Abstract
Tandem systems that integrate CO-generating catalysts with copper have shown promise for enhanced carbon dioxide reduction reaction (CO2RR) performance. Sulfur-containing single-atom catalysts are particularly effective for CO production; however, the role and positioning of sulfur in facilitating both CO2-to-CO conversion and tandem CO2RRs remain elusive. Here we show model thiophene-decorated nickel porphyrins as model single-atom catalysts that exhibit tandem activities in the CO2RR. Spectroscopic and theoretical analyses reveal that thiophene substituents induce ligand holes, regulating the d orbitals and d-band centre of the nickel centre to reduce the reaction barrier and promote CO formation. Coupling these single-atom catalysts with a copper catalyst achieves a Faradaic efficiency of 74.3% and a partial current density of 445.8 mA cm−2 for C2 products in a neutral solution, a 46% improvement over bare copper. Operando studies confirm the formation of CO intermediates from the single-atom catalysts, highlighting their role in facilitating tandem catalysis.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and the Supplementary Information. All data are available from Figshare via https://doi.org/10.6084/m9.figshare.29926913 (ref. 73). Source data are provided with this paper.
References
Dresselhaus, M. S. & Thomas, I. L. Alternative energy technologies. Nature 414, 332–337 (2001).
Chu, S. & Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 488, 294–303 (2012).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Segets, D., Andronescu, C. & Apfel, U.-P. Accelerating CO2 electrochemical conversion towards industrial implementation. Nat. Commun. 14, 7950 (2023).
Xu, A. et al. Copper/alkaline earth metal oxide interfaces for electrochemical CO2-to-alcohol conversion by selective hydrogenation. Nat. Catal. 5, 1081–1088 (2022).
Liang, Y. et al. Stabilizing copper sites in coordination polymers toward efficient electrochemical C–C coupling. Nat. Commun. 14, 474 (2023).
Fan, M. et al. Single-site decorated copper enables energy- and carbon-efficient CO2 methanation in acidic conditions. Nat. Commun. 14, 3314 (2023).
Hung, S.-F. Electrochemical flow systems enable renewable energy industrial chain of CO2 reduction. Pure Appl. Chem. 92, 1937–1951 (2020).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Hung, S.-F. et al. A metal-supported single-atom catalytic site enables carbon dioxide hydrogenation. Nat. Commun. 13, 819 (2022).
Li, P. et al. p–d orbital hybridization induced by p-block metal-doped Cu promotes the formation of C2+ products in ampere-level CO2 electroreduction. J. Am. Chem. Soc. 145, 4675–4682 (2023).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2020).
Wu, Q. et al. Nanograin-boundary-abundant Cu2O–Cu nanocubes with high C2+ selectivity and good stability during electrochemical CO2 reduction at a current density of 500 mA/cm2. ACS Nano 17, 12884–12894 (2023).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Hong, S. et al. Tuning the C1/C2 selectivity of electrochemical CO2 reduction on Cu–CeO2 nanorods by oxidation state control. Adv. Mater. 35, 2208996 (2023).
Handoko, A. D., Wei, F., Jenndy, Yeo, B. S. & Seh, Z. W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 1, 922–934 (2018).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Li, F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule–metal catalyst interfaces. Nat. Catal. 3, 75–82 (2020).
Möller, T., Filippi, M., Brückner, S., Ju, W. & Strasser, P. A CO2 electrolyzer tandem cell system for CO2–CO co-feed valorization in a Ni–N–C/Cu-catalyzed reaction cascade. Nat. Commun. 14, 5680 (2023).
Chen, Y. et al. Efficient multicarbon formation in acidic CO2 reduction via tandem electrocatalysis. Nat. Nanotechnol. 19, 311–318 (2023).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Li, J. et al. Weak CO binding sites induced by Cu–Ag interfaces promote CO electroreduction to multi-carbon liquid products. Nat. Commun. 14, 698 (2023).
Lee, S., Park, G. & Lee, J. Importance of Ag–Cu biphasic boundaries for selective electrochemical reduction of CO2 to ethanol. ACS Catal. 7, 8594–8604 (2017).
