Abstract
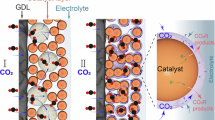
Industrializing the electrosynthesis of formate from CO2 reduction in membrane electrode assembly (MEA) electrolysers necessitates tuning both electrocatalysts and the interfacial water microenvironment. Here we cast a series of Turing-structured topology electrocatalysts, which can control the reorientation of interfacial water through the tuning of surface oxophilicity, for industrial-level conversion of CO2 to formate. Experimental and theoretical results verify the precisely modulated reorientation of interfacial water, with the ratios of four-coordinated to two-coordinated hydrogen-bonded interfacial water ranging from 0.26 to 3.10 over Turing-structured topology catalysts. We further demonstrate the efficiency of these strategies in sustaining high-rate formate electrosynthesis across a wide range of industrial-level current densities (300–1,000 mA cm−2) and formulate a volcano relationship to describe the relation. The optimal Turing Sb0.1Sn0.9O2 catalyst achieves a formate Faradaic efficiency of 92.0% at 1,000 mA cm-2 and exhibits a stability of 200 h at 500 mA cm-2 in a membrane electrode assembly electrolyser. Our findings highlight the prospect of topology-mediated tunings of the interfacial water microenvironment for electrifying the conversion of CO2 to formate, with promising implications for the electrosynthesis of other valuable chemicals.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information files.
References
Gomes, R. J. et al. Modulating water hydrogen bonding within a non-aqueous environment controls its reactivity in electrochemical transformations. Nat. Catal. 7, 689–701 (2024).
Chu, A. T. et al. Organic non-nucleophilic electrolyte resists carbonation during selective CO2 electroreduction. J. Am. Chem. Soc. 145, 9617–9623 (2023).
Zhang, H. et al. Promoting Cu-catalysed CO2 electroreduction to multicarbon products by tuning the activity of H2O. Nat. Catal. 6, 807–817 (2023).
Fang, W. et al. Durable CO2 conversion in the proton-exchange membrane system. Nature 626, 86–91 (2024).
Fernández-Caso, K. et al. Electroreduction of CO2: advances in the continuous production of formic acid and formate. ACS Energy Lett. 8, 1992–2024 (2023).
Yu, X. et al. Coverage enhancement accelerates acidic CO2 electrolysis at ampere-level current with high energy and carbon efficiencies. Nat. Commun. 15, 1711 (2024).
Ren, B. et al. Nano-crumples induced Sn–Bi bimetallic interface pattern with moderate electron bank for highly efficient CO2 electroreduction. Nat. Commun. 13, 2486 (2022).
Peng, C. et al. Ampere-level CO2-to-formate electrosynthesis using highly exposed bismuth(110) facets modified with sulfur-anchored sodium cations. Chem 9, 2830–2840 (2023).
Ko, Y. J. et al. Exploring dopant effects in stannic oxide nanoparticles for CO2 electro-reduction to formate. Nat. Commun. 13, 2205 (2022).
Zhang, L. et al. Oxophilicity-controlled CO2 electroreduction to C2+ alcohols over Lewis acid metal-doped Cuδ+ catalysts. J. Am. Chem. Soc. 145, 21945–21954 (2023).
Li, Z. et al. Directing CO2 electroreduction pathways for selective C2 product formation using single-site doped copper catalysts. Nat. Chem. Eng. 1, 159–169 (2024).
Zeng, M. et al. Reaction environment regulation for electrocatalytic CO2 reduction in acids. Angew. Chem. Int. Ed. 63, e202404574 (2024).
Wang, D. et al. Modulating microenvironments to enhance CO2 electroreduction performance. eScience 3, 100119 (2023).
Zhang, Z. et al. Probing electrolyte effects on cation-enhanced CO2 reduction on copper in acidic media. Nat. Catal. 7, 807–817 (2024).
Ma, X. et al. Hydrogen-bond network promotes water splitting on the TiO2 surface. J. Am. Chem. Soc. 144, 13565–13573 (2022).
Ge, W. et al. Dynamically formed surfactant assembly at the electrified electrode–electrolyte interface boosting CO2 electroreduction. J. Am. Chem. Soc. 144, 6613–6622 (2022).
Wang, Y. et al. Strong hydrogen-bonded interfacial water inhibiting hydrogen evolution kinetics to promote electrochemical CO2 reduction to C2+. ACS Catal. 14, 3457–3465 (2024).
Chen, X. et al. Promoting water dissociation for efficient solar driven CO2 electroreduction via improving hydroxyl adsorption. Nat. Commun. 14, 751 (2023).
Fan, Y. et al. Mechanistic insights into surfactant-modulated electrode–electrolyte interface for steering H2O2 electrosynthesis. J. Am. Chem. Soc. 146, 7575–7583 (2024).
Wang, Y. H. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 600, 81–85 (2021).
