Abstract
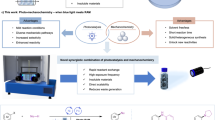
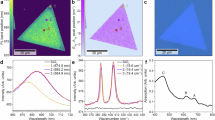
Implementing photochemical reactions through mechanochemical methods can reduce waste generation by eliminating the need for bulk solvents. The absence of solvent results in exceptionally high concentrations of catalysts and reactants in reactions, substantially improving reaction rates and efficiency in terms of both time and energy utilization. However, the integration of mechano- and photochemical approaches is often hindered by the limited transparency of mechanochemical reaction vessels. Here we present a mechano-photoexcitation strategy that utilizes mechanoluminescent materials, in particular SrAl2O4:Eu2+/Dy3+, as internal photon sources activated by mechanical energy. We demonstrate the efficacy of this strategy in two photochemical processes: the Hofmann–Löffler–Freytag reaction and the activation of electron donor–acceptor complexes in sulfonylation reactions. Mechanistic studies confirm the radical nature of these transformations, and control experiments validate the critical role of mechano-photoactivation. By utilizing mechanical energy alone, this method eliminates the need for external light sources and enables gramme-scale photochemical transformations. Our approach represents a valuable application of mechanical energy in synthetic chemistry, providing a complementary means for integrating photochemistry with mechanochemistry.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Hoffmann, N. Photochemical reactions as key steps in organic synthesis. Chem. Rev. 108, 1052–1103 (2008).
Kärkäs, M. D., Porco, J. A. Jr. & Stephenson, C. R. J. Photochemical approaches to complex chemotypes: applications in natural product synthesis. Chem. Rev. 116, 9683–9747 (2016).
Stephenson C., Yoon T., MacMillan D. W. C., Visible Light Photocatalysis in Organic Chemistry (Wiley, 2018).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Crisenza, G. E. M. & Melchiorre, P. Chemistry glows green with photoredox catalysis. Nat. Commun. 11, 803 (2020).
Martinez, V., Stolar, T., Karadeniz, B., Brekalo, I. & Užarević, K. Advancing mechanochemical synthesis by combining milling with different energy sources. Nat. Rev. Chem. 7, 51–65 (2023).
Do, J. L. & Friščić, T. Mechanochemistry: a force of synthesis. ACS Central Sci. 3, 13–19 (2017).
Boulatov, R. (ed.) Polymer Mechanochemistry, Vol. 369, 209–238 (Springer, 2015).
Garay, A. L., Pichon, A. & James, S. L. Solvent-free synthesis of metal complexes. Chem. Soc. Rev. 36, 846–855 (2007).
Friščić, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 20, 7599–7605 (2010).
James, S. L. et al. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 41, 413–447 (2012).
Patel, C. et al. Fluorochemicals from fluorspar via a phosphate-enabled mechanochemical process that bypasses HF. Science 381, 302–306 (2023).
Tan, D. & García, F. Main group mechanochemistry: from curiosity to established protocols. Chem. Soc. Rev. 48, 2274–2292 (2019).
Hernández, J. G. & Bolm, C. Altering product selectivity by mechanochemistry. J. Org. Chem. 82, 4007–4019 (2017).
Friščić, T., Mottillo, C. & Titi, H. M. Mechanochemistry for synthesis. Angew. Chem. Int. Ed. 132, 1030–1041 (2020). (2017).
Howard, J. L., Cao, Q. & Browne, D. L. Mechanochemistry as an emerging tool for molecular synthesis: what can it offer? Chem. Sci. 9, 3080–3094 (2018).
Andersen, J. & Mack, J. Mechanochemistry and organic synthesis: from mystical to practical. Green Chem. 20, 1435–1443 (2018).
Jones, A. C., Leitch, J. A., Raby-Buck, S. E. & Browne, D. L. Mechanochemical techniques for the activation and use of zero-valent metals in synthesis. Nat. Syn. 1, 763–775 (2022).
Sokolov, A. N., Bučar, D. K., Baltrusaitis, J., Gu, S. X. & MacGillivray, L. R. Supramolecular catalysis in the organic solid state through dry grinding. Angew. Chem. Int. Ed. 25, 4273–4277 (2010).
Stojaković, J., Farris, B. S. & MacGillivray, L. R. Liquid-assisted vortex grinding supports the single-step solid-state construction of a [2.2]paracyclophane. Faraday Discuss. 170, 35–40 (2014).
Elacqua, E., Kummer, K. A., Groeneman, R. H., Reinheimer, E. W. & MacGillivray, L. R. Post-application of dry vortex grinding improves the yield of a [2 + 2] photodimerization: addressing static disorder in a cocrystal. J. Photoch. Photobio. A. 331, 42–47 (2016).
