Abstract
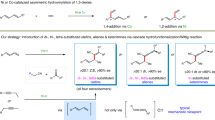
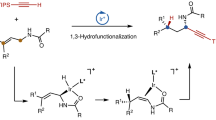
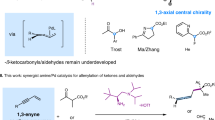
Divergent synthesis approaches generate all isomers of a molecule, but typically focus on single-dimensional selectivity control. The more challenging two-dimensional divergent synthesis dictating two types of selectivity to achieve all four isomers has recently received some attention. As such, three-dimensional divergent synthesis through dictation of three types of selectivity to prepare eight isomers or analogues is very challenging. Here we report a catalyst-controlled three-dimensional divergent protocol for the synthesis of eight types of diaryl allyl skeleton (through combinations of E/Z and R/S stereochemistry and 1,1-/1,3-substitution patterns) from the reaction of alkynes and heteroarenes. Good geometric selectivity, regioselectivity and enantioselectivity are observed for the hydroheteroarylation process of internal alkynes with indolizines via Pd and Rh catalysis. Experimental data and density functional theory calculations reveal that the Pd and Rh catalysts have differing mechanistic pathways for the hydroarylation of the internal alkynes and corresponding allene intermediates.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data are available with the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2463493 (3r) and 2381076 (6p). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/.
References
Harper, M. J. K. & Walpole, A. L. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature 212, 87 (1966).
Caldwell, J. The importance of stereochemistry in drug action and disposition. J. Clin. Pharmacol. 32, 925–929 (1992).
Krautwald, S. & Carreira, E. M. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 139, 5627–5639 (2017).
Beletskaya, I. P., Nájera, C. & Yus, M. Stereodivergent catalysis. Chem. Rev. 118, 5080–5200 (2018).
Lin, L. & Feng, X. Catalytic strategies for diastereodivergent synthesis. Chem. Eur. J. 23, 6464–6482 (2017).
Bihani, M. & Zhao, J. C.-G. Advances in asymmetric diastereodivergent catalysis. Adv. Synth. Catal. 359, 534–575 (2017).
Moser, D., Schmidt, T. A. & Sparr, C. Diastereodivergent catalysis. JACS Au 3, 2612–2630 (2023).
Kim, Y. H. Dual enantioselective control in asymmetric synthesis. Acc. Chem. Res. 34, 955–962 (2001).
Zanoni, G., Castronovo, F., Franzini, M., Vidari, G. & Giannini, E. Toggling enantioselective catalysis—a promising paradigm in the development of more efficient and versatile enantioselective synthetic methodologies. Chem. Soc. Rev. 32, 115–129 (2003).
Bartok, M. Unexpected inversions in asymmetric reactions: reactions with chiral metal complexes, chiral organocatalysts, and heterogeneous chiral catalysts. Chem. Rev. 110, 1663–1705 (2010).
Escorihuela, J., Burguete, M. I. & Luis, S. V. New advances in dual stereocontrol for asymmetric reactions. Chem. Soc. Rev. 42, 5595–5617 (2013).
Funken, N., Zhang, Y.-Q. & Gansäuer, A. Regiodivergent catalysis: a powerful tool for selective catalysis. Chem. Eur. J. 23, 19–32 (2017).
Nájera, C., Beletskaya, I. P. & Yus, M. Metal-catalyzed regiodivergent organic reactions. Chem. Soc. Rev. 48, 4515–4618 (2019).
Neveselý, T., Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E → Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Zhan, G., Du, W. & Chen, Y.-C. Switchable divergent asymmetric synthesis via organocatalysis. Chem. Soc. Rev. 46, 1675–1692 (2017).
Kalita, S. J., Huang, Y.-Y. & Schneider, U. Stereodivergent catalytic asymmetric allylic alkylation. Sci. Bull. 65, 1865–1868 (2020).
Huo, X., Li, G., Wang, X. & Zhang, W. Bimetallic catalysis in stereodivergent synthesis. Angew. Chem. Int. Ed. 61, e202210086 (2022).
