Abstract
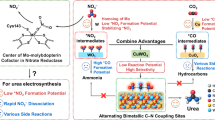
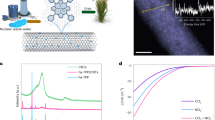
Electrocatalytic urea production from nitrate (NO3−) and carbon dioxide (CO2) provides a promising alternative to the traditional energy-intensive industrial process. However, promoting electrocatalytic carbon–nitrogen coupling and suppressing side reactions remains challenging. Here we report an efficient urea synthesis via electrochemical coupling of NO3− and CO2 using entangled iron porphyrins in three-dimensional covalent organic frameworks. The porous iron catalyst concentrates and cooperatively activates reactants, achieving a high Faradaic efficiency of 90.0%, a nitrogen selectivity of 92.4% and nearly 100% carbon selectivity. The catalyst achieves a urea yield rate of \(135.6\,{\mathrm{mmol}}\,{\mathrm{g}}_{\mathrm{cat}}^{-1}\,{\mathrm{h}}^{-1}\), while maintaining activity for >100 h. Experiments and theoretical calculations suggest the plentiful Fe–N4 sites within porphyrins efficiently facilitate the conversions of CO2 to *CO and NO3− to *NH2, and the spatial localization of twin iron sites overcomes the unordered transfer of intermediates, enabling vectored carbon–nitrogen coupling.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. Supplementary crystallographic data for PCOF-34-Fe, PCOF-90-Fe, PCOF-34 and PCOF-90 can be obtained free of charge via the Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/data_request/cif (CCDC 2345501, 2345502, 2345503 and 2345504, respectively).
References
Zhang, X. et al. Managing nitrogen for sustainable development. Nature 528, 51–59 (2015).
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z. & Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 (2008).
Qiao, J., Liu, Y., Hong, F. & Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 43, 631–675 (2014).
Barzagli, F., Mani, F. & Peruzzini, M. From greenhouse gas to feedstock: formation of ammonium carbamate from CO2 and NH3 in organic solvents and its catalytic conversion into urea under mild conditions. Green Chem. 13, 1267–1274 (2011).
Comer, B. M. et al. Prospects and challenges for solar fertilizers. Joule 3, 1578–1605 (2019).
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6, 303–319 (2022).
Chen, C. et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 12, 717–724 (2020).
Regnier, P., Resplandy, L., Najjar, R. G. & Ciais, P. The land-to-ocean loops of the global carbon cycle. Nature 603, 401–410 (2022).
Schulte-Uebbing, L. F., Beusen, A. H. W., Bouwman, A. F. & de Vries, W. From planetary to regional boundaries for agricultural nitrogen pollution. Nature 610, 507–512 (2022).
Lv, C. et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nat. Sustain. 4, 868–876 (2021).
Wei, X. et al. Oxygen vacancy-mediated selective C–N coupling toward electrocatalytic urea synthesis. J. Am. Chem. Soc. 144, 11530–11535 (2022).
Lv, C. et al. A defect engineered electrocatalyst that promotes high-efficiency urea synthesis under ambient conditions. ACS Nano 16, 8213–8222 (2022).
Geng, J. et al. Ambient electrosynthesis of urea with nitrate and carbon dioxide over iron-based dual-sites. Angew. Chem. Int. Ed. 62, e202210958 (2023).
Meng, N. et al. Oxide-derived core–shell Cu@Zn nanowires for urea electrosynthesis from carbon dioxide and nitrate in water. ACS Nano 16, 9095–9104 (2022).
Leverett, J. et al. Tuning the coordination structure of Cu–N–C single atom catalysts for simultaneous electrochemical reduction of CO2 and NO3- to urea. Adv. Energy Mater. 12, 2201500 (2022).
Zhang, X. et al. Identifying and tailoring C–N coupling site for efficient urea synthesis over diatomic Fe–Ni catalyst. Nat. Commun. 13, 5337 (2022).
Tao, Z., Rooney, C. L., Liang, Y. & Wang, H. Accessing organonitrogen compounds via C–N coupling in electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143, 19630–19642 (2021).
Jiang, M. et al. Review on electrocatalytic coreduction of carbon dioxide and nitrogenous species for urea synthesis. ACS Nano 17, 3209–3224 (2023).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Li, X. et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 4, 690–699 (2019).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Liu, R. et al. Covalent organic frameworks: an ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 50, 120–242 (2021).
