Abstract
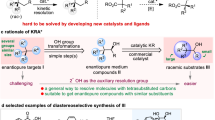
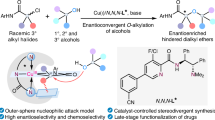
Chiral tertiary alcohols are privileged structures found in numerous bioactive molecules and pharmaceutical agents. However, general and efficient synthetic methods for forming α,β-stereogenic tertiary alcohols bearing two or more contiguous stereocentres are rare. Here we report the development of an enantioconvergent method for the synthesis of α,β-stereogenic tertiary alcohols in a single step by allylation, propargylation or crotylation of racemic α-amino, α-(hetero)aryl and α,α-dialkyl ketones using readily available boronic ester reagents. The identification of the chiral ligand/copper catalytic system enables rapid interconversion and chiral recognition between two enantiomers of racemic ketones, allowing the dynamic kinetic resolution process to occur. The reaction features high levels of diastereoselectivity and enantioselectivity, a wide scope of heterocycle substrates and high functional group compatibility, and provides a general and efficient synthesis of a variety of complex chiral tertiary alcohols otherwise difficult to access, thereby offering a tool for the rapid modification and synthesis of drug molecules.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2266504 (4k), 2266505 (10c), 2266506 (11c) and 2266507 (7n). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Corey, E. J. & Kürti, L. Enantioselective Chemical Synthesis: Methods, Logic and Practice (Direct Book, 2010).
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. Comprehensive Asymmetric Catalysis Vols I–III (Springer, 1999).
Shibasaki, M. & Kanai, M. Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C–C bond formation to ketones and ketimines. Chem. Rev. 108, 2853–2873 (2008).
Stymiest, J. L., Bagutski, V., French, R. M. & Aggarwal, V. K. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature 456, 778–782 (2008).
Zhou, H. et al. Organocatalytic stereoselective cyanosilylation of small ketones. Nature 605, 84–89 (2022).
Peyton, L. R., Gallagher, S. & Hashemzadeh, M. Triazole antifungals: a review. Drugs Today (Barc.) 51, 705–718 (2015).
Liu, R. Y. & Buchwald, S. L. CuH-catalyzed olefin functionalization: from hydroamination to carbonyl addition. Acc. Chem. Res. 53, 1229–1243 (2020).
Yang, Y., Perry, I. B., Lu, G., Liu, P. & Buchwald, S. L. Copper-catalyzed asymmetric addition of olefin-derived nucleophiles to ketones. Science 353, 144–150 (2016).
Bartolo, N. D., Read, J. A., Valentín, E. M. & Woerpel, K. A. Reactions of allylmagnesium reagents with carbonyl compounds and compounds with C–N double bonds: their diastereoselectivities generally cannot be analyzed using the Felkin−Anh and chelation-control models. Chem. Rev. 120, 1513–1619 (2020).
Evans, D. A. Stereoselective organic reactions: catalysts for carbonyl addition processes. Science 240, 420–426 (1988).
Yus, M., González-Gómez, J. C. & Foubelo, F. Diastereoselective allylation of carbonyl compounds and imines: application to the synthesis of natural products. Chem. Rev. 113, 5595–5698 (2013).
Caddick, S. & Jenkins, K. Dynamic resolutions in asymmetric synthesis. Chem. Soc. Rev. 25, 447–456 (1996).
Huerta, F. F., Minidis, A. B. E. & Bäckvall, J. E. Racemization in asymmetric synthesis. dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 30, 321–331 (2001).
Bhat, V., Welin, E. R., Guo, X. & Stoltz, B. M. Advances in stereoconvergent catalysis from 2005 to 2015: transition-metal-mediated stereoablative reactions, dynamic kinetic resolutions, and dynamic kinetic asymmetric transformations. Chem. Rev. 117, 4528–4561 (2017).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
You, H., Rideau, E., Sidera, M. & Fletcher, S. P. Non-stabilized nucleophiles in Cu-catalysed dynamic kinetic asymmetric allylic alkylation. Nature 517, 351–355 (2015).