Wei, L., Fu, C., Wang, Z.-F., Tao, H.-Y. & Wang, C.-J. Synergistic dual catalysis in stereodivergent synthesis. ACS Catal. 14, 3812–3844 (2024).
Sun, H., Ma, Y., Xiao, G. & Kong, D. Stereodivergent dual catalysis in organic synthesis. Trends Chem. 6, 684–701 (2024).
Chen, P., Li, Y., Chen, Z.-C., Du, W. & Chen, Y.-C. Pseudo-stereodivergent synthesis of enantioenriched tetrasubstituted alkenes by cascade 1,3-oxo-allylation/Cope rearrangement. Angew. Chem. Int. Ed. 59, 7083–7088 (2020).
Tang, M.-Q., Yang, Z.-J. & He, Z.-T. Asymmetric formal sp2-hydrocarbonations of dienes and alkynes via palladium hydride catalysis. Nat. Commun. 14, 6303 (2023).
Li, P. et al. Stereodivergent access to non-natural α-amino acids via enantio- and Z/E-selective catalysis. Science 385, 972–979 (2024).
Luo, P. et al. Switchable chemo-, regio- and pseudo-stereodivergence in palladium-catalyzed cycloaddition of allenes. Angew. Chem. Int. Ed. 63, e202412179 (2024).
Wang, J. et al. Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks. Nat. Commun. 15, 3254 (2024).
Koschker, P. & Breit, B. Branching out: rhodium-catalyzed allylation with alkynes and allenes. Acc. Chem. Res. 49, 1524–1536 (2016).
Haydl, A. M., Breit, B., Liang, T. & Krische, M. J. Alkynes as electrophilic or nucleophilic allylmetal precursors in transition-metal catalysis. Angew. Chem. Int. Ed. 56, 11312–11325 (2017).
Li, G., Huo, X., Jiang, X. & Zhang, W. Asymmetric synthesis of allylic compounds via hydrofunctionalisation and difunctionalisation of dienes, allenes, and alkynes. Chem. Soc. Rev. 49, 2060–2118 (2020).
Cera, G. & Maestri, G. Palladium/Brøsted acid catalysis for hydrofunctionalizations of alkynes: from Tsuji–Trost allylations to stereoselective methodologies. ChemCatChem 14, e202200295 (2022).
Kadota, I., Shibuya, A., Gyoung, Y. S. & Yamamoto, Y. Palladium/acetic acid catalyzed allylation of some pronucleophiles with simple alkynes. J. Am. Chem. Soc. 120, 10262–10263 (1998).
Gellrich, U. et al. Mechanistic investigations of the rhodium catalyzed propargylic C–H activation. J. Am. Chem. Soc. 136, 1097–1104 (2014).
Lutete, L. M., Kadota, I. & Yamamoto, Y. Palladium-catalyzed intramolecular asymmetric hydroamination of alkynes. J. Am. Chem. Soc. 126, 1622–1623 (2004).
Lumbroso, A., Koschker, P., Vautravers, N. R. & Breit, B. Redox neutral atom economic rhodium-catalyzed coupling of terminal alkynes with carboxylic acids toward branched allylic esters. J. Am. Chem. Soc. 133, 2386–2389 (2011).
Koschker, P., Kahny, M. & Breit, B. Enantioselective redox-neutral Rh-catalyzed coupling of terminal alkynes with carboxylic acids toward branched allylic esters. J. Am. Chem. Soc. 137, 3131–3137 (2015).
Chen, Q.-A., Chen, Z.-W. & Dong, V. M. Rhodium-catalyzed enantioselective hydroamination of alkynes with indolines. J. Am. Chem. Soc. 137, 8392–8395 (2015).
Liu, Z. & Breit, B. Rhodium-catalyzed enantioselective intermolecular hydroalkoxylation of allenes and alkynes with alcohols: synthesis of branched allylic ethers. Angew. Chem. Int. Ed. 55, 8440–8443 (2016).