Liu, L. et al. Photo-thermo-mechanochemical approach to synthesize quinolines via addition/cyclization of sulfoxonium ylides with 2-vinylanilines catalyzed by iron (II) phthalocyanine. Org. Lett. 24, 1146–1151 (2022).
Toda, F., Tanaka, K. & Sekikawa, A. Host–guest complex formation by a solid–solid reaction. Chem. Commun. 4, 279–280 (1987).
Hernández, J. G. Mechanochemical borylation of aryldiazonium salts; merging light and ball milling. Beilstein J. Org. Chem. 13, 1463–1469 (2017).
Obst, M. & König, B. Solvent-free, visible-light photocatalytic alcohol oxidations applying an organic photocatalyst. Beilstein J. Org. Chem. 12, 2358–2363 (2016).
Obst, M., Shaikh, R. S. & König, B. Solvent-free coupling of aryl halides with pyrroles applying visible-light photocatalysis. React. Chem. Eng. 2, 472–478 (2017).
Štrukil, V. & Sajko, I. Mechanochemically-assisted solid-state photocatalysis (MASSPC). Chem. Commun. 53, 9101–9104 (2017).
Biswas, S. et al. Photomechanochemical control over stereoselectivity in the [2 + 2] photodimerization of acenaphthylene. Faraday Discuss. 241, 266–277 (2023).
Baier, D. M., Spula, C., Fanenstich, S., Grätz, S. & Borchardt, L. The regioselective solid‐state photo‐mechanochemical synthesis of nanographenes with UV light. Angew. Chem. Int. Ed. 62, e202218719 (2023).
Millward, F. & Zysman-Colman, E. Mechanophotocatalysis: a generalizable approach to solvent-minimized photocatalytic reactions for organic synthesis. Angew. Chem. Int. Ed. 63, e202316169 (2024).
Kubota, K., Pang, Y., Miura, A. & Ito, H. Redox reactions of small organic molecules using ball milling and piezoelectric materials. Science 366, 1500–1504 (2019).
Pang, Y., Lee, J. W., Kubota, K. & Ito, H. Solid‐state radical C–H trifluoromethylation reactions using ball milling and piezoelectric materials. Angew. Chem. Int. Ed. 59, 22570–22576 (2020).
Amer, M. M., Hommelsheim, R., Schumacher, C., Kong, D. & Bolm, C. Electro-mechanochemical approach towards the chloro sulfoximidations of allenes under solvent-free conditions in a ball mill. Faraday Discuss. 241, 79–90 (2023).
Schumacher, C., Hernández, J. G. & Bolm, C. Electro‐mechanochemical atom transfer radical cyclizations using piezoelectric BaTiO3. Angew. Chem. Int. Ed. 59, 16357–16360 (2020).
Xie, Y. & Li, Z. Triboluminescence: recalling interest and new aspects. Chem 4, 943–971 (2018).
Zhuang, Y. & Xie, R.-J. Mechanoluminescence rebrightening the prospects of stress sensing: a review. Adv. Mater. 33, 2005925 (2021).
Feng, A. & Smet, P. F. A review of mechanoluminescence in inorganic solids: compounds, mechanisms, models and applications. Materials 11, 484 (2018).
Zhang, J.-C., Wang, X., Marriott, G. & Xu, C.-N. Trap-controlled mechanoluminescent materials. Prog. Mater Sci. 103, 678–742 (2019).
Zhang, H., Wei, Y., Huang, X. & Huang, W. Recent development of elastico-mechanoluminescent phosphors. J. Lumin. 207, 137–148 (2019).
Jha, P. & Chandra, B. P. Survey of the literature on mechanoluminescence from 1605 to 2013. Luminescence 29, 977–993 (2014).
Chandra, B. P. Development of mechanoluminescence technique for impact studies. J. Lumin. 131, 1203–1210 (2011).
Terasaki, N., Zhang, H., Imai, Y., Yamada, H. & Xu, C.-N. Hybrid material consisting of mechanoluminescent material and TiO2 photocatalyst. Thin Solid Films 518, 473–476 (2009).
Terasaki, N., Yamada, H. & Xu, C.-N. Ultrasonic wave induced mechanoluminescence and its application for photocatalysis as ubiquitous light source. Catal. Today 201, 203–208 (2013).
Terasaki, N., Zhang, H., Yamada, H. & Xu, C.-N. Mechanoluminescent light source for a fluorescent probe molecule. Chem. Commun. 47, 8034–8036 (2011).
Protti, S., Ravelli, D. & Fagnoni, M. Designing radical chemistry by visible light-promoted homolysis. Trends Chem. 4, 305–317 (2022).
Lang, Y., Li, C. J. & Zeng, H. Photo-induced transition-metal and external photosensitizer-free organic reactions. Org. Chem. Front. 8, 3594–3613 (2021).
Sumida, Y. & Ohmiya, H. Direct excitation strategy for radical generation in organic synthesis. Chem. Soc. Rev. 50, 6320–6332 (2021).
