Abstract
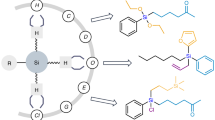
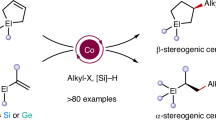
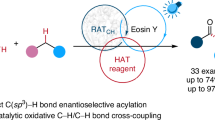
Organosilicon compounds are of great value in chemistry and material science. However, site-selective C–H bond functionalization of simple silanes to prepare more complex organosilicon compounds is challenging because of the presence of multiple C–H bonds in the same molecule. Here we report a broadly applicable photocatalytic site-selective radical functionalization of organosilicon compounds enabled by the β-silicon effect, wherein a silyl group selectively activates β-C(sp3)–H bonds, leading to lower bond dissociation energy than that of α-C(sp3)–H bonds and γ-C(sp3)–H bonds. Various β-C(sp3)–H bond aminoalkylation, alkylation and arylation reactions have been achieved and applied in complex molecule synthesis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or Supplementary Information. Crystallographic data for the structure reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition no. CCDC 2257454 (58). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Lee, V. Y. Organosilicon Compounds (Elsevier, 2017).
Hiyama, T. & Oestreich, M. Organosilicon Chemistry: Novel Approaches and Reactions (Wiley, 2020).
Fan, X. et al. Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y. Nat. Chem. 15, 666–676 (2023).
Rogge, T. et al. C–H activation. Nat. Rev. Methods Primers 1, 43–73 (2021).
Yang, Y., Liu, J., Ouyang, K. & Xi, Z. Transition-metal-catalyzed cleavage of silyl C(sp3)–H bonds. Sci. Sin. Chim. 51, 97–109 (2021).
Liang, Y., Geng, W., Wei, J., Ouyang, K. & Xi, Z. Palladium-catalyzed silyl C(sp3)–H bond activation. Org. Biomol. Chem. 10, 1537–1542 (2012).
Han, J., Qin, Y., Ju, C. & Zhao, D. Divergent synthesis of vinyl-, benzyl-, and borylsilanes: aryl to alkyl 1,5-palladium migration/coupling sequences. Angew. Chem. Int. Ed. 59, 6555–6560 (2020).
Ohmura, T., Torigoe, T. & Suginome, M. Catalytic functionalization of methyl group on silicon: iridium-catalyzed C(sp3)–H borylation of methylchlorosilanes. J. Am. Chem. Soc. 134, 17416–17419 (2012).
Fang, H., Hou, W., Liu, G. & Huang, Z. Ruthenium-catalyzed site-selective intramolecular silylation of primary C–H bonds for synthesis of sila-heterocycles. J. Am. Chem. Soc. 139, 11601–11609 (2017).
Oeschger, R. et al. Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science 368, 736–741 (2020).
Seyferth, D., Washburne, S. S., Attridge, C. J. & Yamamoto, K. Halomethylmetal compounds. XXXIV. Insertion of phenyl(bromodichloromethyl)mercury-derived dichlorocarbene into carbon–hydrogen bonds. Tetraalkylsilicon and tetraalkyltin compounds. J. Am. Chem. Soc. 92, 4405–4417 (1970).
Hatanaka, Y., Watanabe, M., Onozawa, S., Tanaka, M. & Sakurai, H. Rhodium-catalyzed insertion of carbenoids into β-C–H bonds of silacycloalkanes: a facile and general approach to functionalized silacycloalkanes. J. Org. Chem. 63, 422–423 (1998).
Garlets, Z. J. & Davies, H. M. L. Harnessing the β-silicon effect for regioselective and stereoselective rhodium(II)-catalyzed C–H functionalization by donor/acceptor carbenes derived from 1-sulfonyl-1,2,3-triazoles. Org. Lett. 20, 2168–2171 (2018).