Cruz, F. A. & Dong, V. M. Stereodivergent coupling of aldehydes and alkynes via synergistic catalysis using Rh and Jacobsen’s amine. J. Am. Chem. Soc. 139, 1029–1032 (2017).
Cruz, F. A., Zhu, Y.-M., Tercenio, Q. D., Shen, Z.-M. & Dong, V. M. Alkyne hydroheteroarylation: enantioselective coupling of indoles and alkynes via Rh-hydride catalysis. J. Am. Chem. Soc. 139, 10641–10644 (2017).
Su, Y.-L. et al. Asymmetric α-allylation of aldehydes with alkynes by integrating chiral hydridopalladium and enamine catalysis. Org. Lett. 20, 2403–2406 (2018).
Lee, J. T. D. & Zhao, Y. Direct enantioselective α-allylation of unfunctionalized cyclic ketones with alkynes through Pd-amine cooperative catalysis. Chem. Eur. J. 24, 9520–9524 (2018).
Xie, L.-Y., Yang, H.-J., Ma, M.-L. & Xing, D. Rhodium-catalyzed branched and enantioselective direct α-allylic alkylation of simple ketones with alkynes. Org. Lett. 22, 2007–2011 (2020).
Wu, M.-S., Han, Z.-Y. & Gong, L.-Z. Asymmetric α‑pentadienylation of aldehydes with cyclopropylacetylenes. Org. Lett. 23, 636–641 (2021).
Davison, R. T. et al. Enantioselective addition of α‑nitroesters to alkynes. Angew. Chem. Int. Ed. 60, 4599–4603 (2021).
Velasco-Rubio, A. ́, Bernárdez, R., Varela, J. A. & Saá, C. Enantioenriched α-vinyl 1,4-benzodiazepines and 1,4-benzoxazepines via enantioselective rhodium-catalyzed hydrofunctionalizations of alkynes and allenes. J. Org. Chem. 86, 10889–10902 (2021).
Zhang, J. et al. Asymmetric coupling of β-ketocarbonyls and alkynes by chiral primary amine/Rh synergistic catalysis. Org. Lett. 24, 1186–1189 (2022).
Ma, C., Liang Chen, L. & He, Z.-T. Asymmetric intramolecular O-hydroximation of alkynes. CCS Chem. 7, 1168–1176 (2025).
Lin, Y. et al. Asymmetric α‑allylation of amino acid esters with alkynes enabled by chiral aldehyde/palladium combined catalysis. Org. Lett. 26, 7908–7913 (2024).
Chang, C.-Y. & Aponick, A. Enantioselective synthesis of allylic sulfones via rhodium-catalyzed direct hydrosulfonylation of allenes and alkynes. J. Am. Chem. Soc. 146, 16996–17002 (2024).
Liu, Y., Chen, H. & Wang, X. Synergistic homogeneous asymmetric Cu catalysis with Pd nanoparticle catalysis in stereoselective coupling of alkynes with aldimine esters. J. Am. Chem. Soc. 146, 28427–28436 (2024).
Yang, S.-Q., Wang, Y.-F., Zhao, W.-C., Lin, G.-Q. & He, Z.-T. Stereodivergent synthesis of tertiary fluoride-tethered allenes via copper and palladium dual catalysis. J. Am. Chem. Soc. 143, 7285–7291 (2021).
Chen, Y.-W., Liu, Y., Lu, H.-Y., Lin, G.-Q. & He, Z.-T. Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)–H functionalization. Nat. Commun. 12, 5626 (2021).
Wang, Y.-C. et al. Umpolung asymmetric 1,5-conjugate addition via palladium hydride catalysis. Angew. Chem. Int. Ed. 62, e202215568 (2023).
Yang, S.-Q. et al. Catalytic asymmetric hydroalkoxylation and formal hydration and hydroaminoxylation of conjugated dienes. J. Am. Chem. Soc. 145, 3915–3925 (2023).
