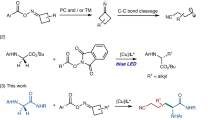
Abstract
Peptide salicylaldehyde esters are the requisite coupling partner in Ser/Thr ligation reactions towards chemical protein synthesis. In general, it would be cost-effective and efficient to use side-chain-protected peptide acids, after Fmoc solid-phase peptide synthesis, for direct C-terminal derivatization; however, this has yet to be achieved, due to an intrinsic epimerization pathway. Here we report the development of 2-(dichloromethyl)phenol as a reagent that can directly form peptide salicylaldehyde esters in an epimerization-free manner. Mechanistic studies reveal that the 2-(dichloromethyl)phenol reagent serves as a source of highly reactive quinone methide species that can be trapped by the peptide C-terminal carboxylate to give α-chloroesters, followed by an Obenzylic-to-Ophenolic acyl transfer and chloride extrusion process. The peptide salicylaldehyde ester reaction products have been applied in the convergent total chemical synthesis of linker histone H1.2 using sequential Ser/Thr ligation reactions.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the main text and Supplementary Information. Source data are provided with this paper.
References
Kürti, L. & Czakó, B. Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms (Elsevier Academic, 2005).
McClelland, R. A. & Santry, L. Reactivity of tetrahedral intermediates. Acc. Chem. Res. 16, 394–399 (1983).
Tailhades, J., Patil, N. A., Hossian, M. A. & Wade, J. D. Intramolecular acyl transfer in peptide and protein ligation and synthesis. J. Pept. Sci. 21, 139–147 (2015).
Burke, H. M., McSweeney, L. & Scanlan, E. M. Exploring chemoselective S-to-N acyl transfer reactions in synthesis and chemical biology. Nat. Commun. 8, 15655 (2017).
Dawson, P. E., Muir, T. M., Clark-Lewis, I. & Kent, S. B. H. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Agouridas, V. et al. Native chemical ligation and extended methods: mechanisms, catalysis, scope, and limitations. Chem. Rev. 119, 7328–7443 (2019).
Quadere, R., Sewing, A. & Hilvert, D. Selenocysteine-mediated native chemical ligation. Helv. Chim. Acta 84, 1197–1206 (2001).
Hondal, R. J., Nilsson, B. L. & Raines, R. T. Selenocysteine in native chemical ligation and expressed protein ligation. J. Am. Chem. Soc. 123, 5140–5141 (2001).
Gieselman, M. D., Xie, L. & van der Donk, W. A. Synthesis of a selenocysteine-containing peptide by native chemical ligation. Org. Lett. 3, 1331–1334 (2001).
Quaderer, R. & Hilvert, D. Selenocysteine-mediated backbone cyclization of unprotected peptides followed by alkylation, oxidative elimination or reduction of the selenol. Chem. Commun. https://doi.org/10.1039/B208288H (2002).
Roelfes, G. & Hilvert, D. Incorporation of selenomethionine into proteins through selenohomocysteine-mediated ligation. Angew. Chem. Int. Ed. 42, 2275–2277 (2003).
Sohma, Y., Sasaki, M., Hayashi, Y., Kimura, T. & Kiso, Y. Novel and efficient synthesis of difficult sequence-containing peptides through O–N intramolecular acyl migration reaction of O-acyl isopeptides. Chem. Commun. https://doi.org/10.1039/B312129A (2004).
Sohma, Y. et al. Development of O-acyl isopeptide method. Pept. Sci. 88, 253–262 (2007).
Popov, V., Panda, S. S. & Katritzky, A. R. Ligations from tyrosine isopeptides via 12- to 19-membered cyclic transition states. J. Org. Chem. 78, 7455–7461 (2013).
Liu, C.-F. & Tam, J. P. Chemical ligation approach to form a peptide bond between unprotected peptide segments. Concept and model study. J. Am. Chem. Soc. 116, 4149–4153 (1994).
Tam, J. P. & Miao, Z. Stereospecific pseudoproline ligation of N-terminal serine, threonine, or cysteine-containing unprotected peptides. J. Am. Chem. Soc. 121, 9013–9022 (1999).
Zhang, Y., Xu, C., Lam, H. Y., Lee, C. L. & Li, X. Protein chemical synthesis by serine and threonine ligation. Proc. Natl Acad. Sci. USA 110, 6657–6662 (2013).
Liu, H. & Li, X. Serine/threonine ligation: origin, mechanistic aspects, and applications. Acc. Chem. Res. 51, 1643–1655 (2018).
Tan, Y. et al. Cysteine/penicillamine ligation independent of terminal steric demands for chemical protein synthesis. Angew. Chem. Int. Ed. 59, 12741–12745 (2020).