Xiong, L. et al. Breaking the linear scaling relationship by compositional and structural crafting of ternary Cu–Au/Ag nanoframes for electrocatalytic ethylene production. Angew. Chem. Int. Ed. 60, 2508–2518 (2021).
Kong, X. et al. Understanding the effect of *CO coverage on C–C coupling toward CO2 electroreduction. Nano Lett. 22, 3801–3808 (2022).
Wang, X. et al. Mechanistic reaction pathways of enhanced ethylene yields during electroreduction of CO2–CO co-feeds on Cu and Cu-tandem electrocatalysts. Nat. Nanotechnol. 14, 1063–1070 (2019).
Lin, L. et al. Synergistic catalysis over iron–nitrogen sites anchored with cobalt phthalocyanine for efficient CO2 electroreduction. Adv. Mater. 31, 1903470 (2019).
Varela, A. S., Ju, W. & Strasser, P. Molecular nitrogen–carbon catalysts, solid metal organic framework catalysts, and solid metal/nitrogen-doped carbon (MNC) catalysts for the electrochemical CO2 reduction. Adv. Energy Mater. 8, 1703614 (2018).
Wang, J. et al. Linkage effect in the heterogenization of cobalt complexes by doped graphene for electrocatalytic CO2 reduction. Angew. Chem. Int. Ed. 58, 13532–13539 (2019).
Yang, H. B. et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Pan, F. et al. Boosting CO2 reduction on Fe–N–C with sulfur incorporation: synergistic electronic and structural engineering. Nano Energy 68, 104384 (2020).
Pan, F. et al. Promoting electrocatalytic CO2 reduction on nitrogen-doped carbon with sulfur addition. Appl. Catal. B 252, 240–249 (2019).
Li, X. et al. Unveiling the in situ generation of a monovalent Fe(I) site in the single-Fe-atom catalyst for electrochemical CO2 reduction. ACS Catal. 11, 7292–7301 (2021).
Chen, Z. et al. Unraveling the origin of sulfur-doped Fe–N–C single-atom catalyst for enhanced oxygen reduction activity: effect of iron spin-state tuning. Angew. Chem. Int. Ed. 60, 25404–25410 (2021).
Lu, Y.-R. et al. Turn the trash into treasure: egg-white-derived single-atom electrocatalysts boost oxygen reduction reaction. ACS Sustain. Chem. Eng. 10, 6736–6742 (2022).
Calogero, G., Bartolotta, A., Di Marco, G., Di Carlo, A. & Bonaccorso, F. Vegetable-based dye-sensitized solar cells. Chem. Soc. Rev. 44, 3244–3294 (2015).
Calogero, G. et al. Natural dye senstizers for photoelectrochemical cells. Energy Environ. Sci. 2, 1162–1172 (2009).
Koenig, J. D. B., Willkomm, J., Roesler, R., Piers, W. E. & Welch, G. C. Electrocatalytic CO2 reduction at lower overpotentials using iron(III) tetra(meso-thienyl)porphyrins. ACS Appl. Energy Mater. 2, 4022–4026 (2019).
Prasath, R., Butcher, R. J. & Bhavana, P. Nitrothienylporphyrins: synthesis, crystal structure and, the effect of position and number of nitro groups on the spectral and electrochemical properties. Spectrochim. Acta A 87, 258–264 (2012).
Zhang, Y., Jiao, L., Yang, W., Xie, C. & Jiang, H.-L. Rational fabrication of low-coordinate single-atom Ni electrocatalysts by MOFs for highly selective CO2 reduction. Angew. Chem. Int. Ed. 60, 7607–7611 (2021).
Zhang, Q. et al. Boosting the proton-coupled electron transfer via Fe−P atomic pair for enhanced electrochemical CO2 reduction. Angew. Chem. Int. Ed. 62, e202311550 (2023).
Kelemen, S. R. et al. Delayed coker coke morphology fundamentals: mechanistic implications based on XPS analysis of the composition of vanadium- and nickel-containing additives during coke formation. Energy Fuels 21, 927–940 (2007).
Krasnikov, S. A. et al. An X-ray absorption and photoemission study of the electronic structure of Ni porphyrins and Ni N-confused porphyrin. J. Phys. Condens. Matter 20, 235207 (2008).
