Abstract
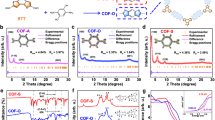
Linkages in covalent organic frameworks (COFs) are the strong covalent bonds that bind molecular building units into porous frameworks. As the stability and properties of the COF structure are often dominated by its linkage, development of suitable linkage chemistry is important. Among the diversity of COF linkages, carbon–carbon bonded linkages are especially interesting for their ultrahigh stabilities. However, due to the highly irreversible nature of carbon–carbon bonds, crystallizing the corresponding COFs remains a major challenge to further expand the scope of this chemistry. Here we develop a postsynthetic linkage conversion strategy for realizing coumarin linkages wherein fused aromatic six-membered rings are constituted by carbon–carbon and carbon–oxygen bonds. With this strategy, five coumarin-linked COFs were synthesized with consecutive Knoevenagel condensation and Pinner reactions. The structures of these COFs were confirmed by powder X-ray diffraction, Fourier-transform infrared spectroscopy and 13C solid-state nuclear magnetic resonance spectroscopy. These COFs showed good porosity (Brunauer–Emmett–Teller surface area up to 981 m2 g−1), outstanding chemical stability in strong acids and bases and high thermal stability up to around 600 °C. The visible-light-induced green luminescence of one of the COFs was also characterized by photoluminescence spectroscopy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The other data that support the findings of this article are available in the Supplementary Information. Source data are provided with this paper.
References
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Martín-Illán, J. A., Rodríguez-San-Miguel, D. & Zamora, F. Evolution of covalent organic frameworks: from design to real-world applications. Coord. Chem. Rev. 495, 215342 (2023).
El-Kaderi, H. M. et al. Designed synthesis of 3D covalent organic frameworks. Science 316, 268–272 (2007).
Côté, A. P. et al. Reticular synthesis of microporous and mesoporous 2D covalent organic frameworks. J. Am. Chem. Soc. 129, 12914–12915 (2007).
Uribe-Romo, F. J. et al. A crystalline imine-linked 3-D porous covalent organic framework. J. Am. Chem. Soc. 131, 4570–4571 (2009).
Ding, S.-Y. et al. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki–Miyaura coupling reaction. J. Am. Chem. Soc. 133, 19816–19822 (2011).
Pyles, D. A. et al. Synthesis of benzobisoxazole-linked two-dimensional covalent organic frameworks and their carbon dioxide capture properties. ACS Macro Lett. 5, 1055–1058 (2016).
Wei, P.-F. et al. Benzoxazole-linked ultrastable covalent organic frameworks for photocatalysis. J. Am. Chem. Soc. 140, 4623–4631 (2018).
Waller, P. J. et al. Conversion of imine to oxazole and thiazole linkages in covalent organic frameworks. J. Am. Chem. Soc. 140, 9099–9103 (2018).
Zhang, B. et al. Crystalline dioxin-linked covalent organic frameworks from irreversible reactions. J. Am. Chem. Soc. 140, 12715–12719 (2018).
Guan, X. et al. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 11, 587–594 (2019).
Zhao, C. et al. Ester-linked crystalline covalent organic frameworks. J. Am. Chem. Soc. 142, 14450–14454 (2020).
Zhuang, X. et al. A two-dimensional conjugated polymer framework with fully sp2-bonded carbon skeleton. Polym. Chem. 7, 4176–4181 (2016).
Jin, E. et al. Two-dimensional sp2 carbon–conjugated covalent organic frameworks. Science 357, 673–676 (2017).
Lyu, H. et al. Porous crystalline olefin-linked covalent organic frameworks. J. Am. Chem. Soc. 141, 6848–6852 (2019).
Li, X. et al. Facile transformation of imine covalent organic frameworks into ultrastable crystalline porous aromatic frameworks. Nat. Comm. 9, 2998 (2018).
Feng, J. et al. Fused-ring-linked covalent organic frameworks. J. Am. Chem. Soc. 144, 6594–6603 (2022).
Yang, Y. et al. Constructing chemical stable 4-carboxyl-quinoline linked covalent organic frameworks via Doebner reaction for nanofiltration. Nat. Comm. 13, 2615 (2022).
Zhang, Q. et al. Synthesis of two-dimensional C–C bonded truxene-based covalent organic frameworks by irreversible Brønsted acid-catalyzed aldol cyclotrimerization. Research 2021, 9790705 (2021).
Han, X. et al. Crystalline polyphenylene covalent organic frameworks. J. Am. Chem. Soc. 146, 89–94 (2024).
Haase, F. & Lotsch, B. V. Solving the COF trilemma: towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 49, 8469–8500 (2020).
Bi, S. et al. Covalent organic frameworks with trans-dimensionally vinylene-linked π-conjugated motifs. Chem. Res. Chin. Univ. 38, 382–395 (2022).