Bode, J. W., Fox, R. M. & Baucom, K. D. Chemoselective amide ligations by decarboxylative condensations of N-alkylhydroxylamines and α-ketoacids. Angew. Chem. Int. Ed. 45, 1248–1252 (2006).
Bode, J. W. Chemical protein synthesis with the α-ketoacid-hydroxylamine ligation. Acc. Chem. Res. 50, 2104–2115 (2017).
Panda, S. S., Hall, C. D., Oliferenko, A. A. & Katritzky, A. R. Traceless chemical ligation from S-, O-, and N-acyl isopeptides. Acc. Chem. Res. 47, 1076–1087 (2014).
Mhidia, R. et al. Exploration of an imide capture/N,N-acyl shift for asparagine native peptide bond formation. Bioorg. Med. Chem. 21, 3479–3485 (2013).
Kawakami, T. & Aimoto, S. Sequential peptide ligation by using a controlled cysteinyl prolyl ester (CPE) autoactivating unit. Tetrahedron Lett. 48, 1903–1905 (2007).
Tsuda, S., Shigenaga, A., Bando, K. & Otaka, A. N→S acyl-transfer-mediated synthesis of peptide thioesters using anilide derivatives. Org. Lett. 11, 823–826 (2009).
Ollivier, N., Dheur, J., Mhidia, R., Blanpain, A. & Melnyk, O. Bis(2-sulfanylethyl)amino native peptide ligation. Org. Lett. 12, 5238–5241 (2010).
Kang, J. & Macmillan, D. Peptide and protein thioester synthesis via N→S acyl transfer. Org. Biomol. Chem. 8, 1993–2002 (2010).
Raibaut, L. et al. Accelerating chemoselective peptide bond formation using bis(2-selenylethyl)amido peptide selenoester surrogates. Chem. Sci. 7, 2657–2665 (2016).
Chen, G. et al. Studies related to the relative thermodynamic stability of C-terminal peptidyl esters of o-hydroxy thiophenol: emergence of a doable strategy for non-cysteine ligation applicable to the chemical synthesis of glycopeptides. J. Am. Chem. Soc. 128, 7460–7462 (2006).
Botti, P., Villain, M., Manganiello, S. & Gaertner, H. Native chemical ligation through in situ O to S acyl shift. Org. Lett. 6, 4861–4864 (2004).
Nagano, M., Huang, Y., Obexer, R. & Suga, H. Chemical peptide macrolactonization via intramolecular S-to-S-to-O acyl transfer. Pept. Sci. 114, e24259 (2022).
Roslund, M. U. et al. Acyl group migration and cleavage in selectively protected β-D-galactopyranosides as studied by NMR spectroscopy and kinetic calculations. J. Am. Chem. Soc. 130, 8769–8772 (2008).
Lassfolk, R. et al. Acetyl group migration across the saccharide units in oligosaccharide model compound. J. Am. Chem. Soc. 141, 1646–1654 (2019).
Wang, Z. et al. Synthetic zwitterionic Streptococcus pneumoniae type 1 oligosaccharides carrying labile O-acetyl esters. Angew. Chem. Int. Ed. 62, e202211940 (2023).
Liang, X. et al. Syntheses, biological evaluation and SAR of ingenol mebutate analogues for treatment of actinic keratosis and non-melanoma skin cancer. Bioorg. Med. Chem. Lett. 23, 5624–5629 (2013).
Filice, M. et al. Chemo-biocatalytic regioselective one-pot synthesis of different deprotected monosaccharides. Catal. Today 140, 11–18 (2009).
Graziani, A., Passacantilli, P., Piancatelli, P. & Tani, S. A mild and efficient approach for the regioselective silyl-mediated protection-deprotection of C-4 hydroxyl group on carbohydrates. Tetrahedron Lett. 42, 3857–3860 (2001).
Deng, S. & Chang, C.-W. T. Cesium trifluoroacetate or silver oxide mediated acyl migration for the construction of disaccharide building blocks. Synlett https://doi.org/10.1055/s-2006-933105 (2006).
An, Y.-H. & Chang, Y.-T. Molecular evolution on chiro-inositol dibenzoate using intramolecular acyl migration and selection by phenyl boronic acid. J. Comb. Chem. 6, 293–296 (2004).
An, Y.-H. & Chang, Y.-T. Molecular evolution using intramolecular acyl migration on myo-inositol benzoates with thermodynamic and kinetic selectors. Chem. Eur. J. 10, 3543–3547 (2004).
Lee, C. L., Liu, H., Wong, C. T. T., Chow, H. Y. & Li, X. Enabling N-to-C Ser/Thr ligation for convergent protein synthesis via combining chemical ligation approaches. J. Am. Chem. Soc. 138, 10477–10484 (2016).
