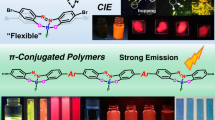
Abstract
Polyenes with an extensively cross-conjugated backbone, namely poly(1,1-vinylene)s or dendralenes, have fascinated chemists owing to their unique opto-electronic properties and reactivities. Existing synthetic strategies rely on coupling vinylic building blocks. Here we report a chain-growth approach that streamlines the synthesis of dendralene skeletons. An in-situ-generated strained [3]cumulene can interact with an organocopper species via its central in-plane π-bond, unlocking a previously unknown 2,3-polymerization pathway. This process rapidly generates cross-conjugated polyenes containing up to ~80–100 consecutive vinylene units on average with well-defined end groups. These π-electron-rich molecules adopt coiled, helical conformations as suggested by spectroscopic analysis, computational modelling and analogy to known foldamer systems, which induces two-photon absorption without compromising visible transparency. Their application in visible-range two-photon lithography with subdiffraction-limit resolution is demonstrated.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information.
References
Nielsen, M. B. & Diederich, F. Conjugated oligoenynes based on the diethynylethene unit. Chem. Rev. 105, 1837–1868 (2005).
Gholami, M. & Tykwinski, R. R. Oligomeric and polymeric systems with a cross-conjugated π-framework. Chem. Rev. 106, 4997–5027 (2006).
Limacher, P. A. & Lüthi, H. P. Cross conjugation. Wiley Interdiscip. Rev. Comput. Mol. Sci. 1, 477–647 (2011).
Mackay, E. G. & Sherburn, M. S. Demystifying the dendralenes. Pure Appl. Chem. 85, 1227–1239 (2013).
Wilson, J. N., Windscheif, P. M., Evans, U., Myrick, M. L. & Bunz, U. H. F. Band gap engineering of poly(p-phenyleneethynylene)s: cross-conjugated PPE-PPV hybrids. Macromolecules 35, 8681–8683 (2002).
Voortman, T. P., Bartesaghi, D., Koster, L. J. A. & Chiech, R. C. Cross-conjugated n-dopable aromatic polyketone. Macromolecules 48, 7007–7014 (2015).
Solomon, G. C. et al. Quantum interference in acyclic systems: conductance of cross-conjugated molecules. J. Am. Chem. Soc. 130, 17301–17308 (2008).
Guédon, C. M. et al. Observation of quantum interference in molecular charge transport. Nat. Nanotechnol. 7, 305–309 (2012).
Gu, J. et al. Cross conjugation in polyenes and related hydrocarbons: what can be learned from valence bond theory about single-molecule conductance? J. Am. Chem. Soc. 141, 6030–6047 (2019).
Zhao, Y. & Tykwinski, R. R. Iterative synthesis and properties of cross-conjugated iso-polydiacetylene oligomers. J. Am. Chem. Soc. 121, 458–459 (1999).
Zhao, Y., McDonald, R. & Tykwinski, R. R. Synthesis and characterization of cross-conjugated polyenynes. Chem. Commun. 77–78 (2000).
Tykwinski, R. R. & Zhao, Y. Cross-conjugated oligo(enynes). Synlett 12, 1939–1953 (2002).
Zhao, Y. et al. Synthesis, structure, and nonlinear optical properties of cross-conjugated perphenylated iso-polydiacetylenes. Chem. Eur. J. 11, 321–329 (2005).
Swager, T. M. & Grubbs, R. H. Synthesis and properties of a novel cross-conjugated conductive polymer precursor: poly(3,4-diisopropylidenecyclobutene). J. Am. Chem. Soc. 109, 894–896 (1987).
Mao, S. S. H. & Tilley, T. D. Cross-conjugated polymers via condensation of a zirconocene alkynyl(benzyne) derivative generated by thermolysis of Cp2ZrMe(C6H4C≡CSiMe3). J. Organomet. Chem. 521, 425–428 (1996).
