Abstract
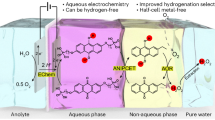
Hydrogen peroxide (H2O2) electrosynthesis via the oxygen reduction reaction offers a sustainable alternative to the industrial anthraquinone process. However, the poor energy efficiency (EE) of current catalysts and systems hinders their industrial application. Here a techno-economic analysis indicates that this electrochemical process becomes economically viable if the EE exceeds 39% at a current density of 300 mA cm−2. Guided by theoretical calculations, we report a class of single-site catalysts with oxygen functional group-coordinated p-block main-group metals. We find that oxygen functional groups induce electron-deficient Sn sites via electronic interactions, optimizing the adsorption strength of key H2O2 intermediates. Using the Sn1/C(O) as the cathodic catalyst in an electrolyser, an industrial current density of 300 mA cm−2 is realized with an ultralow cell voltage of 1.17 V, achieving an EE of 43% and stability exceeding 200 h. This work contributes towards the industrial implementation and economic viability of large-scale electrochemical H2O2 synthesis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the conclusions of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Hydrogen Peroxide Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2020–2025 (Imarc Market Research, 2020).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Lim, J. S., Sa, Y. J. & Joo, S. H. Catalyst design, measurement guidelines, and device integration for H2O2 electrosynthesis from oxygen reduction. Cell Rep. Phys. Sci. 3, 100987 (2022).
Siahrostami, S. et al. A review on challenges and successes in atomic-scale design of catalysts for electrochemical synthesis of hydrogen peroxide. ACS Catal. 10, 7495–7511 (2020).
Lee, B.-H. et al. Supramolecular tuning of supported metal phthalocyanine catalysts for hydrogen peroxide electrosynthesis. Nat. Catal. 6, 234–243 (2023).
Xia, C., Xia, Y., Zhu, P., Fan, L. & Wang, H. T. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 366, 226–231 (2019).
Yang, S. et al. Toward the decentralized electrochemical production of H2O2: a focus on the catalysis. ACS Catal. 8, 4064–4081 (2018).
Hu, J. et al. Uncovering dynamic edge-sites in atomic Co–N–C electrocatalyst for selective hydrogen peroxide production. Angew. Chem. Int. Ed. Engl. 62, e202304754 (2023).
Jiang, K. et al. Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination. Nat. Commun. 10, 3997 (2019).
Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 143, 7819–7827 (2021).
Tian, Z. et al. Constructing interfacial boron–nitrogen moieties in turbostratic carbon for electrochemical hydrogen peroxide production. Angew. Chem. Int. Ed. 61, 202206915 (2022).
Long, Y. et al. Tailoring the atomic‐local environment of carbon nanotube tips for selective H2O2 electrosynthesis at high current densities. Adv. Mater. 35, 202303905 (2023).
Liu, X. & Dai, L. L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 1, 16064 (2016).
Morimoto, N., Kubo, T. & Nishina, Y. Tailoring the oxygen content of graphite and reduced graphene oxide for specific applications. Sci. Rep. 6, 21715 (2016).
Xu, Y. X., Sheng, K. X., Li, C. & Shi, G. Q. Highly conductive chemically converted graphene prepared from mildly oxidized graphene oxide. J. Mater. Chem. 21, 7376–7380 (2011).
Sebastián, D., Suelves, I., Moliner, R. & Lázaro, M. J. The effect of the functionalization of carbon nanofibers on their electronic conductivity. Carbon 48, 4421–4431 (2010).
Xia, Y. et al. Highly active and selective oxygen reduction to H2O2 on boron-doped carbon for high production rates. Nat. Commun. 12, 4225 (2021).
Fellinger, T. P., Hasche, F., Strasser, P. & Antonietti, M. Mesoporous nitrogen-doped carbon for the electrocatalytic synthesis of hydrogen peroxide. J. Am. Chem. Soc. 134, 4072–4075 (2012).
Iglesias, D. et al. N-doped graphitized carbon nanohorns as a forefront electrocatalyst in highly selective O2 reduction to H2O2. Chem 4, 106–123 (2018).
Chen, S. C. et al. Defective carbon-based materials for the electrochemical synthesis of hydrogen peroxide. ACS Sustain. Chem. Eng. 6, 311–317 (2018).
Lin, Z. et al. Atomic Co decorated free-standing graphene electrode assembly for efficient hydrogen peroxide production in acid. Energy Environ. Sci. 15, 1172–1182 (2022).
Liu, C. et al. Heterogeneous molecular Co–N–C catalysts for efficient electrochemical H2O2 synthesis. Energy Environ. Sci. 16, 446–459 (2023).
