Abstract
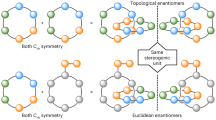
Catenanes are formed by the mechanical interlocking of two or more rings. Enantiomers of a catenane can exist even if the rings themselves are achiral. Here we demonstrate that two achiral rings, each featuring a polarized cavity and two mirror planes, in addition to a two-fold axis of symmetry, can form a catenane with mechanical chirality. The catenane has been designed using an isostructural desymmetrization strategy, enabling the catenane to adopt a compact co-conformation similar to that of its achiral isostructural counterpart. Mechanical chirality in the catenane occurs when its two rings become interlocked in the compact co-conformation, leading to the loss of the two planes of symmetry present in its individual rings. The resulting enantiomers, which both have two-fold axes of symmetry, exist as a racemic modification in the solid state. Dynamic 1H NMR spectroscopy carried out in acetonitrile-d3 reveals a barrier of 16.4 kcal mol−1 to racemization between the two enantiomeric catenanes, the equilibrium of which can be influenced by the addition of chiral disulfonate anions, which support induced chirality and exhibit optical activity. One of the salts crystallizes to give only one diastereoisomer in the solid state. This research highlights the potential of using the isostructural desymmetrization strategy to create and study mechanical chirality along with its properties.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings of this investigation are available in the Article and its Supplementary Information. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2367987 (BPBox·2TFA), 2367986 (BPHC·4TFA), 2367990 (ExBPBox·4PF6) and 2367988 (BPHC·K·2BINSA). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Sauvage, J.-P. & Dietrich-Buchecker, C. Molecular Catenanes, Rotaxanes and Knots: a Journey through the World of Molecular Topology (Wiley, 2008).
Bruns, C. J. & Stoddart, J. F. The Nature of the Mechanical Bond: from Molecules to Machines (Wiley, 2016).
Amabilino, D. B. & Stoddart, J. F. Interlocked and intertwined structures and superstructures. Chem. Rev. 95, 2725–2828 (1995).
Champin, B., Mobian, P. & Sauvage, J.-P. Transition metal complexes as molecular machine prototypes. Chem. Soc. Rev. 36, 358–366 (2007).
Jamieson, E. M. G., Modicom, F. & Goldup, S. M. Chirality in rotaxanes and catenanes. Chem. Soc. Rev. 47, 5266–5311 (2018).
Evans, N. H. Chiral catenanes and rotaxanes: fundamentals and emerging applications. Chem. Eur. J. 24, 3101–3112 (2018).
Goldup, S. M. The end of the beginning of mechanical stereochemistry. Acc. Chem. Res. 57, 1696–1708 (2024).
Cakmak, Y., Erbas-Cakmak, S. & Leigh, D. A. Asymmetric catalysis with a mechanically point-chiral rotaxane. J. Am. Chem. Soc. 138, 1749–1751 (2016).
Maynard, J. R. J. & Goldup, S. M. Strategies for the synthesis of enantiopure mechanically chiral molecules. Chem 6, 1914–1932 (2020).
de Juan, A. et al. A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes. Nat. Chem. 14, 179–187 (2022).
Corra, S. et al. Chemical on/off switching of mechanically planar chirality and chiral anion recognition in a [2]rotaxane molecular shuttle. J. Am. Chem. Soc. 141, 9129–9133 (2019).
Frisch, H. L. & Wasserman, E. Chemical topology. J. Am. Chem. Soc. 83, 3789–3795 (1961).
Stoddart, J. F. The master of chemical topology. Chem. Soc. Rev. 38, 1521–1529 (2009).
Forgan, R. S., Sauvage, J.-P. & Stoddart, J. F. Chemical topology: complex molecular knots, links, and entanglements. Chem. Rev. 111, 5434–5464 (2011).
Stoddart, J. F. Dawning of the age of molecular nanotopology. Nano Lett. 20, 5597–5600 (2020).
Rodríguez-Rubio, A., Savoini, A., Modicom, F., Butler, P. & Goldup, S. M. A co-conformationally ‘topologically’ chiral catenane. J. Am. Chem. Soc. 144, 11927–11932 (2022).
Ashton, P. R. et al. Molecular mosaics formed by a square cyclophane and its inclusion complex with ferrocene. Angew. Chem. Int. Ed. 34, 1862–1865 (1995).