Dehovitz, J. S. et al. Static to inducibly dynamic stereocontrol: the convergent use of racemic ketones. Science 369, 1113–1118 (2020).
Huang, M., Zhang, L., Pan, T. & Luo, S. Deracemization through photochemical E/Z isomerization of enamines. Science 375, 869–874 (2022).
Noyori, R. et al. Stereoselective hydrogenation via dynamic kinetic resolution. J. Am. Chem. Soc. 111, 9134–9135 (1989).
Xie, J.-H. & Zhou, Q.-L. Catalytic asymmetric hydrogenation of α-substituted ketones and aldehydes via dynamic kinetic resolution: efficient approach to chiral alcohols. Aldrichimica Acta. 48, 33–40 (2015).
Steward, K. M., Gentry, E. C. & Johnson, J. S. Dynamic kinetic resolution of α-keto esters via asymmetric transfer hydrogenation. J. Am. Chem. Soc. 134, 7329–7332 (2012).
Wang, F. et al. Asymmetric transfer hydrogenation of α‑substituted-β-keto carbonitriles via dynamic kinetic resolution. J. Am. Chem. Soc. 143, 2477–2483 (2021).
Liu, J., Krajangsri, S., Yang, J., Li, J. & Andersson, P. G. Iridium-catalysed asymmetric hydrogenation of allylic alcohols via dynamic kinetic resolution. Nat. Catal. 1, 438–443 (2018).
Bartlett, S. L. & Johnson, J. S. Synthesis of complex glycolates by enantioconvergent addition reactions. Acc. Chem. Res. 50, 2284–2296 (2017).
Bartlett, S. L., Keiter, K. M. & Johnson, J. S. Synthesis of complex tertiary glycolates by enantioconvergent arylation of stereochemically labile α-keto esters. J. Am. Chem. Soc. 139, 3911–3916 (2017).
Zavesky, B. P. & Johnson, J. S. Direct zinc(II)-catalyzed enantioconvergent additions of terminal alkynes to α-keto esters. Angew. Chem. Int. Ed. 56, 8805–8808 (2018).
Liu, W., Cao, W., Hu, H., Lin, L. & Feng, X. Dynamic kinetic asymmetric transformations of β-halo-α-keto esters by N,N′-dioxide/Ni(II)-catalyzed carbonyl-ene reaction. Chem. Commun. 54, 8901–8904 (2018).
Ruan, L.-X., Sun, B., Liu, J. & Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 379, 662–670 (2023).
Carreira, M. E. & Kvaerno, L. Classics in Stereoselective Synthesis (Wiley-VCH, 2009).
Yus, M., González-Gómez, J. C. & Foubelo, F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 111, 7774–7854 (2011).
Ding, C. & Hou, X. Catalytic asymmetric propargylation. Chem. Rev. 111, 1914–1937 (2011).
Zanghi, J. M. & Meek, S. J. Cu-catalyzed diastereo- and enantioselective reactions of γ,γ-disubstituted allyldiboron compounds with ketones. Angew. Chem. Int. Ed. 59, 8451–8455 (2020).
Karasawa, T., Oriez, R., Kumagai, N. & Shibasaki, M. anti-Selective catalytic asymmetric nitroaldol reaction of α‑keto esters: intriguing solvent effect, flow reaction, and synthesis of active pharmaceutical ingredients. J. Am. Chem. Soc. 140, 12290–12295 (2018).
Li, K., Shao, X., Tseng, L. & Malcolmson, S. J. 2-Azadienes as reagents for preparing chiral amines: synthesis of 1,2-amino tertiary alcohols by Cu-catalyzed enantioselective reductive couplings with ketones. J. Am. Chem. Soc. 140, 598–601 (2018).
Cai, Y. et al. Copper-catalyzed enantioselective Markovnikov protoboration of α-olefins enabled by a buttressed N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 57, 1376–1380 (2018).
de Jesús Cruz, P., Cassels, W. R., Chen, C. & Johnson, J. S. Doubly stereoconvergent crystallization enabled by asymmetric catalysis. Science 376, 1224–1230 (2022).
Shi, S.-L., Wong, Z. & Buchwald, S. L. Copper-catalysed enantioselective stereodivergent synthesis of amino alcohols. Nature 532, 353–356 (2016).
