Abstract
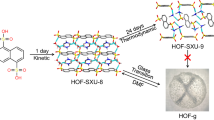
Framework structures such as metal–organic frameworks (MOFs) and hydrogen-bonded organic frameworks (HOFs) can facilitate proton conduction through various proton-carrying sites within the pores or along the backbones, demonstrating their viability as proton-conducting materials for fuel cells. However, the lack of inherent proton-carrying sites on typical MOF backbones and the architectural instability of HOFs pose a challenge for further applications. Here we report the synthesis of a framework that complementarily entangles a MOF and HOF through meticulous control of the deprotonation equilibrium of the linker. The hybrid entangled framework shows higher architectural stability than the MOF net alone through the mutual support of the two isotopological nets. Furthermore, the HOF architecture and plentiful H2O molecules in the well-sized channels provide a proton conductivity of 1.1 × 10−2 S cm−1 at 95 °C and 100% relative humidity. The crossover of different porous frameworks provides a method to integrate various materials seamlessly into a cohesive and functional system.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this study are available in the paper and its Supplementary Information. X-ray crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2384370 (FDM-150), 2384371 (FDM-150-hydrated), 2384372 (FDM-151-36H2O) and 2384373 (FDM-151-hydrated). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Fuel Cell Technologies Office Multi-year Research, Development, and Demonstration Plan Section 1.0 (US DOE, 2024).
Cano, Z. P. et al. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 3, 279–289 (2018).
Staffell, I. et al. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 12, 463–491 (2019).
Qu, E. et al. Proton exchange membranes for high temperature proton exchange membrane fuel cells: challenges and perspectives. J. Power Sources 533, 231386 (2022).
Jiao, K. et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 595, 361–369 (2021).
Scofield, M. E., Liu, H. & Wong, S. S. A concise guide to sustainable PEMFCs: recent advances in improving both oxygen reduction catalysts and proton exchange membranes. Chem. Soc. Rev. 44, 5836–5860 (2015).
Haider, R. et al. High temperature proton exchange membrane fuel cells: progress in advanced materials and key technologies. Chem. Soc. Rev. 50, 1138–1187 (2021).
Mauritz, K. A. & Moore, R. B. State of understanding of Nafion. Chem. Rev. 104, 4535–4585 (2004).
Miyatake, K., Chikashige, Y., Higuchi, E. & Watanabe, M. Tuned polymer electrolyte membranes based on aromatic polyethers for fuel cell applications. J. Am. Chem. Soc. 129, 3879–3887 (2007).
Miyake, J. et al. Design of flexible polyphenylene proton-conducting membrane for next-generation fuel cells. Sci. Adv. 3, eaao0476 (2017).
Sone, Y., Ekdunge, P. & Simonsson, D. Proton conductivity of Nafion 117 as measured by a four‐electrode AC impedance method. J. Electrochem. Soc. 143, 1254–1259 (1996).
Vichi, F. M., Tejedor-Tejedor, M. I. & Anderson, M. A. Effect of pore-wall chemistry on proton conductivity in mesoporous titanium dioxide. Chem. Mater. 12, 1762–1770 (2000).
Karim, M. R. et al. Graphene oxide nanosheet with high proton conductivity. J. Am. Chem. Soc. 135, 8097–8100 (2013).
Chalkova, E., Fedkin, M. V., Wesolowski, D. J. & Lvov, S. N. Effect of TiO2 surface properties on performance of Nafion-based composite membranes in high temperature and low relative humidity PEM fuel cells. J. Electrochem. Soc. 152, A1742–A1747 (2005).
Di Vona, M. L. et al. SPEEK/PPSU-based organic–inorganic membranes: proton conducting electrolytes in anhydrous and wet environments. J. Membr. Sci. 279, 186–191 (2006).
Yang, J. et al. Oxygen- and proton-transporting open framework ionomer for medium-temperature fuel cells. Science 385, 1115–1120 (2024).
Liu, X. et al. Oriented proton-conductive nano-sponge-facilitated polymer electrolyte membranes. Energy Environ. Sci. 13, 297–309 (2020).
Li, H., Eddaoudi, M., O’Keeffe, M. & Yaghi, O. M. Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature 402, 276–279 (1999).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Zhang, J.-P., Zhang, Y.-B., Lin, J.-B. & Chen, X.-M. Metal azolate frameworks: from crystal engineering to functional materials. Chem. Rev. 112, 1001–1033 (2011).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Li, B. et al. Emerging multifunctional metal–organic framework materials. Adv. Mater. 28, 8819–8860 (2016).
Yuan, S. et al. Stable metal–organic frameworks: design, synthesis, and applications. Adv. Mater. 30, 1704303 (2018).
Xu, W. et al. Anisotropic reticular chemistry. Nat. Rev. Mater. 5, 764–779 (2020).
Jiang, H., Alezi, D. & Eddaoudi, M. A reticular chemistry guide for the design of periodic solids. Nat. Rev. Mater. 6, 466–487 (2021).
Simard, M., Su, D. & Wuest, J. D. Use of hydrogen bonds to control molecular aggregation. Self-assembly of three-dimensional networks with large chambers. J. Am. Chem. Soc. 113, 4696–4698 (1991).
