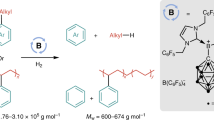
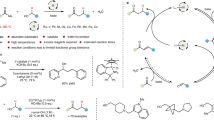
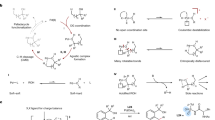
Abstract
Hydrogenolysis, a fundamental chemical process with broad applications, has traditionally been performed using heterogeneous catalysis at high temperatures and pressures with limited selectivity. Recent advancements highlight the potential of homogeneous catalysis as a promising alternative, offering improved selectivity under milder conditions. However, the development of a general homogeneous catalysis approach capable of hydrogenolysing carbon–halogen bonds—one of the most fundamental, versatile and extensively studied functional groups—remains an unresolved challenge. Here we present a comprehensive rationale for the fundamental mechanistic prerequisites crucial to achieving general homogeneous carbon–halogen bond hydrogenolysis, with a particular focus on the tritiation of challenging yet abundant alkyl chlorides. We demonstrate how cooperative interplay of bioinspired carbon–halogen activation and hydrogenation can efficiently catalyse the selective hydrogenolysis of unactivated organohalides. The utility of this approach is demonstrated through its capability to enable deuteration and tritiation of pharmaceutically relevant organohalides with simultaneous control over reactivity and selectivity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data generated and analysed during this study, which include experimental and spectroscopic data, are included in this article and its Supplementary Information. Source data are provided with this paper.
References
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Nakano, M. M. & Zuber, P. In Strict and Facultative Anaerobes Medical and Environmental Aspects 303–317 (CRC, 2004).
Ellis, L. D. et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 4, 539–556 (2021).
Shen, X., Zhang, C., Han, B. & Wang, F. Catalytic self-transfer hydrogenolysis of lignin with endogenous hydrogen: road to the carbon-neutral future. Chem. Soc. Rev. 51, 1608–1628 (2022).
Yang, Y. & McCarty, P. L. Biomass, oleate, and other possible substrates for chloroethene reductive dehalogenation. Bioremediat. J. 4, 125–133 (2000).
Kumar, A., Daw, P. & Milstein, D. Homogeneous catalysis for sustainable energy: hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 122, 385–441 (2022).
Cabrero-Antonino, J. R., Adam, R., Papa, V. & Beller, M. Homogeneous and heterogeneous catalytic reduction of amides and related compounds using molecular hydrogen. Nat. Commun. 11, 3893–3910 (2020).
Iwasaki, T., Tsuge, K., Naito, N. & Nozaki, K. Chemoselectivity change in catalytic hydrogenolysis enabling urea-reduction to formamide/amine over more reactive carbonyl compounds. Nat. Commun. 14, 3279 (2023).
Nishimura, S. In Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis 572–663 (John Wiley & Sons, 2001).
Ghosh B. & Maleczka, R. E., Jr. In Science of Synthesis: Catalytic Reduction in Organic Synthesis 355–373 (Thieme, 2017).
Alonso, F., Beletskaya, I. P. & Yus, M. Metal-mediated reductive hydrodehalogenation of organic halides. Chem. Rev. 102, 4009–4091 (2002).
Sergeev, A. G. & Hartwig, J. F. Selective, nickel-catalyzed hydrogenolysis of aryl ethers. Science 332, 439–443 (2011).
Bart, S. C. & Chirik, P. J. Selective, catalytic carbon–carbon bond activation and functionalization promoted by late transition metal catalysts. J. Am. Chem. Soc. 125, 886–887 (2003).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. Deuterium- and tritium-labelled compounds: applications in the life sciences. Angew. Chem. Int. Ed. 57, 1758–1784 (2018).
Atzrodt, J., Derdau, V., Kerr, W. J. & Reid, M. C–H Functionalisation for hydrogen isotope exchange. Angew. Chem. Int. Ed. 57, 3022–3047 (2018).
Yu, R. P., Hesk, D., Rivera, N., Pelczer, I. & Chirik, P. J. Iron-catalysed tritiation of pharmaceuticals. Nature 529, 195–199 (2016).
Zarate, C., Yang, H., Bezdek, M. J., Hesk, D. & Chirik, P. J. Ni(I)–X complexes bearing a bulky α‑diimine ligand: synthesis, structure, and superior catalytic performance in the hydrogen isotope exchange in pharmaceuticals. J. Am. Chem. Soc. 141, 5034–5044 (2019).
Yang, H., Dormer, P. G., Rivera, N. R. & Hoover, A. J. Palladium(II)-mediated C–H tritiation of complex pharmaceuticals. Angew. Chem. Int. Ed. 57, 1883–1887 (2018).
Daniel-Bertrand, M. et al. Multiple site hydrogen isotope labelling of pharmaceuticals. Angew. Chem. Int. Ed. 59, 21114–21120 (2020).