Wolff, M. E. Cyclization of N-halogenated amines (the Hofmann–Löffler reaction). Chem. Rev. 63, 55–64 (1963).
Dorta, R. L., Francisco, C. G. & Suárez, E. Hypervalent organoiodine reagents in the transannular functionalisation of medium-sized lactams: synthesis of 1-azabicyclo compounds. Chem. Commun. 1168–1169 (1989).
Stateman, L. M., Nakafuku, K. M. & Nagib, D. A. Remote C–H functionalization via selective hydrogen atom transfer. Synthesis 50, 1569–1586 (2018).
Martínez, C. & Muñiz, K. An iodine-catalyzed Hofmann–Löffler reaction. Angew. Chem. Int. Ed. 54, 8287–8291 (2015).
Wappes, E. A., Fosu, S. C., Chopko, T. C. & Nagib, D. A. Triiodide-mediated δ-amination of secondary C–H bonds. Angew. Chem. Int. Ed. 55, 9974–9978 (2016).
Dean, J. A. & Lange, N. A. Lange’s Handbook of Chemistry, 15th edn (McGraw-Hill, 1999).
Sakai, K., Koga, T., Imai, Y., Maehara, S. & Xu, C.-N. Observation of mechanically induced luminescence from microparticles. Phys. Chem. Chem. Phys. 8, 2819–2822 (2006).
Crisenza, G. E., Mazzarella, D. & Melchiorre, P. Synthetic methods driven by the photoactivity of electron donor–acceptor complexes. J. Am. Chem. Soc. 142, 5461–5476 (2020).
Wortman, A. K. & Stephenson, C. R. EDA photochemistry: mechanistic investigations and future opportunities. Chem. 9, 2390–2415 (2023).
Lasso, J. D. et al. A general platform for visible light sulfonylation reactions enabled by catalytic triarylamine EDA complexes. J. Am. Chem. Soc. 146, 2583–2592 (2024).
Kong, D., Amer, M. M. & Bolm, C. Stainless steel-initiated chloro sulfoximidations of allenes under solvent-free conditions in a ball mill. Green Chem. 24, 3125–3129 (2022).
Matsuzawa, T., Aoki, Y., Takeuchi, N. & Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4: Eu2+, Dy3+. J. Electrochem. Soc. 143, 2670 (1996).
Srivastava, V., Singh, P. K. & Singh, P. P. Recent advances of visible-light photocatalysis in the functionalization of organic compounds. J. Photochem. Photobiol., C 50, 100488 (2022).
Srivastava, V. & Singh, P. P. Retracted article: Eosin Y catalysed photoredox synthesis: a review. RSC Adv. 7, 31377–31392 (2017).
Jang, Y. J. et al. Green-light-driven Fe(III)(btz)3 photocatalysis in the radical cationic [4 + 2] cycloaddition reaction. Org. Lett. 24, 4479–4484 (2022).
Zhang, Z. et al. Titanocenes as photoredox catalysts using green‐light irradiation. Angew. Chem. Int. Ed. 59, 9355–9359 (2020).
Gisbertz, S. & Pieber, B. Heterogeneous photocatalysis in organic synthesis. ChemPhotoChem 4, 456–475 (2020).
Acknowledgements
We thank C. Bolm for proofreading and providing helpful suggestions on preparing the paper. This work is supported by the National Natural Science Foundation of China (22201230, Han Wang) and the Science and Technology Base and Talent Special Project of Guangxi (AD21220087, D.Z.).
Author information
Authors and Affiliations
Contributions
Han Wang conceived the concept. X.X. and Han Wang developed the mechanical HLF reactions. J.G., D.Z. and Han Wang developed the mechanical sulfonylation reactions. D.Z., J.W., H.T.A., R.W.T., Hui Wang, Y.L. and Y.C. designed the mechanistic investigations. X.X., J.G. and H.T.A. performed the control experiments. H.T.A., R.W.T., J.W. and Han Wang wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Eli Zysman-Colman, Sven Grätz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary Figs. 1–11 and Tables 1–15.
Source data
Source Data Fig. 5
Source data for Fig. 5.
Source Data Fig. 6
Source data for Fig. 6.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xin, X., Geng, J., Zhang, D. et al. Mechano-photoexcitation for organic synthesis using mechanoluminescent materials as photon sources. Nat. Synth 4, 177–187 (2025). https://doi.org/10.1038/s44160-024-00681-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00681-8
This article is cited by
-
Consecutive mechanical-force-induced electron transfer for reduction of aryl halides with high reduction potentials
Nature Communications (2025)
-
A scalable photo-mechanochemical platform for sustainable photoredox catalysis by resonant acoustic mixing
Nature Communications (2025)
-
A burst of light for mechanochemistry
Nature Synthesis (2024)