Liu, C., Xie, J.-H., Li, Y.-L., Chen, J.-Q. & Zhou, Q.-L. Asymmetric hydrogenation of α,α‘-disubstituted cycloketones via dynamic kinetic resolution: an efficient construction of chiral diols with three contiguous stereocenters. Angew. Chem. Int. Ed. 52, 593–596 (2013).
Jiang, B. & Shi, S.-L. Pd-catalyzed cross-coupling of alkylzirconocenes and aryl chlorides. Chin. J. Chem. 40, 1813–1820 (2022).
Li, T. et al. Asymmetric trapping of zwitterionic intermediates by sulphur ylides in a palladium-catalysed decarboxylation-cycloaddition sequence. Nat. Commun. 5, 5500 (2014).
Liu, Z. et al. Balancing skeleton and functional groups in total syntheses of complex natural products: a case study of tigliane, daphnane and ingenane diterpenoids. Nat. Prod. Rep. 38, 1589–1617 (2021).
Hasuoka, A. & Yamamoto, S. Cyclic amine compound. WO 2008/066117 A1 (2008).
Evans, D. A., Siska, S. J. & Cee, V. J. Resurrecting the Cornforth model for carbonyl addition: studies on the origin of 1,2-asymmetric induction in enolate additions to heteroatom-substituted aldehydes. Angew. Chem. Int. Ed. 42, 1761–1765 (2003).
Cherest, M., Felkin, H. & Prudent, N. Torsional strain involving partial bonds. The stereochemistry of the lithium aluminium hydride reduction of some simple open-chain ketones. Tetrahedron Lett. 9, 2199–2204 (1968).
Anh, N. T. & Eisenstein, O. Induction asymetrique 1–2: comparaison ab initio des modeles de cram, de cornforth, de karabatsos et de felkin. Tetrahedron Lett. 17, 155–158 (1976).
Acknowledgements
Financial support was provided by the National Key R&D Program of China (2022YFA1503702, 2021YFF0701600), the National Natural Science Foundation of China (22325110, 92256303, 21821002, 22171280), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0610000), the Program of Shanghai Academic Research Leader (22XD1424900), the CAS Youth Interdisciplinary Team (JCTD-2021-11), the Ningbo Natural Science Foundation (2022J017) (S.-L.S.), the Natural Science Foundation of Shandong Province (ZR2019BB011) and the Scientific Research Foundation of Qingdao University of Science & Technology (12030430010799) (B.S.).
Author information
Authors and Affiliations
Contributions
B.S. developed the catalytic methods, investigated the substrate scope and conducted mechanistic studies. L.-X.R., R.Z., J.Z., R.N., Q.L., Y.Z. and L.G. synthesized substrates and performed partial substrate scope studies. B.S. and S.-L.S. wrote the manuscript. S.-L.S. conceived the project and directed the investigations.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jeffrey Johnson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, Figs. 1–6, synthetic procedures, scale-up experiments, synthetic transformations and application of the reaction products, control experiments, compound characterization, X-ray crystallography, HPLC analysis and NMR spectra.
Supplementary Data 1
Crystallographic data for compound 4k, CCDC 2266504.
Supplementary Data 2
Crystallographic data for compound 7n, CCDC 2266507.
Supplementary Data 3
Crystallographic data for compound 10c, CCDC 2266505.
Supplementary Data 4
Crystallographic data for compound 11c, CCDC 2266506.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, B., Ruan, LX., Zhao, R. et al. Dynamic kinetic asymmetric allylation, propargylation and crotylation of ketones using copper catalysis. Nat. Synth 3, 1091–1103 (2024). https://doi.org/10.1038/s44160-024-00567-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00567-9
This article is cited by
-
Selective dynamic kinetic asymmetric aldehyde–alkyne reductive coupling
Nature Synthesis (2025)
-
Ni(II)-catalyzed asymmetric alkenylation and arylation of aryl ketones with organoborons via 1,5-metalate shift
Nature Communications (2024)
-
Shattering the mirror with copper catalysis
Nature Synthesis (2024)