Londergan, T. M., You, Y., Thompson, M. E. & Weber, W. P. Ruthenium catalyzed synthesis of cross-conjugated polymers and related hyperbranched materials. copoly(arylene/1,1-vinylene)s. Macromolecules 31, 2784–2788 (1998).
Itami, K., Ohashi, Y. & Yoshida, J.-i Triarylethene-based extended π-systems: programmable synthesis and photophysical properties. J. Org. Chem. 70, 2778–2792 (2005).
Nishioka, N., Hayashi, S. & Koizumi, T. Palladium(0)-catalyzed synthesis of cross-conjugated polymers: transformation into linear-conjugated polymers through the Diels–Alder reaction. Angew. Chem. Int. Ed. 51, 3682–3685 (2012).
van Pruissen, G. W. P., Brebels, J., Hendriks, K. H., Wienk, M. M. & Janssen, R. A. J. Effects of cross-conjugation on the optical absorption and frontier orbital levels of donor–acceptor polymers. Macromolecules 48, 2435–2443 (2015).
Jiang, K., Zhang, L., Zhao, Y., Lin, J. & Chen, M. Palladium-catalyzed cross-coupling polymerization: a new access to cross-conjugated polymers with modifiable structure and tunable optical/conductive properties. Macromolecules 51, 9662–9668 (2018).
Zhou, Q. et al. Palladium-catalyzed carbene coupling of N-tosylhydrazones and arylbromides to synthesize cross-conjugated polymers. Polym. Chem. 10, 569–573 (2019).
Hopf, H. The dendralenes-a neglected group of highly unsaturated hydrocarbons. Angew. Chem. Int. Ed. 23, 948–959 (1984).
Sherburn, M. S. Preparation and synthetic value of π-bond-rich branched hydrocarbons. Acc. Chem. Res. 48, 1961–1970 (2015).
Saglam, M. F., Fallon, T., Paddon-Row, M. N. & Sherburn, M. S. Discovery and computational rationalization of diminishing alternation in [n]dendralenes. J. Am. Chem. Soc. 138, 1022–1032 (2016).
Chen, M. et al. Stereospecific alkenylidene homologation of organoboronates by SNV reaction. Nature 631, 328–334 (2024).
Wu, B. et al. Copper-catalyzed formal dehydration polymerization of propargylic alcohols via cumulene intermediates. J. Am. Chem. Soc. 144, 4315–4320 (2022).
Su, H.-Z., Wu, B., Wang, J. & Zhu, R. Copper-catalyzed C(3+1) copolymerization of propargyl carbonates and aryldiazomethanes. Giant 13, 100139 (2023).
Wang, Z.-L. & Zhu, R. Regioselective condensation polymerization of propargylic electrophiles enabled by catalytic element-cupration. J. Am. Chem. Soc. 146, 19377–19385 (2024).
Johnson, R. P. Strained cyclic cumulenes. Chem. Rev. 89, 1111–1124 (1989).
Shakespeare, W. C. & Johnson, R. P. 1,2,3-Cyclohexatriene and cyclohexen-3-yne: two new highly strained C6H6 isomers. J. Am. Chem. Soc. 112, 8578–8579 (1990).
Kelleghan, A. V., Bulger, A. S., Witkowski, D. C. & Garg, N. K. Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives. Nature 618, 748–755 (2023).
Sakura, M., Ando, S., Hattori, A. & Saito, K. Reaction of cyclohexa-1,2,3-triene with N,α-diphenylnitrone: formation of seven-membered cyclic amines via piradone derivatives. Heterocycles 51, 547–556 (1999).
Angus, R. O., Janakiraman, M. N., Jacobson, R. A. & Johnson, R. P. Small-ring cyclic cumulenes: synthesis and X-ray crystal structure of bis(triphenylphosphine)chloro(η2-1,2,3-cyclononatriene)rhodium. Organometallics 6, 1909–1912 (1987).