Brunet, P., Simard, M. & Wuest, J. D. Molecular tectonics. Porous hydrogen-bonded networks with unprecedented structural integrity. J. Am. Chem. Soc. 119, 2737–2738 (1997).
Yang, W. et al. Exceptional thermal stability in a supramolecular organic framework: porosity and gas storage. J. Am. Chem. Soc. 132, 14457–14469 (2010).
He, Y., Xiang, S. & Chen, B. A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature. J. Am. Chem. Soc. 133, 14570–14573 (2011).
Mastalerz, M. & Oppel, I. M. Rational construction of an extrinsic porous molecular crystal with an extraordinary high specific surface area. Angew. Chem. Int. Ed. 51, 5252–5255 (2012).
Lin, R.-B. & Chen, B. Hydrogen-bonded organic frameworks: chemistry and functions. Chem 8, 2114–2135 (2022).
Song, X. et al. Design rules of hydrogen-bonded organic frameworks with high chemical and thermal stabilities. J. Am. Chem. Soc. 144, 10663–10687 (2022).
Liu, B.-T. et al. A solution processible single-crystal porous organic polymer. Nat. Synth. 2, 873–879 (2023).
Yoon, M. et al. High and highly anisotropic proton conductivity in organic molecular porous materials. Angew. Chem. Int. Ed. 50, 7870–7873 (2011).
Bazaga-García, M. et al. Guest molecule-responsive functional calcium phosphonate frameworks for tuned proton conductivity. J. Am. Chem. Soc. 136, 5731–5739 (2014).
Nguyen, N. T. T. et al. Three-dimensional metal-catecholate frameworks and their ultrahigh proton conductivity. J. Am. Chem. Soc. 137, 15394–15397 (2015).
Karmakar, A. et al. Hydrogen‐bonded organic frameworks (HOFs): a new class of porous crystalline proton‐conducting materials. Angew. Chem. Int. Ed. 55, 10667–10671 (2016).
Wang, S. et al. A robust zirconium amino acid metal–organic framework for proton conduction. Nat. Commun. 9, 4937 (2018).
Xing, G. et al. Synthesis of crystalline porous organic salts with high proton conductivity. Angew. Chem. Int. Ed. 57, 5345–5349 (2018).
Lim, D.-W. & Kitagawa, H. Proton transport in metal–organic frameworks. Chem. Rev. 120, 8416–8467 (2020).
Lim, D.-W. & Kitagawa, H. Rational strategies for proton-conductive metal–organic frameworks. Chem. Soc. Rev. 50, 6349–6368 (2021).
Pal, S. C. et al. Proton-conducting hydrogen-bonded organic frameworks. ACS Energy Lett. 6, 4431–4453 (2021).
Sharma, A. et al. Superprotonic conductivity of MOF‐808 achieved by controlling the binding mode of grafted sulfamate. Angew. Chem. Int. Ed. 60, 14334–14338 (2021).
Sun, Y. et al. Bio‐inspired synthetic hydrogen‐bonded organic frameworks for efficient proton conduction. Adv. Mater. 35, 2208625 (2022).
Chen, X. et al. A proton conductive porous framework of an 18‐crown‐6‐ether derivative networked by rigid hydrogen bonding modules. Angew. Chem. Int. Ed. 61, e202211686 (2022).
Chen, S. et al. Photo responsive electron and proton conductivity within a hydrogen‐bonded organic framework. Angew. Chem. Int. Ed. 62, e202308418 (2023).
Dong, X.-Y. et al. Highly selective Fe3+ sensing and proton conduction in a water-stable sulfonate–carboxylate Tb–organic-framework. J. Mater. Chem. A 3, 641–647 (2015).
Phang, W. J. et al. Superprotonic conductivity of a UiO‐66 framework functionalized with sulfonic acid groups by facile postsynthetic oxidation. Angew. Chem. Int. Ed. 54, 5142–5146 (2015).
Taylor, J. M. et al. The role of a three dimensionally ordered defect sublattice on the acidity of a sulfonated metal–organic framework. J. Am. Chem. Soc. 137, 11498–11506 (2015).
Yang, F. et al. A flexible metal–organic framework with a high density of sulfonic acid sites for proton conduction. Nat. Energy 2, 877–883 (2017).
Li, X.-M. et al. Superprotonic conductivity of a functionalized metal–organic framework at ambient conditions. ACS Appl. Mater. Interfaces 14, 9264–9271 (2022).
Ramaswamy, P., Wong, N. E., Gelfand, B. S. & Shimizu, G. K. H. A water stable magnesium MOF that conducts protons over 10−2 S cm−1. J. Am. Chem. Soc. 137, 7640–7643 (2015).
Shigematsu, A., Yamada, T. & Kitagawa, H. Wide control of proton conductivity in porous coordination polymers. J. Am. Chem. Soc. 133, 2034–2036 (2011).
Ponomareva, V. G. et al. Imparting high proton conductivity to a metal–organic framework material by controlled acid impregnation. J. Am. Chem. Soc. 134, 15640–15643 (2012).