Li, T., Liu, H. & Li, X. Chemical synthesis of HMGA1a proteins with post-translational modifications via Ser/Thr ligation. Org. Lett. 18, 5944–5947 (2016).
Wu, H. et al. Chemical synthesis and biological evaluations of adiponectin collagenous domain glycoforms. J. Am. Chem. Soc. 143, 7808–7818 (2021).
Wu, H., Wei, T., Ngai, W. L., Zhou, H. & Li, X. Ligation embedding aggregation disruptor strategy enables the chemical synthesis of PD-1 immunoglobulin and extracellular domains. J. Am. Chem. Soc. 144, 14748–14757 (2022).
Wu, H., Tan, Y., Ngai, W. L. & Li, X. Total synthesis of interleukin-2 via a tunable backbone modification strategy. Chem. Sci. 14, 1582–1589 (2023).
Ma, W. et al. Chemical synthesis of proteins with base-labile posttranslational modifications enabled by a Boc-SPPS based general strategy towards peptide C-terminal salicylaldehyde esters. Angew. Chem. Int. Ed. 62, e202214053 (2023).
Lam, H. Y. et al. Total synthesis of daptomycin by cyclization via a chemoselective serine ligation. J. Am. Chem. Soc. 135, 6272–6279 (2013).
Wong, C. T. T., Lam, H. Y., Song, T., Chen, G. & Li, X. Synthesis of constrained head-to-tail cyclic tetrapeptides by an imine-induced ring-closing/contraction strategy. Angew. Chem. Int. Ed. 52, 10212–10215 (2013).
Jin, K. et al. Total synthesis of teixobactin. Nat. Commun. 7, 12394 (2016).
Sun, Z. et al. Total synthesis of malacidin A by β-hydroxyaspartic acid ligation-mediated cyclization and absolute structure establishment. Angew. Chem. Int. Ed. 59, 19868–19872 (2020).
Wang, J. et al. Total synthesis of mannopeptimycin β via β-hydroxyenduracididine ligation. J. Am. Chem. Soc. 143, 12784–12790 (2021).
Dolci, S., Marchetti, F., Pampaloni, G. & Zacchini, S. A crystallographic and spectroscopic study on the reactions of WCl6 with carbonyl compounds. Dalton Trans. 42, 5635–5648 (2013).
Aghapour, G. & Afzali, A. Facile conversion of aldehydes and ketones to gem-dichlorides using chlorodiphenylphosphine/N-chlorosuccinimide as a new and neutral system. Synth. Commun. 38, 4023–4035 (2008).
Barlos, K. et al. Darstellung geschützter peptid-fragmente unter einsatz substituierter triphenylmethyl-harze. Tetrahedron Lett. 30, 3943–3946 (1989).
Barlos, K. et al. Veresterung von partiell geschützten peptid-fragmenten mit harzen. Einsatz von 2-chlortritylchlorid zur synthese von Leu15-gastrin I. Tetrahedron Lett. 30, 3947–3950 (1989).
Wong, C. T. T., Li, T., Lam, H. Y., Zhang, Y. & Li, X. Realizing serine/threonine ligation: scope and limitations and mechanistic implication thereof. Front. Chem. 2, 28 (2014).
Carpino, L. A. & El-Faham, A. Efficiency in peptide coupling: 1-hydroxy-7-azabenzotriazole vs 3,4-dihydro-3-hydroxy-4-oxo-1,2,3-benzotriazine. J. Org. Chem. 60, 3561–3564 (1995).
Li, H. et al. 3-(Diethoxyphosphoryloxy)-1,2,3-benzotriazin-4(3H)-one (DEPBT): a new coupling reagent with remarkable resistance to racemization. Org. Lett. 1, 91–93 (1999).
Ye, Y., Li, H. & Jiang, X. DEPBT as an efficient coupling reagent for amide bond formation with remarkable resistance to racemization. Biopolymers 80, 172–178 (2005).
Hu, L. et al. Ynamides as racemization-free coupling reagents for amide and peptide synthesis. J. Am. Chem. Soc. 138, 13135–13138 (2016).
Yang, Y., Hansen, L. & Baldi, A. Suppression of simultaneous Fmoc-His(Trt)-OH racemization and Nα-DIC-endcapping in solid-phase peptide synthesis through design of experiments and its implication for an amino acid activation strategy in peptide synthesis. Org. Process Res. Dev. 26, 2464–2474 (2022).
Zhou, Y. et al. Suppression of alpha-carbon racemization in peptide synthesis based on a thiol-labile amino protecting group. Nat. Commun. 14, 53204 (2023).
Snella, B., Diemer, V., Drobeca, H., Agouridas, V. & Melnyk, O. Native chemical ligation at serine revisited. Org. Lett. 20, 7616–7619 (2018).