Su, Y. et al. Crystalline and stable benzofuran-linked covalent organic frameworks from irreversible cascade reactions. J. Am. Chem. Soc. 142, 13316–13321 (2020).
Chen, Z. et al. Manipulating grain boundary defects in π-conjugated covalent organic frameworks enabling intrinsic radical generation for photothermal conversion. Sol. RRL 5, 2100762 (2021).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal158 (2017).
Zhou, Z. et al. Carbon dioxide capture from open air using covalent organic frameworks. Nature 635, 96–101 (2024).
Chen, J.-M. et al. Improving lithium–sulfur batteries’ performance via inverse vulcanization of vinylene-linked covalent organic frameworks. Energy Fuels 36, 5998–6004 (2022).
Haldar, S. et al. Covalent trapping of cyclic-polysulfides in perfluorinated vinylene-linked frameworks for designing lithium-organosulfide batteries. ACS Energy Lett. 8, 5098–5106 (2023).
Yang, Y. et al. On-water surface synthesis of vinylene-linked cationic two-dimensional polymer films as the anion-selective electrode coating. Angew. Chem. Int. Ed. 63, e202316299 (2024).
Yuan, C. et al. Crystalline C–C and C=C bond-linked chiral covalent organic frameworks. J. Am. Chem. Soc. 143, 369–381 (2021).
Wang, Y. et al. Linkages make a difference in the photoluminescence of covalent organic frameworks. Angew. Chem. Int. Ed. 62, e202310794 (2023).
Cao, D. et al. Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev. 119, 10403–10519 (2019).
Wagner, B. D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 14, 210–237 (2009).
Tian, G. et al. Design, synthesis and application in analytical chemistry of photo-sensitive probes based on coumarin. Crit. Rev. Anal. Chem. 51, 565–581 (2021).
Stringlis, I. A., de Jonge, R. & Pieterse, C. M. J. The age of coumarins in plant–microbe interactions. Plant Cell Physiol. 60, 1405–1419 (2019).
de Kiewiet, T. & Stephen, H. Condensation of aromatic aldehydes with phenylacetonitrile. J. Chem. Soc. 0, 639–640 (1931).
Brufola, G. et al. Simple and efficient one-pot preparation of 3-substituted coumarins in water. Heterocycles 43, 1257–1266 (1996).
Irgashev, R. A. et al. A convenient approach to the design and synthesis of indolo[3,2-c]coumarins via the microwave-assisted Cadogan reaction. Tetrahedron Lett. 54, 5734–5738 (2013).
Bourda, L. et al. Conquering the crystallinity conundrum: efforts to increase quality of covalent organic frameworks. Mater. Adv. 2, 2811–2845 (2021).
Greene, T. W. & Wuts, P. G. M. Protective Groups in Organic Synthesis (Wiley-Interscience, 1999).
Pinner, A. & Klein, F. Umwandlung der nitrile in imide. Ber. Dtsch. Chem. Ges. 10, 1889–1897 (1877).
Chen, S. et al. Synthesis and studies of axial chiral bisbenzocoumarins: aggregation-induced emission enhancement properties and aggregation-annihilation circular dichroism effects. Spectrochim. Acta, Part A 193, 141–146 (2018).
Pawley, G. S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 14, 357–361 (1981).
Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts (Wiley, 2001).
Bovey, F. A. NMR Data Tables for Organic Compounds (Interscience, 1967).
Beamson, G. & Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database (Wiley, 1992).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Ji, W. et al. Removal of GenX and perfluorinated alkyl substances from water by amine-functionalized covalent organic frameworks. J. Am. Chem. Soc. 140, 12677–12681 (2018).
Woodliffe, J. L. et al. Evaluating the purification and activation of metal–organic frameworks from a technical and circular economy perspective. Coord. Chem. Rev. 428, 213578 (2021).
Lastoskie, C., Gubbins, K. E. & Quirke, N. Pore size distribution analysis of microporous carbons: a density functional theory approach. J. Phys. Chem. 97, 4786–4796 (1993).
Grimm, J. B., Heckman, L. M. & Lavi, L. D. Progress in Molecular Biology and Translational Science: Fluorescence-Based Biosensors Ch. 1 (Academic, 2013).
Yang, M. et al. Acrylonitrile-linked covalent organic frameworks enable fast stimulus-responsive fluorescence with high quantum yield via fluorine chemistry. Adv. Funct. Mater. 8, 2200008 (2022).
Xue, R. et al. Fluorescence properties and analytical applications of covalent organic frameworks. Anal. Methods 9, 3737–3750 (2017).
Fung, B. M., Khitrin, A. K. & Ermolaev, K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 142, 97–101 (2000).
Johnson, R. L. & Schmidt-Rohr, K. Quantitative solid-state 13C NMR with signal enhancement by multiple cross polarization. J. Magn. Reson. 239, 44–49 (2014).