Wang, X. et al. Homochiral 2D porous covalent organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 138, 12332–12335 (2016).
Xu, X., Cai, P., Chen, H., Zhou, H.-C. & Huang, N. Three-dimensional covalent organic frameworks with she topology. J. Am. Chem. Soc. 144, 18511–18517 (2022).
Meng, Y. et al. 2D and 3D porphyrinic covalent organic frameworks: the influence of dimensionality on functionality. Angew. Chem. Int. Ed. 59, 3624–3629 (2020).
Li, H. et al. Three-dimensional covalent organic frameworks with dual linkages for bifunctional cascade catalysis. J. Am. Chem. Soc. 138, 14783–14788 (2016).
Lin, S. et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 349, 1208–1213 (2015).
Lv, F. et al. Near-unity electrochemical conversion of nitrate to ammonia on crystalline nickel porphyrin-based covalent organic frameworks. Energy Environ. Sci. 16, 201–209 (2023).
Shan, Z. et al. 3D covalent organic frameworks with interpenetrated pcb topology based on 8-connected cubic nodes. J. Am. Chem. Soc. 144, 5728–5733 (2022).
Gong, C. et al. Synthesis and visualization of entangled 3D covalent organic frameworks with high-valency stereoscopic molecular nodes for gas separation. Angew. Chem. Int. Ed. 61, e202204899 (2022).
Zhu, Y. et al. Unravelling surface and interfacial structures of a metal–organic framework by transmission electron microscopy. Nat. Mater. 16, 532–536 (2017).
Zhang, D. et al. Atomic-resolution transmission electron microscopy of electron beam-sensitive crystalline materials. Science 359, 675–679 (2018).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Dong, C., Kou, T., Gao, H., Peng, Z. & Zhang, Z. Eutectic-derived mesoporous Ni–Fe–O nanowire network catalyzing oxygen evolution and overall water splitting. Adv. Energy Mater. 8, 1701347 (2018).
Wang, D. et al. Fe–N4 and Co–N4 dual sites for boosting oxygen electroreduction in Zn–air batteries. J. Mater. Chem. A 9, 13678–13687 (2021).
Zhang, R. et al. Edge-site engineering of defective Fe–N4 nanozymes with boosted catalase-like performance for retinal vasculopathies. Adv. Mater. 34, 2205324 (2022).
Kobayashi, H., Maeda, Y. & Yanagawa, Y. Mössbauer spectra of iron tetraphenylporphins. Bull. Chem. Soc. Jpn. 43, 2342–2346 (1970).
Genoux, A. et al. Well-defined iron sites in crystalline carbon nitride. J. Am. Chem. Soc. 145, 20739–20744 (2023).
Zhu, K. et al. Unraveling the role of interfacial water structure in electrochemical semihydrogenation of alkynes. ACS Catal. 12, 4840–4847 (2022).
Xu, M. et al. Atomically dispersed Cu sites on dual-mesoporous N-doped carbon for efficient ammonia electrosynthesis from nitrate. ChemSusChem 15, e202200231 (2022).
Wu, Z.-Y. et al. Electrochemical ammonia synthesis via nitrate reduction on Fe single atom catalyst. Nat. Commun. 12, 2870 (2021).
Lin, G. et al. 3D porphyrin-based covalent organic frameworks. J. Am. Chem. Soc. 139, 8705–8709 (2017).
Ren, W.-X. et al. A covalent organic framework with a self-contained light source for photodynamic therapy. Chem. Commun. 58, 5245–5248 (2022).
Liu, X. et al. Carbon nanotubes with fluorine-rich surface as metal-free electrocatalyst for effective synthesis of urea from nitrate and CO2. Appl. Catal. B 316, 121618 (2022).
Liu, S. et al. AuCu nanofibers for electrosynthesis of urea from carbon dioxide and nitrite. Cell Rep. Phys. Sci. 3, 100869 (2022).
Zhang, D., Xue, Y., Zheng, X., Zhang, C. & Li, Y. Multi-heterointerfaces for selective and efficient urea production. Natl Sci. Rev. 10, nwac209 (2023).
