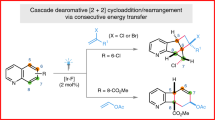
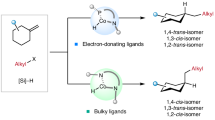
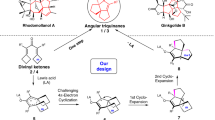
Abstract
Highly strained rings serve as privileged building blocks for the synthesis of saturated, three-dimensional scaffolds, which are increasingly recognized as critical components in modern drug discovery. Here we disclose a substrate-dependent, divergent strategy to access a broad family of housanes through an intramolecular-energy-transfer-mediated [2 + 2] cycloaddition of 1,4-dienes—a transformation that has long been considered challenging. This method rapidly builds up strain while suppressing the di-π-methane rearrangement, thereby expanding the toolkit for efficient exploration of housane chemical space. Substituent engineering enables switching between single and double energy-transfer pathways to deliver 1,3- and 1,2-disubstituted housanes with excellent stereocontrol and broad functional-group tolerance. Mechanistic studies and density functional theory calculations support an energy-transfer pathway and rationalize the observed selectivity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2486494 (2k), 2486495 (2ao), 2486496 (3av) and 2504328 (7). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Computational data (ORCA input and output files) can be accessed in the IOChemBD repository (https://doi.org/10.19061/iochem-bd-6-583).
References
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next?. Org. Biomol. Chem. 17, 2839–2849 (2019).
Sodano, T. M., Combee, L. A. & Stephenson, C. R. J. Recent advances and outlook for the isosteric replacement of anilines. ACS Med. Chem. Lett. 11, 1785–1788 (2020).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. Med. Chem. Commun. 4, 515–519 (2013).
Ritchie, T. J. & Macdonald, S. J. F. The impact of aromatic ring count on compound developability—are too many aromatic rings a liability in drug design?. Drug Discov. Today 14, 1011–1020 (2009).
Wei, W., Cherukupalli, S., Jing, L., Liu, X. & Zhan, P. Fsp3: a new parameter for drug-likeness. Drug Discov. Today 25, 1839–1845 (2020).
Denisenko, A. et al. 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring. Nat. Chem. 15, 1155–1163 (2023).
Dong, W. et al. Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane. Nat. Chem. 14, 1068–1077 (2022).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Lopchuk, J. M. et al. Strain-release heteroatom functionalization: development, scope, and stereospecificity. J. Am. Chem. Soc. 139, 3209–3226 (2017).
Cohen, Y., Cohen, A. & Marek, I. Creating stereocenters within acyclic systems by C–C bond cleavage of cyclopropanes. Chem. Rev. 121, 140–161 (2021).
Harmata, A. S., Roldan, B. J. & Stephenson, C. R. J. Formal cycloadditions driven by the homolytic opening of strained, saturated ring systems. Angew. Chem. Int. Ed. 62, e202213003 (2023).
Sterling, A. J., Smith, R. C., Anderson, E. A. & Duarte, F. Beyond strain release: delocalization-enabled organic reactivity. J. Org. Chem. 89, 9979–9989 (2024).
Turkowska, J., Durka, J. & Gryko, D. Strain release—an old tool for new transformations. Chem. Commun. 56, 5718–5734 (2020).
Zhou, X., Hu, Y., Huang, Y. & Xiong, Y. Recent advances in photochemical strain-release reactions of bicyclo[1.1.0]butanes. Chem. Commun. 61, 23–32 (2025).
Hu, Q.-Q., Chen, J., Yang, Y., Yang, H. & Zhou, L. Strain-release transformations of bicyclo[1.1.0]butanes and [1.1.1]propellanes. Tetrahedron Chem 9, 100070 (2024).
Bellotti, P. & Glorius, F. Strain-release photocatalysis. J. Am. Chem. Soc. 145, 20716–20732 (2023).
Pramanik, M. M. D., Qian, H., Xiao, W.-J. & Chen, J.-R. Photoinduced strategies towards strained molecules. Org. Chem. Front. 7, 2531–2537 (2020).