Stork, C. M. et al. Hydrogen isotope exchange by homogeneous iridium catalysis in aqueous buffers with deuterium or tritium gas. Angew. Chem. Int. Ed. 62, e202301512 (2023).
Levernier, E. et al. Easy-to-implement hydrogen isotope exchange for the labeling of N‑heterocycles, alkylkamines, benzylic scaffolds, and pharmaceuticals. J. Am. Chem. Soc. Au 2, 801–808 (2022).
Loh, Y. Y. et al. Photoredox-catalyzed deuteration and tritiation of pharmaceutical compounds. Science 358, 1182–1187 (2017).
Yang, H. et al. Efficient aliphatic hydrogen-isotope exchange with tritium gas through the merger of photoredox and hydrogenation catalysts. J. Am. Chem. Soc. 144, 5010–5022 (2022).
Kramp, H. et al. In situ generated iridium nanoparticles as hydride donors in photoredox-catalyzed hydrogen isotope exchange reactions with deuterium and tritium gas. Angew. Chem. Int. Ed. 62, e202308983 (2023).
Kopf, S. et al. Recent developments for the deuterium and tritium labeling of organic molecules. Chem. Rev. 122, 6634–6718 (2022).
Zhao, D., Petzold, R., Yan, J., Muri, D. & Ritter, T. Tritiation of aryl thianthrenium salts with a molecular palladium catalyst. Nature 600, 444–449 (2021).
Wilkinson, D. J., Hickey, M. J., Kingston, L. P. & Mather, A. N. In Synthesis and Applications of Isotopically Labeled Compounds (eds. Dean, D. C., Filer, C. N. & McCarthy, K. E.) Vol. 8, 47–50 (John Wiley & Sons, 2004).
Marek, A., Patil, M. R. & Elbert, T. The labeling of brassinosteroids by tritium. RSC Adv. 5, 65214–65220 (2015).
Elbert, T., Patil, M. R. & Marek, A. Enantiospecific tritium labeling of 28-homocastasterone. J. Labelled Compd. Radiopharm. 60, 176–182 (2017).
Kriegelstein, M., Nováková, G. & Marek, A. Synthesis of [3H]Org24598 using in-house prepared [3H]MeI. J. Labelled Compd. Radiopharm. 67, 91–103 (2024).
Saljoughian, M., Morimoto, H., Dorsky, A. M., Rapoport, H. & Andres, H. A new and efficient synthesis of monotritiomethyl iodide. J. Labelled Compd. Radiopharm. 27, 767–776 (1989).
Saljoughian, M., Morimoto, H. & Rapoport, H. Synthesis of monotritiomethyl iodide from thioethers. Hydrogenolysis in the presence of thioethers. J. Org. Chem. 54, 4689–4691 (1989).
Marek, A., Klepetářová, B. & Elbert, T. A facile method for steroid labeling by heavy isotopes of hydrogen. Tetrahedron 71, 4874–4882 (2015).
Kubas, G. J. Activation of dihydrogen and coordination of molecular H2 on transition metals. J. Organomet. Chem. 751, 33–49 (2014).
Fan, L., Parkin, S. & Ozerov, O. V. Halobenzenes and Ir(I): kinetic C–H oxidative addition and thermodynamic C–Hal oxidative addition. J. Am. Chem. Soc. 127, 16772–16773 (2005).
Crabtree, R. H. Dihydrogen complexation. Chem. Rev. 116, 8750–8769 (2016).
van Santen, R. A. In Modern Heterogeneous Catalysis: An Introduction 293–344 (John Wiley, 2017).
Agarwal, V. et al. Enzymatic halogenation and dehalogenation reactions: pervasive and mechanistically diverse. Chem. Rev. 117, 5619–5674 (2017).
Fincker, M. & Spormann, A. M. Biochemistry of catabolic reductive dehalogenation. Annu. Rev. Biochem. 86, 357–386 (2017).
Voges, R., Heys, J. R. & Moenius, T. In Preparation of Compounds Labeled with Tritium and Carbon-14 109–209 (John Wiley & Sons, 2009).
Giedyk, M., Goliszewska, K. & Gryko, D. Vitamin B12 catalysed reactions. Chem. Soc. Rev. 44, 3391–3404 (2015).
Hidalgo, N., Moreno, J. J., García-Rubio, I. & Campos, J. Enhanced dihydrogen activation by mononuclear iridium(II) compounds: a mechanistic study. Angew. Chem. Int. Ed. 61, e202206831 (2022).
Ravetz, B. D., Wang, J. Y., Ruhl, K. E. & Rovis, T. Photoinduced ligand-to-metal charge transfer enables photocatalyst-independent light-gated activation of Co(II). ACS Catal. 9, 200–204 (2019).