Norskov, J. K., Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 1, 37–46 (2009).
Yang, X. X. et al. Tuning two-electron oxygen-reduction pathways for H2O2 electrosynthesis via engineering atomically dispersed single metal site catalysts. Adv. Mater. 34, 2107954 (2022).
Fan, W. et al. Rational design of heterogenized molecular phthalocyanine hybrid single-atom electrocatalyst towards two-electron oxygen reduction. Nat. Commun. 14, 1426 (2023).
Mok, D. H., Back, S. & Siahrostami, S. Validating ΔΔG selectivity descriptor for electrosynthesis of H2O2 from oxygen reduction reaction. Angew. Chem. Int. Ed. Engl. 63, e202404677 (2024).
Du, J. et al. CoIn dual-atom catalyst for hydrogen peroxide production via oxygen reduction reaction in acid. Nat. Commun. 14, 4766 (2023).
Gu, Y., Xi, B. J., Zhang, H., Ma, Y. C. & Xiong, S. L. Activation of main‐group antimony atomic sites for oxygen reduction catalysis. Angew. Chem. Int. Ed. 134, e202202200 (2022).
Luo, F. et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 19, 1215–1223 (2020).
Han, G. F. et al. Building and identifying highly active oxygenated groups in carbon materials for oxygen reduction to H2O2. Nat. Commun. 11, 2209 (2020).
Cao, P. et al. Metal single-site catalyst design for electrocatalytic production of hydrogen peroxide at industrial-relevant currents. Nat. Commun. 14, 172 (2023).
Acknowledgements
This study was financially supported by National Science Fund for Distinguished Young Scholars (grant no. 52025133), the National Key R&D Program of China (grant no. 2022YFE0128500), Beijing Natural Science Foundation (grant no. Z220020), Beijing Outstanding Young Scientist Program (JWZQ20240102004 (S.G.)), Tencent Foundation through the XPLORER PRIZE, National Natural Science Foundation of China (grant nos. 22309006 to Y.G., 22205010 to Y.H., 52202201 to S.Z. and 22309005 to H.T.) and the China Postdoctoral Science Foundation (grant no. 2023M730045 to Y.G.). We thank the photoemission BL14W1 and BL11B in the Shanghai Synchrotron Radiation Facility (SSRF) and BL1W1B in the Beijing Synchrotron Radiation Facility (BSRF) for the help with characterizations. We thank Q. Fu from the University of Science and Technology of China and R. Jun from North University of China for their assistance with the theoretical calculations.
Author information
Authors and Affiliations
Contributions
S.G. conceived and supervised the project. M.L. guided and supervised all of the research. Y.G. designed the experiments. Y.G. and Y.T. performed the experiments and data analysis. H.T., Y.H. and F.L. contributed to senior aberration-corrected TEM characterization. Y.G. and D.C. contributed to the DFT calculations. L.Z. and S.Z. helped with X-ray absorption fine structure experiments. Z.Q. and R.Z. participated in part of the basic experiments. H.G. and Y.L. assisted with the electrochemical tests. Y.G. wrote the paper. All authors took part in the discussion of data and gave comments on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Samira Siahrostami, Shuangliang Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–38 and Tables 1–13.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1a,b.
Source Data Fig. 2
Statistical source data for Fig. 2b–e.
Source Data Fig. 3
Statistical source data for Fig. 3b–f.
Source Data Fig. 4
Statistical source data for Fig. 4a–d.
Source Data Fig. 5
Statistical source data for Fig. 5a–f.
Source Data Fig. 6
Statistical source data for Fig. 6a,b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gu, Y., Tan, Y., Tan, H. et al. Industrial electrosynthesis of hydrogen peroxide over p-block metal single sites. Nat. Synth 4, 614–621 (2025). https://doi.org/10.1038/s44160-024-00722-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-024-00722-2
This article is cited by
-
A self-breathing electrode enabled by interface regulation and gradient wettability engineering for industrial H2O2 electrosynthesis
Nature Communications (2026)
-
Carbon-based electrocatalysts for selective two-electron oxygen reduction
Catal (2026)
-
Supercritical CO2-assisted rapid synthesis of covalent organic framework-based electrocatalyst for efficient two-electron oxygen reduction reaction
Nature Communications (2025)
-
Arraying faceted manganese oxides for selective ethylene electro-oxidation to ethylene glycol in aqueous electrolytes
Nature Communications (2025)
-
Oxygen functionalization of carbon quantum dots enables efficient acidic hydrogen peroxide electrosynthesis
Nature Communications (2025)