Golfmann, M. & Walker, J. C. L. Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis. Commun. Chem. 6, 9 (2023).
Kelly, C. B., Milligan, J. A., Tilley, L. J. & Sodano, T. M. Bicyclobutanes: from curiosities to versatile reagents and covalent warheads. Chem. Sci. 13, 11721–11737 (2022).
Tyler, J. L. & Aggarwal, V. K. Synthesis and applications of bicyclo[1.1.0]butyl and azabicyclo[1.1.0]butyl organometallics. Chem. Eur. J. 29, e202300008 (2023).
Xiao, Y. et al. Catalytic asymmetric strategies for bicyclo[1.1.0]butane transformations: advances and applications. CCS Chemistry 7, 1903–1934 (2025).
Dilmaç, A. M., Spuling, E., de Meijere, A. & Bräse, S. Propellanes—from a chemical curiosity to “explosive” materials and natural products. Angew. Chem. Int. Ed. 56, 5684–5718 (2017).
He, F.-S., Xie, S., Yao, Y. & Wu, J. Recent advances in the applications of [1.1.1]propellane in organic synthesis. Chin. Chem. Lett. 31, 3065–3072 (2020).
Kanazawa, J. & Uchiyama, M. Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 30, 1–11 (2019).
Ma, X. & Nhat Pham, L. Selected topics in the syntheses of bicyclo[1.1.1]pentane (BCP) analogues. Asian J. Org. Chem. 9, 8–22 (2020).
Shire, B. R. & Anderson, E. A. Conquering the synthesis and functionalization of bicyclo[1.1.1]pentanes. JACS Au 3, 1539–1553 (2023).
Liu, X., He, J., Lin, K., Wang, X. & Cao, H. State-of-the-art strategies for Lewis acid-catalyzed strain-release cycloadditions of bicyclo[1.1.0]butanes (BCBs). Org. Chem. Front. 11, 6942–6957 (2024).
Chang, Y.-C., Salome, C., Fessard, T. & Brown, M. K. Synthesis of 2-azanorbornanes via strain-release formal cycloadditions initiated by energy transfer. Angew. Chem. Int. Ed. 62, e202314700 (2023).
Roy, D. et al. SmI2-catalyzed coupling of alkyl housane ketones and alkenes in an approach to norbornanes. Angew. Chem. Int. Ed. 64, e202512018 (2025).
Jung, M. & Lindsay, V. N. G. One-pot synthesis of strain-release reagents from methyl sulfones. J. Am. Chem. Soc. 144, 4764–4769 (2022).
Liu, Y. et al. Facile synthesis of housanes by an unexpected strategy. J. Am. Chem. Soc. 147, 6318–6325 (2025).
Park, Y. S., Wang, S. C., Tantillo, D. J. & Little, R. D. A highly selective rearrangement of a housane-derived cation radical: an electrochemically mediated transformation. J. Org. Chem. 72, 4351–4357 (2007).
Semeno, V. V. et al. Building the housane: diastereoselective synthesis and characterization of bicyclo[2.1.0]pentane carboxylic acids. J. Org. Chem. 85, 2321–2337 (2020).
Coto, D. et al. From cyclopropene to housane derivatives via intramolecular cyclopropanation. Angew. Chem. Int. Ed. 63, e202409226 (2024).
Keen, B. et al. Stereoselective synthesis of highly functionalized bicyclo[2.1.0]pentanes by sequential [2 + 1] and [2 + 2] cycloadditions. Org. Lett. 27, 1673–1678 (2025).
Mondal, A. R. S., Bapat, N. A., Mishra, H. & Hari, D. P. Highly stereoselective synthesis of polysubstituted housanes and spiro-oxa-housanes: application and mechanistic insights. Chem. Sci. 16, 12350–12361 (2025).
