Abstract
Metabolic dysregulation, including perturbed glutamine–glutamate homeostasis, is common among patients with cardiovascular diseases, but the underlying mechanisms remain largely unknown. Using the human MESA cohort, here we show that plasma glutamine–glutamate ratio is an independent risk factor for carotid plaque progression. Mice deficient in glutaminase-2 (Gls2), the enzyme that mediates hepatic glutaminolysis, developed accelerated atherosclerosis and susceptibility to catastrophic cardiac events, while Gls2 overexpression partially protected from disease progression. High-throughput transcriptional profiling and high-resolution structural biology imaging of aortas showed that Gls2 deficiency perturbed extracellular matrix composition and increased vessel stiffness. This results from an imbalance of glutamine- and glutamate-dependent cross-linked proteins within atherosclerotic lesions and cellular remodeling of plaques. Thus, hepatic glutaminolysis functions as a potent regulator of glutamine homeostasis, which affects the aortic wall structure during atherosclerotic plaque progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its supplementary information. Other information is available from the corresponding authors upon reasonable request. snRNA-seq data have been deposited at the Gene Expression Omnibus (accession number GSE269682). RNA-seq data of aortas, livers and SMC line (MOVAS) have been deposited at the Gene Expression Omnibus under accession numbers GSE270056 and GSE270058, respectively. Source data are provided with this paper.
References
Sotos-Prieto, M. et al. Association of changes in diet quality with total and cause-specific mortality. N. Engl. J. Med. 377, 143–153 (2017).
Joseph, P. et al. Reducing the global burden of cardiovascular disease, part 1: the epidemiology and risk factors. Circ. Res. 121, 677–694 (2017).
DeBerardinis, R. J. & Cheng, T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29, 313–324 (2010).
O’Neill, L. A. J. & Pearce, E. J. Immunometabolism governs dendritic cell and macrophage function. J. Exp. Med. 213, 15–23 (2016).
Qi, L. et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA 310, 821 (2013).
Suzuki, S. et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl Acad. Sci. USA 107, 7461–7466 (2010).
Miller, R. A. et al. Targeting hepatic glutaminase activity to ameliorate hyperglycemia. Nat. Med. 24, 518–524 (2018).
Shah, S. H. et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 3, 207–214 (2010).
Zheng, Y. et al. Metabolites of glutamate metabolism are associated with incident cardiovascular events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) Trial. J. Am. Heart Assoc. 5, e003755 (2016).
Ma, W. et al. Dietary glutamine, glutamate and mortality: two large prospective studies in US men and women. Int. J. Epidemiol. 47, 311–320 (2018).
Cheng, S. et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 125, 2222–2231 (2012).
Ganda, A. et al. Mild renal dysfunction and metabolites tied to low HDL cholesterol are associated with monocytosis and atherosclerosis. Circulation 127, 988–996 (2013).
Suhre, K. et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature 477, 54–60 (2011).
Kettunen, J. et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet. 44, 269–276 (2012).
Shin, S. Y. et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 46, 543–550 (2014).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023).
Aragam, K. G. et al. Discovery and systematic characterization of risk variants and genes for coronary artery disease in over a million participants. Nat. Genet. 54, 1803–1815 (2022).
Tcheandjieu, C. et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat. Med. 28, 1679–1692 (2022).
Shea, S. et al. Cholesterol mass efflux capacity, incident cardiovascular disease, and progression of carotid plaque: the Multi-ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 39, 89–96 (2019).
Tacke, F. et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Investig. 117, 185–194 (2007).
Jordan, S. et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 178, 1102–1114.e17 (2019).
Rader, D. J. New therapeutic approaches to the treatment of dyslipidemia. Cell Metab. 23, 405–412 (2016).
Tall, A. R., Thomas, D. G., Gonzalez-Cabodevilla, A. G. & Goldberg, I. J. Addressing dyslipidemic risk beyond LDL-cholesterol. J. Clin. Investig. 132, e148559 (2022).
Eckel, R. H., Bornfeldt, K. E. & Goldberg, I. J. Cardiovascular disease in diabetes, beyond glucose. Cell Metab. 33, 1519–1545 (2021).
Chang, M.-K. et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl Acad. Sci. USA 96, 6353–6358 (1999).
Breslow, J. L. Mouse models of atherosclerosis. Science 272, 685–688 (1996).
Nagareddy, P. R. et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 17, 695–708 (2013).
Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 (2010).
Reily, C., Stewart, T. J., Renfrow, M. B. & Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 15, 346–366 (2019).
Karna, E., Szoka, L., Huynh, T. Y. L. & Palka, J. A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 77, 1911–1918 (2020).
Janke, C. & Chloë Bulinski, J. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 12, 773–786 (2011).
Lorand, L. & Graham, R. M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. 4, 140–156 (2003).
Wloga, D., Joachimiak, E., Louka, P. & Gaertig, J. Post-translational modifications of tubulin and cilia. Cold Spring Harbor Perspect. Biol. 9, a028159 (2017).
Wang, Z.-M., Gao, X.-F., Zhang, J.-J. & Chen, S.-L. Primary cilia and atherosclerosis. Front. Physiol. 12, 640774 (2021).
Chen, S. et al. Roles and potential clinical implications of tissue transglutaminase in cardiovascular diseases. Pharmacol. Res. 177, 106085 (2022).