Wang, H. et al. Nickel L-edge soft X-ray spectroscopy of nickel−iron hydrogenases and model compounds: evidence for high-spin nickel(II) in the active enzyme. J. Am. Chem. Soc. 122, 10544–10552 (2000).
de Groot, F. M. F. X-ray absorption and dichroism of transition metals and their compounds. J. Electron Spectrosc. 67, 529–622 (1994).
Glatzel, P. et al. Electronic structure of Ni complexes by X-ray resonance Raman spectroscopy (resonant inelastic X-ray scattering). J. Am. Chem. Soc. 124, 9668–9669 (2002).
Hu, Z. et al. On the electronic structure of Cu(III) and Ni(III) in La2Li1/2Cu1/2O4, Nd2Li1/2Ni1/2O4, and Cs2KCuF6. Chem. Phys. 232, 63–74 (1998).
Merrien, N. et al. XAS and XPS study of electronic structure of the trivalent cuprate La2Li0.5Cu0.5O4−δ. J. Phys. Chem. Solids 54, 499–506 (1993).
Weekes, D. M., Salvatore, D. A., Reyes, A., Huang, A. & Berlinguette, C. P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 51, 910–918 (2018).
Liu, M. et al. Potential alignment in tandem catalysts enhances CO2-to-C2H4 conversion efficiencies. J. Am. Chem. Soc. 146, 468–475 (2024).
Chen, J. et al. Accelerated transfer and spillover of carbon monoxide through tandem catalysis for kinetics-boosted ethylene electrosynthesis. Angew. Chem. Int. Ed. 62, e202215406 (2023).
Yin, Z. et al. Hybrid catalyst coupling single-atom Ni and nanoscale Cu for efficient CO2 electroreduction to ethylene. J. Am. Chem. Soc. 144, 20931–20938 (2022).
Duan, G.-Y. et al. Highly efficient electrocatalytic CO2 reduction to C2+ products on a poly(ionic liquid)-based Cu0–CuI tandem catalyst. Angew. Chem. Int. Ed. 61, e202110657 (2022).
Wu, J., Sharifi, T., Gao, Y., Zhang, T. & Ajayan, P. M. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals. Adv. Mater. 31, 1804257 (2019).
Hung, S.-F. et al. Unraveling geometrical site confinement in highly efficient iron-doped electrocatalysts toward oxygen evolution reaction. Adv. Energy Mater. 8, 1701686 (2018).
Hung, S. F. et al. Identification of stabilizing high-valent active sites by operando high-energy resolution fluorescence-detected X-ray absorption spectroscopy for high-efficiency water oxidation. J. Am. Chem. Soc. 140, 17263–17270 (2018).
Hung, S. F. et al. Operando X-ray absorption spectroscopic studies of the carbon dioxide reduction reaction in a modified flow cell. Catal. Sci. Technol. 12, 2739–2743 (2022).
Lin, Z.-Y. et al. Operando studies for CO2/CO reduction in flow-based devices. ChemNanoMat 10, e202400070 (2024).
Zhou, Y. et al. Asymmetric dinitrogen-coordinated nickel single-atomic sites for efficient CO2 electroreduction. Nat. Commun. 14, 3776 (2023).
Li, H., Wei, P., Gao, D. & Wang, G. In situ Raman spectroscopy studies for electrochemical CO2 reduction over Cu catalysts. Curr. Opin. Green Sustain. Chem. 34, 100589 (2022).
Wu, F.-Y. et al. Copper–barium-decorated carbon-nanotube composite for electrocatalytic CO2 reduction to C2 products. J. Mater. Chem. A 11, 13217–13222 (2023).
Schindler, J. et al. Sterically induced distortions of nickel(II) porphyrins—comprehensive investigation by DFT calculations and resonance Raman spectroscopy. Coord. Chem. Rev. 360, 1–16 (2018).
Shelnutt, J. A. et al. Resonance Raman spectroscopy of non-planar nickel porphyrins. J. Raman Spectrosc. 23, 523–529 (1992).
Shelnutt, J. A., Medforth, C. J., Berber, M. D., Barkigia, K. M. & Smith, K. M. Relationships between structural parameters and Raman frequencies for some planar and nonplanar nickel(II) porphyrins. J. Am. Chem. Soc. 113, 4077–4087 (1991).