Yuan, M. et al. Unveiling electrochemical urea synthesis by co-activation of CO2 and N2 with Mott–Schottky heterostructure catalysts. Angew. Chem. Int. Ed. 60, 10910–10918 (2021).
Yuan, M. et al. Highly selective electroreduction of N2 and CO2 to urea over artificial frustrated Lewis pairs. Energy Environ. Sci. 14, 6605–6615 (2021).
Yuan, M. et al. Host–guest molecular interaction promoted urea electrosynthesis over a precisely designed conductive metal–organic framework. Energy Environ. Sci. 15, 2084–2095 (2022).
Yu, Y. et al. Activation of Ga liquid catalyst with continuously exposed active sites for electrocatalytic C–N coupling. Angew. Chem. Int. Ed. 63, e202402236 (2024).
Zhao, Y. et al. Efficient urea electrosynthesis from carbon dioxide and nitrate via alternating Cu–W bimetallic C–N coupling sites. Nat. Commun. 14, 4491 (2023).
Luo, Y. et al. Selective electrochemical synthesis of urea from nitrate and CO2 via relay catalysis on hybrid catalysts. Nat. Catal. 6, 939–948 (2023).
Gao, Y. et al. Tandem catalysts enabling efficient C–N coupling toward the electrosynthesis of urea. Angew. Chem. Int. Ed. 63, e202402215 (2024).
Liu, Y. et al. C-bound or O-bound surface: which one boosts electrocatalytic urea synthesis? Angew. Chem. Int. Ed. 62, e202300387 (2023).
Xu, M. et al. Kinetically matched C–N coupling toward efficient urea electrosynthesis enabled on copper single-atom alloy. Nat. Commun. 14, 6994 (2023).
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2021YFA1501501, 2021YFA1200402 and 2022YFE0113800), the National Natural Science Foundation of China (22331007, 22375179 and 223B2117), the Key Project of Basic Research of Shanghai (22JC1402000), and a start-up grant (project number 2019125016829) from Zhejiang University of Technology. Y.Z. acknowledges the National Natural Science Foundation of China (22075250, 22122505, 21771161). W.Y. acknowledges the University Leading Talents Program of Zhejiang Province (4095C502222140203). We gratefully acknowledge the staff of BL16B1 at the Shanghai Synchrotron Light Source for their assistance with the synchrotron radiation measurements.
Author information
Authors and Affiliations
Contributions
Y.C. and Y.P. conceived and designed the research project. C.G. designed, synthesized and characterized the materials. G.S. and Y.Z. performed low-dose cryo-EM measurements. M.X. and W.Y. conducted the catalytic experiments. X. Wei. and F.D. performed the theoretical calculations. J.L. assisted in the conduct of the experiment. X. Wu., X.H., J.D. and Z.C. helped with discussion of the paper. C.G., M.X., X. Wei., W.Y., Y.P. and Y.C. co-wrote and revised the paper. All authors contributed to this work and read the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–127, Discussion and Tables 1–10.
Supplementary Video 1
Assembly and metallization of PCOF-34.
Supplementary Video 2
Electrocatalytic urea synthesis processes via twin iron sites.
Supplementary Data 1
Structure model data for PCOF-34-Fe, CCDC 2345501.
Supplementary Data 2
Structure model data for PCOF-90-Fe, CCDC 2345502.
Supplementary Data 3
Structure model data for PCOF-34, CCDC 2345503.
Supplementary Data 4
Structure model data for PCOF-90, CCDC 2345504.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, C., Peng, Y., Xu, M. et al. Selective electrocatalytic synthesis of urea using entangled iron porphyrins in covalent organic frameworks. Nat. Synth 4, 720–729 (2025). https://doi.org/10.1038/s44160-025-00742-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00742-6
This article is cited by
-
Sequential-chain coupling over hierarchical click-sites enables highly selective urea electrosynthesis
Nature Communications (2026)
-
Electrocatalytic C‒N bond construction from inorganic nitrogen sources in water
Nature Protocols (2026)
-
Dual spillover of carbon monoxide and hydrogen initiates tandem urea electrosynthesis
Nature Communications (2026)
-
CeOx-Integrated dual site enhanced urea electrosynthesis from nitrate and carbon dioxide
Nature Communications (2025)
-
Boosting the brightness of aggregation-caused quenching chromophore-based covalent organic frameworks via energy level matching strategy
Nature Communications (2025)