Abstract
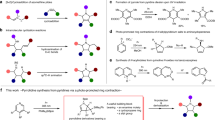
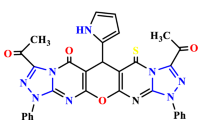
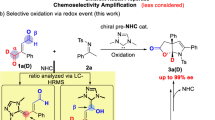
Pyrrole and 2-pyrrolone derivatives are valuable heterocyclic compounds and while classical condensation methods for their synthesis have a long history, many of the recent developments for their preparation involve the use of transition metal catalysis. Here we report a complementary, metal-free strategy for constructing structurally diverse derivatives of these heterocycles. The key feature of the approach is the in situ creation of a reactive intermediate by an initial facile event that simultaneously generates a pyrrole ring bearing a free carbene. This is straightforwardly accessed via a spontaneous, net [3 + 2] cyclization reaction of a linear alkynyl O-silylimidate or alkynyl aldimine with an electrophilic alkyne. The carbene then undergoes either 1,4-silyl migration (to produce 2-pyrrolone derivatives) or C–H insertion, cycloaddition, cyclopropanation or macrocyclization reactions (leading to pyrrole derivatives).

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The Supplementary Information includes preparation procedures and characterization data for all new compounds, computational methodology and results, and static copies of NMR spectra of all new compounds. The X-ray diffraction structure of 44a can be found at the Cambridge Crystallographic Data Centre under Deposition Number 2431543. These data, available free of charge, can be accessed at https://www.ccdc.cam.ac.uk/structures/. A master.mnova file containing the raw data files of NMR spectra for all new compounds is available from figshare via https://doi.org/10.6084/m9.figshare.29649548 (ref. 34).
References
Bhardwaj, V., Gumber, D., Abbot, V., Dhiman, S. & Sharma, P. Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 5, 15233–15266 (2015).
Petri, G. L. et al. Bioactive pyrrole-based compounds with target selectivity. Eur. J. Med. Chem. 208, 112783 (2020).
Jeelan Basha, N., Basavarajaiah, S. M. & Shyamsunder, K. Therapeutic potential of pyrrole and pyrrolidine analogs: An update. Mol. Divers. 26, 2915–2937 (2022).
Singh, N. et al. Recent progress in the total synthesis of pyrrole-containing natural products (2011–2020). Org. Chem. Front. 8, 5550–5573 (2021).
Forte, B. et al. A submarine journey: The pyrrole-imidazole alkaloids. Mar. Drugs 7, 705–753 (2009).
Yen, Y.-S. et al. Pyrrole-based organic dyes for dye-sensitized solar cells. J. Phys. Chem. C 112, 12557–12567 (2008).
Pang, A. L., Arsad, A. & Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 32, 1428–1454 (2021).
Guo, J.-L., Feng, Z.-M., Yang, Y.-J., Zhang, Z.-W. & Zhang, P.-C. Pollenopyrroside A and B, novel pyrrole ketohexoside derivatives from bee-collected Brassica campestris pollen. Chem. Pharm. Bull. 58, 983–985 (2010).
Tong, X.-G. et al. Acortatarins A and B, two novel antioxidative spiroalkaloids with a naturally unusual morpholine motif from Acorus tatarinowii. Org. Lett. 12, 1844–1847 (2010).
Shuda, M. et al. CDK1 substitutes for mTOR kinase to activate mitotic cap-dependent protein translation. Proc. Natl Acad. Sci. USA 112, 5875–5882 (2015).
Khajuria, R., Dham, S. & Kapoor, K. K. Active methylenes in the synthesis of a pyrrole motif: An imperative structural unit of pharmaceuticals, natural products and optoelectronic materials. RSC Adv. 6, 37039–37066 (2016).
Philkhana, S. C., Badmus, F. O., Dos Reis, I. C. & Kartika, R. Recent advancements in pyrrole synthesis. Synth. 53, 1531–1555 (2021).
Estévez, V., Villacampa, M. & Menéndez, J. C. Multicomponent reactions for the synthesis of pyrroles. Chem. Soc. Rev. 39, 4402–4421 (2010).
Kel’in, A. V., Sromek, A. W. & Gevorgyan, V. A novel Cu-assisted cycloisomerization of alkynyl imines: Efficient synthesis of pyrroles and pyrrole-containing heterocycles. J. Am. Chem. Soc. 123, 2074–2075 (2001).
Gilbert, Z. W., Hue, R. J. & Tonks, I. A. Catalytic formal [2 + 2 + 1] synthesis of pyrroles from alkynes and diazenes via TiII/TiIV redox catalysis. Nat. Chem. 8, 63–68 (2016).
Zhou, Y., Zhou, L., Jesikiewicz, L. T., Liu, P. & Buchwald, S. L. Synthesis of pyrroles through the CuH-catalyzed coupling of enynes and nitriles. J. Am. Chem. Soc. 142, 9908–9914 (2020).