Ninomiya, R. et al. β-Silicon-effect-promoted intermolecular site-selective C(sp3)–H amination with dirhodium nitrenes. Chem. Commun. 56, 5759–5762 (2020).
Golden, D. L., Suh, S.-E. & Stahl, S. S. Radical C(sp3)–H functionalization and cross-coupling reactions. Nat. Rev. Chem. 6, 405–427 (2022).
Sarkar, S., Cheung, K. P. S. & Gevorgyan, V. C–H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals. Chem. Sci. 11, 12974–12993 (2020).
Yi, H. et al. Recent advances in radical C–H activation/radical cross-coupling. Chem. Rev. 117, 9016–9085 (2017).
Bellotti, P., Huang, H.-M., Faber, T. & Glorius, F. Photocatalytic late-stage C–H functionalization. Chem. Rev. 123, 4237–4352 (2023).
Ye, Z., Lin, Y. & Gong, L. The merger of photocatalyzed hydrogen atom transfer with transition metal catalysis for C–H functionalization of alkanes and cycloalkanes. Eur. J. Org. Chem. 2021, 5545–5556 (2021).
Ravelli, D., Protti, S. & Fagnoni, M. Decatungstate anion for photocatalyzed “Window Ledge” reactions. Acc. Chem. Res. 49, 2232–2242 (2016).
Stateman, L., Nakafuku, K. & Nagib, D. Remote C–H functionalization via selective hydrogen atom transfer. Synthesis 50, 1569–1586 (2018).
Cao, H., Tang, X., Tang, H., Yuan, Y. & Wu, J. Photoinduced intermolecular hydrogen atom transfer reactions in organic synthesis. Chem. Catal. 1, 523–598 (2021).
Jeffrey, J. L., Terrett, J. A. & MacMillan, D. W. C. O–H hydrogen bonding promotes H-atom transfer from α-C–H bonds for C-alkylation of alcohols. Science 349, 1532–1536 (2015).
Loh, Y. Y. et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Le, C., Liang, Y., Evans, R. W., Li, X. & MacMillan, D. W. C. Selective sp3 C–H alkylation via polarity-match-based cross-coupling. Nature 547, 79–83 (2017).
Zhong, P.-F. et al. Photoelectrochemical oxidative C(sp3)–H borylation of unactivated hydrocarbons. Nat. Commun. 14, 6530 (2023).
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
van Staden, L. F., Gravestock, D. & Agerc, D. J. New developments in the Peterson olefination reaction. Chem. Soc. Rev. 31, 195–200 (2002).
Roberts, D. D. & McLaughlin, M. G. Strategic applications of the β-silicon effect. Adv. Synth. Catal. 364, 2307–2332 (2022).
Wilt, J. W. & Aznavoorian, P. M. Favored reduction of α-chlorosilanes vs α-chloroalkanes with tri-n-butyltin hydride. J. Org. Chem. 43, 1285–1286 (1978).
Kawamura, T. & Kochi, J. K. Hyperconjugative and p–d homoconjugative effects of silicon, germanium, and tin on alkyl radicals from electron spin resonance studies. J. Am. Chem. Soc. 94, 648–650 (1972).
Landais, Y. Stereocontrol in reactions of cyclic and acyclic β-silyl radicals. C. R. Chim. 8, 823–832 (2005).
Rubinsztajn, S. & Cella, J. A. A new polycondensation process for the preparation of polysiloxane copolymers. Macromolecules 38, 1061–1063 (2005).
Mu, Q.-C., Chen, J., Xia, C.-G. & Xu, L.-W. Synthesis of silacyclobutanes and their catalytic transformations enabled by transition-metal complexes. Coord. Chem. Rev. 374, 93–113 (2018).
Huang, J., Liu, F., Wu, X., Chen, J.-Q. & Wu, J. Recent advances in the reactions of silacyclobutanes and their applications. Org. Chem. Front. 9, 2840–2855 (2022).