Stoddart, J. F. et al. Molecular meccano. 3. Constitutional and translational isomerism in [2]catenanes and [n]pseudorotaxanes. J. Am. Chem. Soc. 117, 11142–11170 (1995).
Zhu, K., Baggi, G. & Loeb, S. J. Ring-through-ring molecular shuttling in a saturated [3]rotaxane. Nat. Chem. 10, 625–630 (2018).
Bruns, C. J. & Stoddart, J. F. Rotaxane-based molecular muscles. Acc. Chem. Res. 47, 2186–2199 (2014).
Vukotic, V. N., Harris, K. J., Zhu, K., Schurko, R. W. & Loeb, S. J. Metal–organic frameworks with dynamic interlocked components. Nat. Chem. 4, 456–460 (2012).
Iwaso, K., Takashima, Y. & Harada, A. Fast response dry-type artificial molecular muscles with [c2]daisy chains. Nat. Chem. 8, 625–632 (2016).
Meng, W. et al. An elastic metal–organic crystal with a densely catenated backbone. Nature 598, 298–303 (2021).
Huang, Z. et al. Highly compressible glass-like supramolecular polymer networks. Nat. Mater. 21, 103–109 (2022).
Ma, T. et al. Catenated covalent organic frameworks constructed from polyhedra. Nat. Synth. 2, 286–295 (2023).
Schalley, C. A., Beizai, K. & Vögtle, F. On the way to rotaxane-based molecular motors: studies in molecular mobility and topological chirality. Acc. Chem. Res. 34, 465–476 (2001).
Coskun, A., Banaszak, M., Astumian, R. D., Stoddart, J. F. & Grzybowski, B. A. Great expectations: can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 41, 19–30 (2012).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
Pezzato, C., Cheng, C., Stoddart, J. F. & Astumian, R. D. Mastering the non-equilibrium assembly and operation of molecular machines. Chem. Soc. Rev. 46, 5491–5507 (2017).
Jamieson, E. M. G. & Goldup, S. M. Chirality makes a move. Nat. Chem. 11, 765–767 (2019).
Ren, Y., Jamagne, R., Tetlow, D. J. & Leigh, D. A. A tape-reading molecular ratchet. Nature 612, 78–82 (2022).
Wang, Y. et al. Multistate circularly polarized luminescence switching through stimuli-induced co-conformation regulations of pyrene-functionalized topologically chiral [2]catenane. Angew. Chem. Int. Ed. 61, e202210542 (2022).
Vignon, S. A., Wong, J., Tseng, H.-R. & Stoddart, J. F. Helical chirality in donor-acceptor catenanes. Org. Lett. 6, 1095–1098 (2004).
Zhu, K., Vukotic, V. N. & Loeb, S. J. Molecular shuttling of a compact and rigid H-shaped [2]rotaxane. Angew. Chem. Int. Ed. 51, 2168–2172 (2012).
Hasell, T. et al. Triply interlocked covalent organic cages. Nat. Chem. 2, 750–755 (2010).
Power, M. J., Morris, D. T. J., Vitorica-Yrezabal, I. J. & Leigh, D. A. Compact rotaxane superbases. J. Am. Chem. Soc. 145, 8593–8599 (2023).
Barnes, J. C. et al. A radically configurable six-state compound. Science 339, 429–433 (2013).
Barnes, J. C. et al. Solid-state characterization and photoinduced intramolecular electron transfer in a nanoconfined octacationic homo[2]catenane. J. Am. Chem. Soc. 136, 10569–10572 (2014).
Lahlali, H., Jobe, K., Watkinson, M. & Goldup, S. M. Macrocycle size matters: ‘small’ functionalized rotaxanes in excellent yield using the CuAAC active template approach. Angew. Chem. Int. Ed. 50, 4151–4155 (2011).
Lewis, J. E. M., Modicom, F. & Goldup, S. M. Efficient multicomponent active template synthesis of catenanes. J. Am. Chem. Soc. 140, 4787–4791 (2018).
Rowan, S. J., Cantrill, S. J., Cousins, G. R. L., Sanders, J. K. M. & Stoddart, J. F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 41, 898–952 (2002).
Caprice, K. et al. Diastereoselective amplification of a mechanically chiral [2]catenane. J. Am. Chem. Soc. 143, 11957–11962 (2021).
Wu, G. et al. Controllable self-assembly of macrocycles in water for isolating aromatic hydrocarbon isomers. J. Am. Chem. Soc. 140, 5955–5961 (2018).