Ye, Y. et al. Straightforward loading of imidazole molecules into metal–organic framework for high proton conduction. J. Am. Chem. Soc. 139, 15604–15607 (2017).
Nagarkar, S. S. et al. Two‐in‐one: inherent anhydrous and water‐assisted high proton conduction in a 3D metal–organic framework. Angew. Chem. Int. Ed. 53, 2638–2642 (2013).
Horike, S., Umeyama, D. & Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal–organic frameworks. Acc. Chem. Res. 46, 2376–2384 (2013).
Lin, R.-B. et al. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 48, 1362–1389 (2019).
Yang, F.-F. et al. Vitrification-enabled enhancement of proton conductivity in hydrogen-bonded organic frameworks. Nat. Commun. 15, 3930 (2024).
Yahiaoui, O. et al. 3D anionic silicate covalent organic framework with srs topology. J. Am. Chem. Soc. 140, 5330–5333 (2018).
Zhao, Z.-H., Huang, J.-R., Liao, P.-Q. & Chen, X.-M. Isolated tin(IV) active sites for highly efficient electroreduction of CO2 to CH4 in neutral aqueous solution. Angew. Chem. Int. Ed. 62, e202301767 (2023).
Perl, D., Lee, S. J., Ferguson, A., Jameson, G. B. & Telfer, S. G. Hetero-interpenetrated metal–organic frameworks. Nat. Chem. 15, 1358–1364 (2023).
O’Keeffe, M., Peskov, M. A., Ramsden, S. J. & Yaghi, O. M. The Reticular Chemistry Structure Resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 41, 1782–1789 (2008).
Bonneau, C. & O’Keeffe, M. High-symmetry embeddings of interpenetrating periodic nets. Essential rings and patterns of catenation. Acta Crystallogr. A 71, 82–91 (2015).
Barthram, A. M., Cleary, R. L., Kowallick, R. & Ward, M. D. A new redox-tunable near-IR dye based on a trinuclear ruthenium(II) complex of hexahydroxytriphenylene. Chem. Commun. 1998, 2695–2696 (1998).
Yang, L., He, X. & Dincă, M. Triphenylene-bridged trinuclear complexes of Cu: models for spin interactions in two-dimensional electrically conductive metal–organic frameworks. J. Am. Chem. Soc. 141, 10475–10480 (2019).
Leubner, S. et al. Hexahydroxytriphenylene for the synthesis of group 13 MOFs—a new inorganic building unit in a β-cristobalite type structure. Dalton Trans. 49, 3088–3092 (2020).
Yang, L. & Dincă, M. Redox ladder of Ni3 complexes with closed‐shell, mono‐, and diradical triphenylene units: molecular models for conductive 2D MOFs. Angew. Chem. Int. Ed. 60, 23784–23789 (2021).
Willems, T. F. et al. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 149, 134–141 (2012).
Liu, X., Wang, X. & Kapteijn, F. Water and metal–organic frameworks: from interaction toward utilization. Chem. Rev. 120, 8303–8377 (2020).
Zhao, X., Mao, C., Bu, X. & Feng, P. Direct observation of two types of proton conduction tunnels coexisting in a new porous indium–organic framework. Chem. Mater. 26, 2492–2495 (2014).
Kim, S. et al. Achieving superprotonic conduction in metal–organic frameworks through iterative design advances. J. Am. Chem. Soc. 140, 1077–1082 (2018).
Sadakiyo, M. et al. Promotion of low-humidity proton conduction by controlling hydrophilicity in layered metal–organic frameworks. J. Am. Chem. Soc. 134, 5472–5475 (2012).
Acknowledgements
This work was supported by the National Key Research and Development Project of China (grant no. 2018YFA0209401, Q.L.) and the National Natural Science Foundation of China (grant nos. 22088101, 21922103 and 21961132003, Q.L.). We thank X. Kong and J. Zhu for the NMR measurements and data analysis, M. Li for the structure topology analysis and Z. Zhang for assistance with the structure refinement.
Author information
Authors and Affiliations
Contributions
Q.L. conceived and supervised the project. Z.J. and Q.L. designed the experiments. Z.J. and Y.S. performed the syntheses, structural characterizations and proton conductivity studies. Y.R., L.Y. and H.X. also performed structural characterizations. Z.J. and Q.L. wrote the paper. All authors contributed to the data analysis, discussion and revision of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Banglin Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Discussion, Figs. 1–29 and Tables 1–12.
Supplementary Data 1
Crystallographic data for FDM-150, CCDC 2384370.
Supplementary Data 2
Crystallographic data for FDM-150-hydrated, CCDC 2384371.
Supplementary Data 3
Crystallographic data for FDM-151-36H2O, CCDC 2384372.
Supplementary Data 4
Crystallographic data for FDM-151-hydrated, CCDC 2384373.
Source data
Source Data Fig. 2
Source data for the PXRD patterns in Fig. 2a,c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, Z., Sun, Y., Rao, Y. et al. Isotopological entanglement of a metal–organic framework and a hydrogen-bonded organic framework for proton conduction. Nat. Synth 4, 622–631 (2025). https://doi.org/10.1038/s44160-025-00738-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00738-2