Sharland, J. C. & Davies, H. M. L. One-pot synthesis of difluorobicyclo[1.1.1]pentanes from α-allyldiazoacetates. Org. Lett. 25, 5214–5219 (2023).
Eckart-Frank, I. K., Arnold, E. S., Murphy, L. P. & Wilkerson-Hill, S. M. Synthesis of bicyclo[2.1.0]pentanes and vinylcyclopropanes using palladium carbenes: ligand-controlled carbene reactivity. J. Am. Chem. Soc. 147, 33923–33931 (2025).
Chintawar, C. C. et al. Photoredox-catalysed amidyl radical insertion to bicyclo[1.1.0]butanes. Nat. Catal. 7, 1232–1242 (2024).
Dutta, S. et al. Photoredox-enabled dearomative [2π + 2σ] cycloaddition of phenols. J. Am. Chem. Soc. 146, 2789–2797 (2024).
Dutta, S. et al. Double strain-release [2π + 2σ]-photocycloaddition. J. Am. Chem. Soc. 146, 5232–5241 (2024).
Kleinmans, R. et al. Ortho-selective dearomative [2π + 2σ] photocycloadditions of bicyclic aza-arenes. J. Am. Chem. Soc. 145, 12324–12332 (2023).
Kleinmans, R. et al. Intermolecular [2π + 2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Liang, Y., Nematswerani, R., Daniliuc, C. G. & Glorius, F. Silver-enabled cycloaddition of bicyclobutanes with isocyanides for the synthesis of polysubstituted 3-azabicyclo[3.1.1]heptanes. Angew. Chem. Int. Ed. 63, e202402730 (2024).
Liang, Y., Paulus, F., Daniliuc, C. G. & Glorius, F. Catalytic formal [2π + 2σ] cycloaddition of aldehydes with bicyclobutanes: expedient access to polysubstituted 2-oxabicyclo[2.1.1]hexanes. Angew. Chem. Int. Ed. 62, e202305043 (2023).
Tyler, J. L. et al. Bicyclo[1.1.0]butyl radical cations: synthesis and application to [2π + 2σ] cycloaddition reactions. J. Am. Chem. Soc. 146, 16237–16247 (2024).
Wang, H. et al. syn-Selective difunctionalization of bicyclobutanes enabled by photoredox-mediated C–S σ-bond scission. J. Am. Chem. Soc. 145, 23771–23780 (2023).
Wang, H. et al. Dearomative ring expansion of thiophenes by bicyclobutane insertion. Science 381, 75–81 (2023).
Zhang, F. et al. Solvent-dependent divergent cyclization of bicyclo[1.1.0]butanes. Angew. Chem. Int. Ed. 64, e202418239 (2025).
Meinwald, J. & Smith, G. W. Mercury-photosensitized reactions of 1,4-dienes. J. Am. Chem. Soc. 89, 4923–4932 (1967).
Tsuno, T., Hoshino, H., Okuda, R. & Sugiyama, K. Allenyl(vinyl)methane photochemistry. Photochemistry of γ-(3-methyl-1-phenyl-1,2-butadienyl)-substituted α,β-unsaturated ester and nitrile derivatives. Tetrahedron 57, 4831–4840 (2001).
Tsuno, T. & Sugiyama, K. Allenyl(vinyl)methane photochemistry. Photochemistry of methyl 4,4-dimethyl-2,5,6-heptatrienoate derivatives. Bull. Chem. Soc. Jpn. 72, 519–531 (1999).
Zimmerman, H. E., Penn, J. H. & Johnson, M. R. New reactions and theory in organic photochemistry: the 1,3-vinyl migration and its relevance to exchange integral control. Proc. Natl Acad. Sci. USA 78, 2021–2025 (1981).
Hixson, S. S., Mariano, P. S. & Zimmerman, H. E. Di-π-methane and oxa-di-π-methane rearrangements. Chem. Rev. 73, 531–551 (1973).
Zimmerman, H. E. & Armesto, D. Synthetic aspects of the di-π-methane rearrangement. Chem. Rev. 96, 3065–3112 (1996).