Shafizadeh, N., Poisson, L. & Soep, B. Ultrafast electronic relaxation of excited state vitamin B12 in the gas phase. Chem. Phys. 350, 2–6 (2008).
Lockley, W. J. S., Venanzi, N. A. E. & Crane, G. J. Studies of hydrogen isotope scrambling during the dehalogenation of aromatic chloro-compounds with deuterium gas over palladium catalysts. J. Labelled Compd. Radiopharm. 63, 531–552 (2020).
Smith, D. M., Pulling, M. E. & Norton, J. R. Tin-free and catalytic radical cyclizations. J. Am. Chem. Soc. 129, 770–771 (2007).
Gansäuer, A., Fan, C.-A. & Piestert, F. Sustainable radical reduction through catalytic hydrogen atom transfer. J. Am. Chem. Soc. 130, 6916–6917 (2008).
Yao, C., Dahmen, T., Gansäuer, A. & Norton, J. R. Anti-Markovnikov alcohols via epoxide hydrogenation through cooperative catalysis. Science 364, 764–767 (2019).
Park, Y. et al. Visible light enables catalytic formation of weak chemical bonds with molecular hydrogen. Nat. Chem. 13, 969–976 (2021).
Shimakoshi, H., Li, L., Nishi, M. & Hisaeda, Y. Photosensitizing catalysis of the B12 complex without an additional photosensitizer. Chem. Commun. 47, 10921–10923 (2011).
Shimakoshi, H., Sakumori, E., Kaneko, K. & Hisaeda, Y. B12–TiO2 hybrid catalyst for dehalogenation of organic halides. Chem. Lett. 38, 468–469 (2009).
Hu, Y. & Norton, J. R. Kinetics and thermodynamics of H–/H•/H+ transfer from a rhodium(III) hydride. J. Am. Chem. Soc. 136, 5938–5948 (2014).
Chuentragool, P., Kurandina, D. & Gevorgyan, V. Catalysis with palladium complexes photoexcited by visible light. Angew. Chem. Int. Ed. 58, 11586–11598 (2019).
Sarkar, S., Cheung, K. P. S. & Gevorgyan, V. Recent advances in visible light induced palladium catalysis. Angew. Chem. Int. Ed. 63, e202311972 (2024).
Brož, B. & Marek, A. Tritiodefluorination of alkyl C–F groups. J. Labelled Compd. Radiopharm. 62, 743–750 (2019).
Xu, J. et al. Unveiling extreme photoreduction potentials of donor–acceptor cyanoarenes to access aryl radicals from aryl chlorides. J. Am. Chem. Soc. 143, 13266–13273 (2021).
Schmidt, V. A., Quinn, R. K., Brusoe, A. T. & Alexanian, E. J. Site-selective aliphatic C–H bromination using N-bromoamides and visible light. J. Am. Chem. Soc. 136, 14389–14392 (2014).
Di Martino, R. M. C., Maxwell, B. D. & Pirali, T. Deuterium in drug discovery: progress, opportunities and challenges. Nat. Rev. Drug Discov. 22, 562–584 (2023).
Derdau, V. et al. The future of (radio)-labeled compounds in research and development within the life science industry. Angew. Chem. Int. Ed. 62, e202306019 (2023).
Acknowledgements
We thank V. Derdau and C. Loewe (Sanofi-Aventis Deutschland GmbH) for NMR spectroscopy analysis. We thank T. Ritter (Max-Planck-Institut für Kohlenforschung) and D. Muri (Roche Innovation Center Basel) for insightful discussions and suggestions. This project was supported by the Fundamental Research Funds for the Central Universities, the National Natural Science Foundation of China (grant no. 22101278 to D.Z.), the Chinese Academy of Sciences, the Special Educating Project of the Talent for Carbon Peak and Carbon Neutrality of University of Chinese of Academy of Sciences, University of Chinese Academy of Sciences and the Project of Talent Cultivation for Carbon Peak and Carbon Neutrality of the University of Chinese of Academy of Sciences.
Author information
Authors and Affiliations
Contributions
D.Z. conceived the project. B.B.Z. optimized the hydrogenolysis reaction and explored the substrate scope. B.B.Z. and Z.Y.Z. investigated the mechanism. D.Z. wrote the manuscript with input from all authors. Y.W. and D.Z. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Aleš Marek and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental Details, Supplementary Discussion, Figs. 1–108, Schemes 1–3 and Tables 1–14.
Source data
Source Data Fig. 2
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Zhang, Z., Wang, Y. et al. Late-stage deuteration and tritiation through bioinspired cooperative hydrogenolysis. Nat. Synth 4, 444–452 (2025). https://doi.org/10.1038/s44160-024-00716-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00716-0
This article is cited by
-
Selective hydrogenolysis of organohalides
Nature Synthesis (2025)