Mizukoshi, Y., Mikami, K. & Uchiyama, M. Aryne polymerization enabling straightforward synthesis of elusive poly(ortho-arylene)s. J. Am. Chem. Soc. 137, 74–77 (2015).
Saglam, M. F. et al. Synthesis and Diels–Alder reactivity of substituted [4]dendralenes. J. Org. Chem. 81, 1461–1475 (2016).
Bürger, M. Phosphine-catalyzed aryne oligomerization: direct access to α,ω‑bisfunctionalized oligo(ortho-arylenes). J. Am. Chem. Soc. 143, 16796–16803 (2021).
Jing, Z. et al. Alkali metal ion recognition by 18-crown‑6 in aqueous solutions: evidence from local structures. J. Phys. Chem. B 127, 4858–4869 (2023).
Hartley, C. S. Folding of ortho-phenylenes. Acc. Chem. Res. 49, 646–654 (2016).
Ando, S. et al. Remarkable effects of terminal groups and solvents on helical folding of o-phenylene oligomers. J. Am. Chem. Soc. 134, 11084–11087 (2012).
Wang, S., Feng, X., Zhao, Z., Zhang, J. & Wan, X. Reversible cis-cisoid to cis-transoid helical structure transition in poly(3,5-disubstituted phenylacetylene)s. Macromolecules 49, 8407–8417 (2016).
Yuan, L. et al. Vitamin C stabilizes n-type organic semiconductors. Nat. Mater. 23, 1268–1275 (2024).
Maruo, S., Nakamura, O. & Kawata, S. Three-dimensional microfabrication with two-photon-absorbed. Opt. Lett. 22, 132–134 (1997).
O’Halloran, S., Pandit, A., Heise, A. & Kellett, A. Two-photon polymerization: fundamentals, materials, and chemical modification strategies. Adv. Sci. 10, 2204072 (2023).
Pawlicki, M., Collins, H. A., Denning, R. G. & Anderson, H. L. Two-photon absorption and the design of two-photon dyes. Angew. Chem. Int. Ed. 48, 3244–3266 (2009).
Totzeck, M., Ulrich, W., Göhnermeier, A. & Kaiser, W. Pushing deep ultraviolet lithography to its limits. Nat. Photonics 1, 629–631 (2007).
Liu, T. et al. Ultrahigh-printing-speed photoresists for additive manufacturing. Nat. Nanotechnol. 19, 51–57 (2024).
Acknowledgements
Financial support was provided by the Natural Science Foundation of China (grant numbers 223B2102, 22350006, 22222101 and 22171012). We thank Z. Gu, X. Liu, X. Zhou and G. Wang from the State Key Laboratory of Digital Medical Engineering at Southeast University, China, for their help and discussion in TPL. We thank S. Li (Institute of Chemistry, Chinese Academy of Sciences) for assistance with MALDI-TOF mass spectroscopy.
Author information
Authors and Affiliations
Contributions
R.Z. and Z.-Y.W. proposed the transformation. Z.-Y.W., Y.-Z.X., L.Z. and J.-Y.L. performed compound synthesis, polymer preparation and characterizations. Z.C. performed femtosecond laser lithography. R.Z. and Z.-Y.W. wrote the paper. R.Z. and Y.-Q.Z. directed the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alison Stoddart, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods and references.
Source data
Source Data Fig. 2
Source data for Fig. 2a.
Source Data Fig. 3
Source data for Fig. 3c.
Source Data Fig. 3
Source data for Fig. 3d.
Source Data Fig. 4
Source data for Fig. 4b.
Source Data Fig. 5
Source data for Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, ZY., Xu, YZ., Cui, Z. et al. Chain-growth synthesis of extensively cross-conjugated polyenes. Nat. Synth 4, 702–709 (2025). https://doi.org/10.1038/s44160-025-00760-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00760-4