Tsuno, T., Yoshida, M., Iwata, T. & Sugiyama, K. Allenyl(vinyl)methane photochemistry. Photochemistry of γ-allenyl-substituted α,β-unsaturated enone derivatives. Tetrahedron 58, 7681–7689 (2002).
Kunio, S. & Takashi, T. Novel chemistry of 5-methylene-substituted 1,3-dioxane-4,6-dione derivatives. Trends Heterocycl. Chem. 7, 91–106 (2001).
Tyler, J. L., Trauner, D. & Glorius, F. Reaction development: a student’s checklist. Chem. Soc. Rev. 54, 3272–3292 (2025).
Pitzer, L., Schäfers, F. & Glorius, F. Rapid assessment of the reaction-condition-based sensitivity of chemical transformations. Angew. Chem. Int. Ed. 58, 8572–8576 (2019).
Schäfer, F., Lückemeier, L. & Glorius, F. Improving reproducibility through condition-based sensitivity assessments: application, advancement and prospect. Chem. Sci. 15, 14548–14555 (2024).
Collins, K. D. & Glorius, F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 5, 597–601 (2013).
Hirbawi, N., Lin, P. C. & Jarvo, E. R. Halogenation reactions of alkyl alcohols employing methyl Grignard reagents. J. Org. Chem. 87, 12352–12369 (2022).
Wang, M., Huang, Y., Li, C. & Lu, P. Diastereoselective synthesis of 1,1,3,3-tetrasubstituted cyclobutanes enabled by cycloaddition of bicyclo[1.1.0]butanes. Org. Chem. Front. 9, 2149–2153 (2022).
Nandy, M. et al. Total Synthesis of (+)-brevianamides A and B. Org. Lett. 26, 10424–10429 (2024).
Dhote, P. S., Patel, P., Vanka, K. & Ramana, C. V. Total synthesis of the pseudoindoxyl class of natural products. Org. Biomol. Chem. 19, 7970–7994 (2021).
Joshi-Pangu, A. et al. Acridinium-based photocatalysts: a sustainable option in photoredox catalysis. J. Org. Chem. 81, 7244–7249 (2016).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Popescu, M. V. & Paton, R. S. Dynamic vertical triplet energies: understanding and predicting triplet energy transfer. Chem 10, 3428–3443 (2024).
Carpenter, B. K. Understanding the puzzling chemistry of bicyclo[2.1.0]pentane. Org. Biomol. Chem. 2, 103–109 (2004).
Acknowledgements
This work is supported by Muenster University. J.D. acknowledges PhD fellowship funding by the Hans und Ria Messer Stiftung. N.H. acknowledges PhD fellowship funding by the German National Academic Foundation (Studienstiftung des deutschen Volkes). The authors thank D. Rana and L. Schlosser for helpful discussions.
Author information
Authors and Affiliations
Contributions
F.G. and F.Z. conceived the project. F.Z. designed all the experiments. F.Z. and J.D. performed synthetic experiments. N.H. conducted computational investigations. All authors analysed the data. C.G.D. analysed X-ray structures. F.G. and F.Z. supervised the research and wrote the manuscript with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
Authors declare that they have no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Joel Cejas-Sánchez, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Supplementary sections 1–12, Figs. 1–19 and Tables 1–8.
Supplementary Data 1
Crystallographic data for compound 2ao, CCDC 2486495.
Supplementary Data 2
Crystallographic data for compound 2k, CCDC 2486494.
Supplementary Data 3
Crystallographic data for compound 3av, CCDC 2486496.
Supplementary Data 4
Crystallographic data for compound 7, CCDC 2504328.
Source data
Source Data Fig. 5
Statistical source data for Fig. 5a–c.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, F., Domack, J., Hölter, N. et al. Divergent housane synthesis via intramolecular [2 + 2] cycloaddition of 1,4-dienes. Nat. Synth (2026). https://doi.org/10.1038/s44160-026-00997-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44160-026-00997-7