Edgell, W. F. & Dunkle, M. P. The infrared and Raman spectra of a triphenylphosphine derivative of Ni(CO)4. Inorg. Chem. 4, 1629–1636 (1965).
Betoni Momo, P., Pavani, C., Baptista, M. S., Brocksom, T. J. & Thiago de Oliveira, K. Chemical transformations and photophysical properties of meso-tetrathienyl-substituted porphyrin derivatives. Eur. J. Org. Chem. 2014, 4536–4547 (2014).
Rayati, S., Zakavi, S., Bohloulbandi, E., Jafarian, M. & avei, M. R. Comparative study of the catalytic activity of a series of β-brominated Mn–porphyrins in the oxidation of olefins and organic sulfides: better catalytic performance of the partially brominated ones. Polyhedron 34, 102–107 (2012).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Lu, Y. H. et al. Model thiophene-decorated nickel porphyrins for tandem CO2 reduction. Figshare https://doi.org/10.6084/m9.figshare.29926913 (2025).
Acknowledgements
Support from the National Science and Technology Council, Taiwan (contract numbers NSTC 113-2628-M-A49-008 and NSTC 114-2628-M-A49-005) is gratefully acknowledged. We also thank the Yushan Young Scholar Program (MOE 114-YSFMS-0010-003-P2) and the Center for Emergent Functional Matter Science, Ministry of Education, Taiwan for support. This work was supported by the Higher Education Sprout Project of the National Yang Ming Chiao Tung University and the Ministry of Education (MOE), Taiwan. A.X. is grateful for the support of a Sydney Horizon Fellowship and to the ARC Centre of Excellence for Green Electrochemical Transformation of Carbon Dioxide (CE230100017). M. T. Chang and R.-F. Cai are responsible for acquiring the high-angle annular dark-field scanning transmission electron microscopy atomic images and for the analysis of the EELS spectra. S.-Y. Liu and M. Lun Wu are responsible for preparing the plane-view transmission electron microscopy samples and for focused ion beam sample pretreatment. We thank L.-C. Shen for assistance with NMR experiments.
Author information
Authors and Affiliations
Contributions
S.-F.H. supervised the project. S.-F.H., Y.-H. Lu and A.X. conceived the idea and carried out the experiments. Y.-H. Lu and S.-F.H. wrote the paper. A.X., Y.-Y.H. and C.-C.C. carried out the DFT calculations. Y.-H. Lu, Y.-J.S. and Y.-H. Lee synthesized the porphyrin molecules. Y.-H. Lu and Y.-J.S. performed the electrochemical measurements. Y.-H. Lu conducted the in situ Raman and XAS measurements. H.-J.T., Z.-Y.L. and T.-J.L. performed the XAS and RIXS measurements. W.-Y.H., G.-L.C. H.-J.L. and S.-H.H. helped to characterize the materials. N.H. and H.I. analysed the XAS and RIXS results. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Jongwoo Lim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–36 and Tables 1–4.
Supplementary Data 1
Source data for SI Figures
Source data
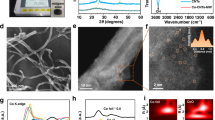
Source Data Fig. 1
Source data for Figs. 1b, 1c, 1d
Source Data Fig. 2
Source data for Figs. 2a, 2b, 2c, 2d, 2e, 2f, 2g, 2h
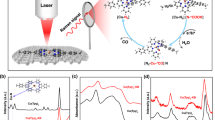
Source Data Fig. 3
Source data for Figs. 3a, 3b, 3c, 3d, 3e, 3f
Source Data Fig. 4
Source data for Figs. 4a, 4b, 4c, 4d, 4e, 4f
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, YH., Shen, YJ., Tsai, HJ. et al. Model thiophene-decorated nickel porphyrins for tandem CO2 reduction. Nat. Synth 5, 189–198 (2026). https://doi.org/10.1038/s44160-025-00903-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44160-025-00903-7
This article is cited by
-
Sulfur enhances electrochemical CO2 reduction over porphyrin catalysts
Nature Synthesis (2026)