Fotie, J., Matherne, C. M. & Wroblewski, J. E. Silicon switch: carbon–silicon bioisosteric replacement as a strategy to modulate the selectivity, physicochemical, and drug like properties in anticancer pharmacophores. Chem. Bio. Drug Des. 102, 235–254 (2023).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem 6, 1877–1887 (2020).
Collins, K. D., Rühling, A. & Glorius, F. Application of a robustness screen for the evaluation of synthetic organic methodology. Nat. Protoc. 9, 1348–1353 (2014).
Dai, Z.-Y., Nong, Z.-S. & Wang, P.-S. Light-mediated asymmetric aliphatic C–H alkylation with hydrogen atom transfer catalyst and chiral phosphoric acid. ACS Catal. 10, 4786–4790 (2020).
Capaldo, L., Fagnoni, M. & Ravelli, D. Vinylpyridines as building blocks for the photocatalyzed synthesis of alkylpyridines. Chem. Eur. J. 23, 6527–6530 (2017).
Fan, P. et al. Photocatalytic hydroacylation of trifluoromethyl alkenes. Chem. Commun. 55, 12691–12694 (2019).
Li, Y., Guo, S., Li, Q.-H. & Zheng, K. Metal-free photoinduced C(sp3)–H/C(sp3)–H cross-coupling to access α-tertiary amino acid derivatives. Nat. Commun. 14, 6225 (2023).
Niu, L., Liu, J., Liang, X.-A., Wang, S. & Lei, A. Visible light-induced direct α C–H functionalization of alcohols. Nat. Commun. 10, 467 (2019).
He, T., Li, B., Liu, L., Ma, W. & He, W. Rhodium-catalyzed intermolecular silylation of Csp–H by silacyclobutanes. Chem. Eur. J. 27, 5648–5652 (2021).
Chen, H. et al. Rhodium-catalyzed reaction of silacyclobutanes with unactivated alkynes to afford silacyclohexenes. Angew. Chem. Int. Ed. 58, 4695–4699 (2019).
Tang, X. et al. Ring expansion of silacyclobutanes with allenoates to selectively construct 2- or 3-(E)-enoate-substituted silacyclohexenes. ACS Catal. 12, 5185–5196 (2022).
Acknowledgements
We are grateful to National Key R&D Program of China (2022YFA1506100, X.S.), National Natural Science Foundation of China (22471201, X.S.; 21901191, X.S.) and Fundamental Research Funds for the Central Universities (2042023kf0202, X.S.) for financial support. We thank T. Ritter (Max-Planck-Institut für Kohlenforschung) for helpful discussions. We thank R. Zhang from Core Facility of Wuhan University for the assistance with X-ray structure analysis. The theoretical calculations were performed on the supercomputing system in the Supercomputing Center of Wuhan University.
Author information
Authors and Affiliations
Contributions
X.S. conceived the idea, guided the project and wrote the paper with revisions by all the other authors; X.H. developed the catalytic methods and performed the mechanistic studies and synthetic applications. X.H., W.Z., Z.L. and Y. Zhao prepared the substrates and studied the scope. Y. Zhang performed the calculations of the BDEs. S.L. co-guided the project and analysed the spectra data. All the authors were involved in the discussion and analysis of the data.
Corresponding author
Ethics declarations
Competing interests
X.S., S.L. and X.H. may benefit from a patent based on the method disclosed in this paper. The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Mark McLaughlin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental Details, Discussion, Sections 1–10, Figs. 1–5 and Tables 1–6.
Supplementary Data 1
Crystallographic data for compound 58, CCDC 2257454.
Supplementary Data 2
Structure factors for compound 58, CCDC 2257454.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, X., Zhang, Y., Liu, S. et al. Photocatalytic site-selective radical C(sp3)–H aminoalkylation, alkylation and arylation of silanes. Nat. Synth 4, 188–195 (2025). https://doi.org/10.1038/s44160-024-00664-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00664-9