Cui, Z. & Jin, G.-X. Construction of a molecular prime link by interlocking two trefoil knots. Nat. Synth. 1, 635–640 (2022).
Miljanić, O. Š. & Stoddart, J. F. Dynamic donor acceptor [2]catenanes. Proc. Natl Acad. Sci. USA 104, 12966–12970 (2007).
Fujita, M., Ibukuro, F., Hagihara, H. & Ogura, K. Quantitative self-assembly of a [2]catenane from two preformed molecular rings. Nature 367, 720–723 (1994).
Garci, A. et al. Mechanical-bond-induced exciplex fluorescence in an anthracene-based homo[2]catenane. J. Am. Chem. Soc. 142, 7956–7967 (2020).
Kruve, A. et al. Ion-mobility mass spectrometry for the rapid determination of the topology of interlocked and knotted molecules. Angew. Chem. Int. Ed. 58, 11324–11328 (2019).
Ashton, P. R. et al. Self-assembling cyclophanes and catenanes possessing elements of planar chirality. Chem. Eur. J. 4, 299–310 (1998).
Dietrich-Buchecker, C. O., Edel, A., Kintzinger, J. P. & Sauvage, J. P. Synthese et etude d’un catenate de cuivre chiral comportant deux anneaux coordinant a 27 atomes. Tetrahedron 43, 333–344 (1987).
Juríček, M. et al. Induced-fit catalysis of corannulene bowl-to-bowl inversion. Nat. Chem. 6, 222–228 (2014).
Garci, A. et al. Mechanically interlocked pyrene-based photocatalysts. Nat. Catal. 5, 524–533 (2022).
Lu, T. & Chen, Q. Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J. Comput. Chem. 43, 539–555 (2022).
Acknowledgements
We acknowledge W. Liu from the University of South Florida and Y. Xu from Henan University for their helpful discussion of NMR spectroscopy line-shape simulations. Financial support from the University of Hong Kong, Northwestern University and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (grant no. SN-ZJU-SIAS-006) is gratefully acknowledged. C.T. has received support from the University Research Committee of the University of Hong Kong. This work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under award DE-FG02-99ER14999 (M.R.W.). This work made use of the IMSERC Crystallography facility at Northwestern University, which has received support from the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633), and Northwestern University. J.F.S. passed away on 30 December 2024 and was a corresponding author when the Article was first submitted.
Author information
Authors and Affiliations
Contributions
C.T., R.Z. and J.F.S. conceived the idea for this project. C.T. synthesized and characterized all the compounds. R.M.Y. and P.J.B. acquired the time-resolved spectroscopy and analysed the data. P.J.D. and S.A. designed and performed the cell viability and confocal microscopy experiments. C.T., R.Z. and J.F.S. wrote the draft of the paper with input from G.W., H.H., X.Z., A.H.G.D., H.W., B.S., A.A., Y.W., Y.Y., Y.F., A.X.-Y.C., C.L.S., Z.L., E.A.S. and M.R.W. All the authors participated in evaluating the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note The editorial team of Nature Synthesis declare that Alison Stoddart has had no involvement in the editorial handling of this article.
Supplementary information
Supplementary Information
Experimental details, Supplementary Figs. 1–70, Supplementary Discussion, Supplementary Schemes 1–5 and Supplementary Tables 1–4.
Supplementary Data 1
X-ray crystallographic data for BPBox·2TFA, CCDC 2367987.
Supplementary Data 2
X-ray crystallographic data for BPHC·4TFA, CCDC 2367986.
Supplementary Data 3
X-ray crystallographic data for ExBPBox·4PF6, CCDC 2367990.
Supplementary Data 4
X-ray crystallographic data for BPHC·K·2BINSA, CCDC 2367988.
Source data
Source Data Fig. 2
Including ultraviolet–visible spectra, fluorescent spectra and TA data.
Source Data Fig. 5
Including Eyring equation analysis and fitting.
Source Data Fig. 6
Including CD spectra and titration analysis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, C., Zhang, R., Almunif, S. et al. A compact catenane with tuneable mechanical chirality. Nat. Synth 4, 956–964 (2025). https://doi.org/10.1038/s44160-025-00781-z
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00781-z
This article is cited by
-
Unravelling the nucleation–elongation mechanism of one-pot catenation
Nature Communications (2026)