health Aspects of Air Pollution: REVIHAAP Project: Technical Report https://www.who.int/europe/publications/i/item/WHO-EURO-2013-4101-43860-61757 (WHO, 2021).
Ishii, M. et al. Association of short-term exposure to air pollution with myocardial infarction with and without obstructive coronary artery disease. Eur. J. Prev. Cardiol. 28, 1435–1444 (2021).
Yamana, H. et al. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J. Epidemiol. 27, 476–482 (2017).
Konishi, T. et al. Validity of operative information in Japanese administrative data: a chart review-based analysis of 1221 cases at a single institution. Surg. Today 52, 1484–1490 (2022).
Ministry of the Environment. Automatic measurement results of fine particulate matter (PM2.5) components (in Japanese) https://www.env.go.jp/air/osen/pm_resultmonitoring/post_25.html.
Pan, X. et al. Synergistic effect of water-soluble species and relative humidity on morphological changes in aerosol particles in the Beijing megacity during severe pollution episodes. Atmos. Chem. Phys. 19, 219–232 (2019).
Kojima, S. et al. Association of fine particulate matter exposure with bystander-witnessed out-of-hospital cardiac arrest of cardiac origin in Japan. JAMA Netw. Open 3, e203043 (2020).
Gasparrini, A., Armstrong, B. & Kenward, M. G. Distributed lag non-linear models. Stat. Med. 29, 2224–2234 (2010).
Ng, C. F. S. et al. Sociogeographic variation in the effects of heat and cold on daily mortality in Japan. J. Epidemiol. 24, 15–24 (2014).
Gasparrini, A. et al. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386, 369–375 (2015).
World Health Organization. Health Relevance of Particulate Matter from Various Sources: Report on a WHO Workshop, Bonn, Germany 26-27 March 2007 (WHO Regional Office for Europe, 2007).
EPA. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2019) https://assessments.epa.gov/isa/document/&deid=347534 (EPA, 2019).
Zhang, Z., Cheng, Y., Liang, L. & Liu, J. The measurement of atmospheric black carbon: a review. Toxics 11, 975 (2023).
Krzyzanowski, M., Kuna-Dibbert, B. & Schneider, J. (ed.). Health effects of transport-related air pollution. WHO Regional Office Europe; (WHO, 2005).
Dominguez-Rodriguez, A., Rodríguez, S., Abreu-Gonzalez, P., Avanzas, P. & Juarez-Prera, R. A. Black carbon exposure, oxidative stress markers and major adverse cardiovascular events in patients with acute coronary syndromes. Int. J. Cardiol. 188, 47–49 (2015).
Zhu, X. et al. Short and long-term association of exposure to ambient black carbon with all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Pollut. 324, 121086 (2023).
Michikawa, T. et al. Effects of exposure to chemical components of fine particulate matter on mortality in Tokyo: a case-crossover study. Sci. Total Environ. 755, 142489 (2021).
Bond, T. C. & Sun, H. Can reducing black carbon emissions counteract global warming? Environ. Sci. Technol. 39, 5921–5926 (2005).
Takemura, T. & Suzuki, K. Weak global warming mitigation by reducing black carbon emissions. Sci. Rep. 9, 4419 (2019).
Armstrong, B. G. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup. Environ. Med. 55, 651–656 (1998).
Nakai, M. et al. Associations among cardiovascular and cerebrovascular diseases: analysis of the nationwide claims-based JROAD-DPC dataset. PLoS ONE 17, e0264390 (2022).
Nakao, K. et al. Comparison of care and outcomes for myocardial infarction by heart failure status between United Kingdom and Japan. ESC Heart Fail 10, 1372–1384 (2023).
Zhou, J., Ito, K., Lall, R., Lippmann, M. & Thurston, G. Time-series analysis of mortality effects of fine particulate matter components in Detroit and Seattle. Environ. Health Perspect. 119, 461–466 (2011).
Acknowledgements
The authors would like to thank the participants of the JROAD-DPC for their contributions and the JROAD-DPC registry facilities for maintaining the registry. This study was supported in part by the Environment Research and Technology Development Fund (grant number JPMEERF20225M02) of the Environmental Restoration and Conservation Agency provided by the Ministry of the Environment of Japan and Grants-in-Aid for Scientific Research (grant numbers 21K07356 and 24K15317) from the Japan Society for the Promotion of Science, Japan. The sponsors had no role in any stage of the study, including the study design, data analysis, and manuscript preparation.
Author information
Authors and Affiliations
Contributions
S.K.: study conceptualisation, methodology, formal analysis, investigation, data curation, writing - original draft, writing - review & editing, visualisation, supervision, project administration, and funding acquisition; T.M.: study conceptualisation, methodology, software, formal analysis, investigation, data curation, writing - review & editing, visualisation, project administration, and funding acquisition; A.Y.: study investigation, resources, and writing - review & editing; K.T.: study resources and writing - review & editing; T.I. contributed to resources and writing - review & editing; Y.N.: study investigation and writing - review & editing; A.T.: study conceptualisation, methodology, investigation, resources, and writing - review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work. [A peer review file is available.]
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kojima, S., Michikawa, T., Yoshino, A. et al. Components of particulate matter as potential risk factors for acute myocardial infarction. Commun Med 5, 362 (2025). https://doi.org/10.1038/s43856-025-01095-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-025-01095-z