Li, P. et al. Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal. 5, 900–911 (2022).
Sun, Q. et al. Understanding hydrogen electrocatalysis by probing the hydrogen-bond network of water at the electrified Pt–solution interface. Nat. Energy 8, 859–869 (2023).
Ma, Z. et al. CO2 electroreduction to multicarbon products in strongly acidic electrolyte via synergistically modulating the local microenvironment. Nat. Commun. 13, 7596 (2022).
Gu, J. et al. Turing structuring with multiple nanotwins to engineer efficient and stable catalysts for hydrogen evolution reaction. Nat. Commun. 14, 5389 (2023).
Gu, J. et al. Twinning engineering of platinum/iridium nanonets as Turing-type catalysts for efficient water splitting. J. Am. Chem. Soc. 146, 5355–5365 (2024).
Tan, Z. et al. Polyamide membranes with nanoscale Turing structures for water purification. Science 360, 518–521 (2018).
Zhang, X. L. et al. An efficient Turing‐type Ag2Se–CoSe2 multi‐interfacial oxygen‐evolving electrocatalyst. Angew. Chem. Int. Ed. 60, 6553–6560 (2021).
Li, X. Y. et al. Constrained minimal-interface structures in polycrystalline copper with extremely fine grains. Science 370, 831–836 (2020).
Hu, C. et al. Misoriented high-entropy iridium ruthenium oxide for acidic water splitting. Sci. Adv. 9, f9144 (2023).
Zhang, Y. et al. Homogeneous solution assembled Turing structures with near zero strain semi-coherence interface. Nat. Commun. 13, 2942 (2022).
Bi, J. et al. High‐rate CO2 electrolysis to formic acid over a wide potential window: an electrocatalyst comprised of indium nanoparticles on chitosan‐derived graphene. Angew. Chem. Int. Ed. 62, e202307612 (2023).
Shen, H. et al. In‐situ constructing of copper‐doped bismuth catalyst for highly efficient CO2 electrolysis to formate in ampere‐level. Adv. Energy Mater. 13, 2202818 (2023).
Li, L. et al. Stable, active CO2 reduction to formate via redox-modulated stabilization of active sites. Nat. Commun. 12, 5223 (2021).
Yan, S. et al. Electron localization and lattice strain induced by surface lithium doping enable ampere-level electrosynthesis of formate from CO2. Angew. Chem. Int. Ed. 60, 25741–25745 (2021).
Chen, X. et al. Revealing the role of interfacial water and key intermediates at ruthenium surfaces in the alkaline hydrogen evolution reaction. Nat. Commun. 14, 5289 (2023).
Liu, Z. et al. Interfacial water tuning by intermolecular spacing for stable CO2 electroreduction to C2+ products. Angew. Chem. Int. Ed. 62, e202309319 (2023).
Ye, F. et al. The role of oxygen-vacancy in bifunctional indium oxyhydroxide catalysts for electrochemical coupling of biomass valorization with CO2 conversion. Nat. Commun. 14, 2040 (2023).
Acknowledgements
This work was financially supported by the Science and Technology Innovation Project of Laoshan Laboratory (LSKJ202205400), the National Science Fund for Distinguished Young Scholars (number 52025133), the Beijing Outstanding Young Scientist Program (JWZQ20240102004), the National Science Fund for Young Scholars (numbers 22102003, 22409009), the China National Petroleum Corporation-Peking University Strategic Cooperation Project of Fundamental Research, the Beijing Natural Science Foundation (number Z220020), the Tencent Foundation through the XPLORER PRIZE, the CNPC Innovation Fund (2021DQ02-1002) and the China Postdoctoral Science Foundation (grant number 2023M730052).
Author information
Authors and Affiliations
Contributions
S.G. conceived the project. N.Y. and M.L. designed the research, and performed the material synthesis, characterization and electrochemical tests. K.W., Y.T., Z.Q., Z.L. and Q.H. participated in assembling and testing the MEA. N.Y. and Y.L. carried out the XAFS data analysis. H.G., C.S., Y.H. and C.Z. performed the HAADF-STEM characterization. L.L. and Y.G. conducted the density functional theory calculations. S.G. and N.Y. wrote the paper. All authors participated in the project discussions and production of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
General information, Syntheses, Methods, Supplementary Figs. 1–36 and Tables 1 and 2.
Source data
Source Data Fig. 2
Statistical source data for Fig. 2a–j.
Source Data Fig. 3
Statistical source data for Fig. 3a–e.
Source Data Fig. 4
Statistical source data for Fig. 4a–i.
Source Data Fig. 5
Statistical source data for Fig. 5b–d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, N., Wang, K., Tan, Y. et al. Industrial-level CO2 to formate conversion on Turing-structured electrocatalysts. Nat. Synth 4, 799–807 (2025). https://doi.org/10.1038/s44160-025-00769-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00769-9