Malins, L. R., Cergol, K. M. & Payne, R. J. Chemoselective sulfenylation and peptide ligation at tryptophan. Chem. Sci. 5, 260–266 (2014).
Toteva, M. M. & Richard, J. P. The generation and reactions of quinone methides. Adv. Phys. Org. Chem. 45, 39–91 (2011).
Singh, M. S., Nagaraju, A., Anand, N. & Chowdhury, S. ortho-Quinone methide (o-QM): a highly reactive, ephemeral and versatile intermediate in organic synthesis. RSC Adv. 4, 55924–55959 (2014).
Huang, Z. et al. Bioorthogonal photocatalytic decaging-enabled mitochondrial proteomics. J. Am. Chem. Soc. 143, 18714–18720 (2021).
Diao, L., Yang, C. & Wan, P. Quinone methide intermediates from the photolysis of hydroxybenzyl alcohols in aqueous solution. J. Am. Chem. Soc. 117, 5369–5370 (1995).
Khan, P. R., Charan, P. H. K. & Rao, M. B. FeCl3 catalyzed Prins cyclization for the synthesis of angularly fused polycyclic scaffolds. Synth. Commun. 50, 432–437 (2020).
Mardirossian, N. & Head-Gordon, M. Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol. Phys. 115, 2315–2372 (2017).
Tiwary, A. S. & Mukherjee, A. K. Performance of the M06 family of functionals in prediction of the charge transfer transition energies of the naphthalene-TCNE and pyrene-TCNE molecular complexes. Chem. Phys. Lett. 610, 19–22 (2014).
Lee, W., Benton, T. R., Sengupta, A. & Houk, K. N. Molecular dynamics of the norbornyl cation in solution and its generation in Winstein-Trifan solvolysis: the timing of sigma bridging. J. Org. Chem. 89, 1140–1146 (2024).
Zhou, Y. & Yu, Z. 1,5‐X insertions of free alkylidene carbenes: a theoretical study. Asian J. Org. Chem. 12, e202300440 (2023).
Harshman, S. W., Young, N. L., Parthun, M. R. & Freitas, M. A. H1 histones: current perspectives and challenges. Nucleic Acids Res. 41, 9593–9609 (2013).
Hergeth, S. P. & Schneider, R. The H1 linker histones: multifunctional proteins beyond the nucleosomal core particle. EMBO Rep. 16, 1439–1453 (2015).
Flanagan, T. W. & Brown, D. T. Molecular dynamics of histone H1. Biochim. Biophys. Acta 1859, 468–475 (2016).
Izzo, A. & Schneider, R. The role of linker histone H1 modifications in the regulation of gene expression and chromatin dynamic. Biochim. Biophys. Acta 1859, 486–495 (2016).
Lai, S., Jia, J., Cao, X., Zhou, P.-K. & Gao, S. Molecular and cellular functions of the linker histone H1.2. Front. Cell Dev. Biol. 9, 773195 (2022).
Fyodorov, D. V., Zhou, B. R., Skoultchi, A. I. & Bai, Y. Emerging roles of linker histones in regulating chromatin structure and function. Nat. Rev. Mol. Cell Biol. 19, 192–206 (2018).
Kamo, N. et al. Organoruthenium-catalyzed chemical protein synthesis to elucidate the functions of epigenetic modifications on heterochromatin factors. Chem. Sci. 12, 5926–5937 (2021).
Hong, Z. Z. et al. Development of convergent hybrid phase ligation for efficient and convenient total synthesis of proteins. Pept. Sci. 115, e24332 (2023).
Acknowledgements
The work was supported by the Grants Research Council of University Grants Committee of Hong Kong (C7017-18G, 17302621, 17306521 and AoE/P-706/16) and the National Natural Science Foundation of China (22177097).
Author information
Authors and Affiliations
Contributions
X.L. conceived and supervised the study. X.L. and W.X. designed experiments. W.X., Z.Z. and J.L. performed experiments. B.-W.L. and Z.-X.Y. performed the computational study. X.L., H.L., W.X. and B.-W.L. analysed the data. X.L. and H.L. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Robert Pollice and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–76, Table 1, additional experimental procedures, computational calculation details and characterization data.
Supplementary Data 1
Cartesian coordinates of intermediates and transition states.
Source data
Source Data Fig. 8b
Scanned SDS–PAGE of synthetic and expressed histone H1.2. The three lanes on the right side are not related to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, W., Li, BW., Zhong, Z. et al. O-to-O acyl transfer for epimerization-free peptide C-terminal salicylaldehyde ester synthesis. Nat. Synth 3, 1049–1060 (2024). https://doi.org/10.1038/s44160-024-00570-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00570-0
This article is cited by
-
Advances in the chemical synthesis of human proteoforms
Science China Life Sciences (2025)