Chen, X.-X., Luo, H., Chen, Y.-W., Liu, Y. & He, Z.-T. Enantioselective palladium-catalyzed directed migratory allylation of remote dienes. Angew. Chem. Int. Ed. 62, e202307628 (2023).
Tang, M.-Q., Yang, Z.-J., Han, A.-J. & He, Z.-T. Diastereoselective and enantioselective hydrophosphinylations of conjugated enynes, allenes and dienes via synergistic Pd/Co catalysis. Angew. Chem. Int. Ed. 64, e202413428 (2025).
Jiang, R., Ding, L., Zheng, C. & You, S.-L. Iridium-catalyzed Z-retentive asymmetric allylic substitution reactions. Science 371, 380–386 (2021).
Roberts, C. C., Matías, D. M., Goldfogel, M. J. & Meek, S. J. Lewis acid activation of carbodicarbene catalysts for Rh-catalyzed hydroarylation of dienes. J. Am. Chem. Soc. 137, 6488–6491 (2015).
Goldfogel, M. L. & Meek, S. J. Diastereoselective synthesis of vicinal tertiary and N-substituted quaternary stereogenic centers by catalytic hydroalkylation of dienes. Chem. Sci. 7, 4079–4084 (2016).
Cooke, M. L., Xu, K. & Breit, B. Enantioselective rhodium-catalyzed synthesis of branched allylic amines by intermolecular hydroamination of terminal allenes. Angew. Chem. Int. Ed. 51, 10876–10879 (2012).
Beck, T. M. & Breit, B. Regioselective rhodium-catalyzed addition of 1,3-dicarbonyl compounds to terminal alkynes. Org. Lett. 18, 124–127 (2016).
Pritzius, A. B. & Breit, B. Z-selective hydrothiolation of racemic 1,3-disubstituted allenes: an atom-economic rhodium-catalyzed dynamic kinetic resolution. Angew. Chem. Int. Ed. 54, 15818–15822 (2015).
Hilpert, L. J. & Breit, B. Rhodium-catalyzed parallel kinetic resolution of racemic internal allenes towards enantiopure allylic 1,3-diketones. Angew. Chem. Int. Ed. 58, 9939–9943 (2019).
Correia, J. T. M., List, B. & Coelho, F. Catalytic asymmetric conjugate addition of indolizines to α,β-unsaturated ketones. Angew. Chem. Int. Ed. 56, 7967–7970 (2017).
Zhang, Y.-Z. et al. Organocatalytic C3-functionalization of indolizines: synthesis of biologically important indolizine derivatives. Org. Biomol. Chem. 18, 5688–5696 (2020).
Singh, K., Staig, S. J. & Weaver, J. D. Facile synthesis of Z-alkenes via uphill catalysis. J. Am. Chem. Soc. 136, 5275–5278 (2014).
Acknowledgements
Z.-T.H acknowledges the National Natural Science Foundation of China (grant no. 22371292), Ningbo Natural Science Foundation (grant no. 2023J036), Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB0610000), State Key Laboratory of Organometallic Chemistry and Shanghai Institute of Organic Chemistry for financial support. X.-S.X. acknowledges the funding from the National Natural Science Foundation of China (grant no. 22193012), the CAS Project for Young Scientists in Basic Research (grant no. YSBR-095) and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB0590000).
Author information
Authors and Affiliations
Contributions
Z.-T.H. conceived the project. A.H. and Z.-J.Y. performed the experiments. H.-R.X. and X.-S.X. conducted the computations. Z.-T.H. and X.-S.X. supervised the project and wrote the manuscript with the feedback from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Zhihui Shao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Stephanie Greed, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary Sections 1–13 and Supplementary Fig. 1.
Supplementary Data 1
X-ray crystallographic data for 3r. CCDC 2463493.
Supplementary Data 2
X-ray crystallographic data for 6p. CCDC 2381076.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, A., Xu, HR., Yang, ZJ. et al. Three-dimensional divergent hydroheteroarylation of internal alkynes with indolizines. Nat. Synth (2025). https://doi.org/10.1038/s44160-025-00870-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44160-025-00870-z