Acknowledgements
H.Z. and Z.Z. thank H. Li (Yaghi Research Group, UC Berkeley) for helpful discussions. We also thank K. Wang and A. Alawadhi (Yaghi Research Group, UC Berkeley) for their time and efforts on maintaining the sorption instruments. We acknowledge H. Celik, R. Giovine and UC Berkeley Pines Magnetic Resonance Center’s Core NMR Facility for spectroscopic assistance. We also thank H. Zhu, Y. Shan, J. Feijoo, X. Chen, P. Yang (Yang Group, UC Berkeley) and the Molecular Foundry for their assistance in photoluminescence spectroscopy, XPS and TEM. We thank M. Kang, R. Zalpuri, D. Jorgens and the UC Berkeley Electron Microscope Laboratory, for access and assistance in electron microscopy data collection. We also thank Z. Zhou and UC Berkeley QB3/Chemistry Mass Spectrometry Facility for mass spectrum assistance. This research was supported by the King Abdulaziz City for Science and Technology (Center of Excellence for Nanomaterials and Clean Energy Applications) and the Bakar Institute of Digital Materials for the Planet. The WAXS data in this research were obtained from beamline 7.3.3 of the Advanced Light Source, which is a DOE Office of Science User Facility (contract no. DE-AC02-05CH11231). The NMR instruments used in this work are supported by National Institutes of Health (grant no. S10OD024998). The SEM instrument used in this work is supported by National Institutes of Health (grant no. S10OD030258-01). Z.Z. and O.M.Y. acknowledge the interest and support of Fifth Generation (Love, Tito’s).
Author information
Authors and Affiliations
Contributions
H.Z., Z.Z. and O.M.Y. conceived of the idea and led the experimental efforts. H.Z. and Z.Z. designed the COFs and developed synthetic methodologies. H.Z. collected and analysed the nitrogen sorption, PXRD, SEM, TGA and FT-IR data. Z.Z. conducted the ssNMR experiments. C.Z. conducted the WAXS experiments. All authors contributed to revising the paper.
Corresponding author
Ethics declarations
Competing interests
COF-991 to COF-995 and their related materials have been filed as US provisional patent application no. 63/797,930 by UC Berkeley. O.M.Y., H.Z. and Z.Z. are the inventors of this patent. C.Z. declares no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Bao-Hang Han, Qichun Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 HRTEM images of COF-991-CN and COF-991.
a, High-resolution TEM image of COF-991-CN, showing lattice fringe of (001) plane. b, High-resolution TEM image of COF-991, showing lattice fringe of (110) plane.
Extended Data Fig. 2 Comparison of XPS curves of COF-991-CN and COF-991.
a, Full XPS curves of COF-991-CN and COF-991. b-d, Experimental data, fitted curves, linear deconvolutions and background curves for O 1s (b), N 1s (c) and C 1s (d) from XPS curves of COF-991-CN (top) and COF-991 (bottom).
Extended Data Fig. 3 Comparison of PXRD patterns and FT-IR spectra of original COF-991 before and after being treated with various reagents.
a,b, Comparison of PXRDs patterns (a) and FT-IR spectra (b) of original COF-991, and after treated with water, boiling water, CF3SO3H, 16 M HNO3, 12 M HCl, 96% H2SO4, 12 M NaOH, 3 M NaOMe in MeOH and saturated NaH in THF.
Extended Data Fig. 4 Comparison of nitrogen sorption isotherms at 77 K of original COF-991 before and after being treated with various reagents.
Nitrogen sorption isotherms at 77K of original COF-991, and after treated with boiling water, HCl, 96% H2SO4, 12 M NaOH, and saturated NaH in THF.
Extended Data Fig. 5 13C CP/MAS solid-state NMR spectra of COF-992-CN to COF-995-CN and COF-992 to COF-995.
a, 13C solid-state NMR spectra of COF-992-CN and COF-992. b, 13C solid-state NMR spectra of COF-993-CN and COF-993. c, 13C solid-state NMR spectra of COF-994-CN and COF-994. d, 13C solid-state NMR spectra of COF-995-CN and COF-995.
Supplementary information
Supplementary Information
Supplementary Figs. 1–92, Tables 1–17, methods, discussions and equation (1).
Source data
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data Fig. 5
Statistical source data for Fig. 5.
Source Data Extended Data Fig. 2
Statistical source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Statistical source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Statistical source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Statistical source data for Extended Data Fig. 5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Zhou, Z., Zhu, C. et al. Crystalline coumarin-linked covalent organic frameworks. Nat. Synth 4, 1376–1385 (2025). https://doi.org/10.1038/s44160-025-00859-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00859-8
This article is cited by
-
Coumarin linkages for robust frameworks
Nature Synthesis (2025)