Xu, Q. & Hoye, T. R. Free carbenes from complementarily paired alkynes. Nat. Chem. 16, 1083–1092 (2024).
Guzman, A. L., Mann, A. N. & Hoye, T. R. Alkynes to (free) carbenes to polycyclic cyclopropanes. J. Am. Chem. Soc. 146, 28642–28647 (2024).
Xu, Q. & Hoye, T. R. A distinct mode of strain-driven cyclic allene reactivity: Group migration to the central allene carbon atom. J. Am. Chem. Soc. 145, 9867–9875 (2023).
Xu, Q. & Hoye, T. R. A cascade of strain-driven events converting benzynes to alkynylbenzocyclobutenes to 1,3-dien-5-ynes to cyclic allenes to benzocyclohexadienones. J. Am. Chem. Soc. 146, 6438–6443 (2024).
Klebe, J. F. Silyl-proton exchange reactions. Acc. Chem. Res. 3, 299–305 (1970).
Kashutina, M. V., Ioffe, S. L. & Tartakovskii, V. A. Silylation of organic compounds. Russ. Chem. Rev. 44, 733–747 (1975).
Deleris, G., Dunogues, J. & Calas, R. Synthese regioselective de nitriles par voie organosilicique. J. Organomet. Chem. 116, C45–C48 (1976).
Seeman, J. I. The Curtin-Hammett principle and the Winstein-Holness equation: New definition and recent extensions to classical concepts. J. Chem. Educ. 63, 42–48 (1986).
Kanzian, T., Nigst, T. A., Maier, A., Pichl, S. & Mayr, H. Nucleophilic reactivities of primary and secondary amines in acetonitrile. Eur. J. Org. Chem. 2009, 6379–6385 (2009).
Maji, B. & Mayr, H. Nucleophilic reactivities of Schiff bases. Z. Naturforsch. B 68, 693–699 (2013).
Minegishi, S., Kobayashi, S. & Mayr, H. Solvent nucleophilicity. J. Am. Chem. Soc. 126, 5174–5181 (2004).
Zanardi, M. M. & Sarotti, A. M. Sensitivity analysis of DP4+ with the probability distribution terms: development of a universal and customizable method. J. Org. Chem. 86, 8544–8548 (2021).
Shen, M. & Schultz, A. G. Preparation and Diels-Alder reactivity of ethyl-β-phenylsulfonylpropiolate. Tetrahedron Lett. 22, 3347–3350 (1981).
Wang, R., Xu, Q. & Hoye, T. R. Reactions of electrophilic allenoates [and isocyanates/isothiocyanates] with a 2-alkynylpyridine via a free carbene intermediate. Org. Lett. 26, 7805–7808 (2024).
Watson, W. On byproducts and side products. Org. Process Res. Dev. 16, 1877 (2012).
David, O., Vanucci-Bacque, C., Fargeau-Bellassoued, M.-C. & Lhommet, G. New access to chiral cyclic ω-oxygenated β-enamino esters by intramolecular aminocyclisation reactions. Heterocycles 62, 839–846 (2004).
Garcia Jimenez, D., Poongavanam, V. & Kihlberg, J. Macrocycles in drug discovery─ learning from the past for the future. J. Med. Chem. 66, 5377–5396 (2023).
Xu, Q., Shi, J. & Hoye, T. R. Master MNova File of all NMR spectra for Nat Synth.zip. figshare https://doi.org/10.6084/m9.figshare.29649548 (2025).
Wiberg, K. B. Application of the Pople-Santry-Segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24, 1083–1096 (1968).
Acknowledgements
This research was enabled by a grant from the United States National Science Foundation (CHE-2155042) (T.R.H.). High-resolution mass spectrometry data (electrospray ionization) were obtained in the Analytical Biochemistry Shared Resource Laboratory of the University of Minnesota (UMN). DFT computations were performed utilizing resources provided by the UMN Supercomputing Institute (MSI). The X-ray diffraction analysis of 44a was performed by A. Lovstedt in the X-Ray Crystallographic Laboratory in the Department of Chemistry, UMN.
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary sections I–VII.
Supplementary Data 1
Excel data sheets for NMR spectroscopy computations.
Supplementary Data 2
Computed geometries for conformers for NMR spectroscopy calculations.
Supplementary Data 3
X-ray crystallographic data for compound 44a cif (.cif) and checkcif (as PDF) files, CCDC 2431543.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Q., Shi, J. & Hoye, T.R. Pyrrole-stabilized free carbenes generated from alkynyl imidates or aldimines and electrophilic alkynes give pyrrolone- or pyrrole-containing products. Nat. Synth 5, 95–102 (2026). https://doi.org/10.1038/s44160-025-00899-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00899-0