Chen, R., McVey, D. G., Shen, D., Huang, X. & Ye, S. Phenotypic switching of vascular smooth muscle cells in atherosclerosis. J. Am. Heart Assoc. 12, e031121 (2023).
Torrino, S. et al. Mechano-induced cell metabolism promotes microtubule glutamylation to force metastasis. Cell Metab. 33, 1342–1357 (2021).
Fuller, M. et al. The effects of diet on occlusive coronary artery atherosclerosis and myocardial infarction in scavenger receptor class B, type 1/low-density lipoprotein receptor double knockout mice. Arterioscler. Thromb. Vasc. Biol. 34, 2394–2403 (2014).
van den Borne, S. W. M. et al. Myocardial remodeling after infarction: the role of myofibroblasts. Nat. Rev. Cardiol. 7, 30–37 (2010).
Merlin, J. et al. Non-canonical glutamine transamination sustains efferocytosis by coupling redox buffering to oxidative phosphorylation. Nat. Metab. 3, 1313–1326 (2021).
Karjalainen, M. K. et al. Genome-wide characterization of circulating metabolic biomarkers. Nature 628, 130–138 (2024).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 30, e34408 (2018).
Clément, M. et al. Vascular smooth muscle cell plasticity and autophagy in dissecting aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 39, 1149–1159 (2019).
Nasse, M. J. et al. High-resolution Fourier-transform infrared chemical imaging with multiple synchroton beams. Nat. Methods 8, 413–416 (2011).
Baker, M. J. et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 9, 1771–1791 (2014).
Palombo, F., Cremers, S. G., Weinberg, P. D. & Kazarian, S. G. Application of Fourier transform infrared spectroscopic imaging to the study of effects of age and dietary L-arginine on aortic lesion composition in cholesterol-fed rabbits. J. R. Soc. Interface 6, 669–680 (2009).
Berquand, A. et al. Revealing the elasticity of an individual aortic fiber during ageing at nanoscale by in situ atomic force microscopy. Nanoscale 13, 1124–1133 (2021).
Acknowledgements
We thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org. We acknowledge the participants and investigators of the FinnGenn study. We thank O. Söhnlein (Universität Münster) for kindly providing the MOVAS cells. We thank F. Labret for assistance with flow cytometry, M. Irondelle for assistance with microscopy and C3M and CDTA animal facilities (all from Inserm U1065). We also thank the GIS-IBISA multi-sites platform Microscopie Imagerie Côte d’Azur and particularly the imaging site of C3M (INSERM U1065) supported by Conseil Régional, Conseil Départemental and IBISA. Some of the experiments were carried out within the Nanomat platform (www.nanomat.eu) supported by the Ministère de l'Enseignement Supérieur et de la Recherche, the Région Grand Est, and FEDER funds from the European Community. We thank L. Robinson of Insight Editing London for her assistance with preparation/critical review/scientific editing of this manuscript. This work was supported by grants from the European Research Council consolidator program (ERC2016COG724838), Agence Nationale de la Recherche (ANR19-CE17-0030-DS, ANR24-CE14-Glutacare) and the Fondation de France to L.Y.C and the Société Francophone du Diabète to B.B.M. MESA is supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040, UL1-TR-001079 and UL1-TR-001420 from the National Center for Advancing Translational Sciences and by grant R01HL127637 to S.S. This publication was developed under STAR research assistance agreements, no. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the US Environmental Protection Agency (EPA). This work has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
L.Y.-C. conceived the project, designed the experiments and wrote the paper. F.M. performed most of the molecular, histological and in vivo experiments. C.B. replicated and finalized molecular and histological experiments and helped with the experimental design. T.B. and N.V. helped with the experimental design, supported most of the histological experiments and assisted with the data analysis. S.G.A., N.O., A.B. and L.D. performed high-resolution infrared and AFM analyses. T.B., M.C. and P.B. helped with snRNA-seq design and analysis. S.T. and T.B. helped with in vitro cell culture experiments. J.M. helped with analysis of human studies. S.D. helped with bioinformatic analyses. M.U.K., E.B.T., E.L.G. and B.B.M. provided scientific advice and helped with the experimental design. M.K.K. and J.K. conducted the two-sample MR analysis. R.L.M. and W.T. planned and conducted the statistical analysis of the MESA data. J.S. and S.S. supervised the human study, assisted with the data analysis and provided intellectual discussion. W.T. assisted with the data analysis. L.Y.-C. also designed and supervised the study and obtained funding. All the authors read, edited and approved the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cardiovascular Research thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
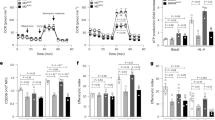
Extended Data Fig. 1 Glutamine synthesis regulates the Gln-to-Glu cycle but not atherosclerosis.
(a) Experimental outline. Apoe−/− mice were fed an atherogenic diet (Western Diet, WD) with i.p injection of saline or 20 mg/kg BW of methionine sulfoximine (MSO) every other day for 12 weeks (left panel). Schematic representation of whole-body Gln-to-Glu cycle (upper right panel). Western blot of GS protein in the liver and muscle of these mice (lower right panel). (b) Liver and muscle Gln (left panel) and Glu (right panel) levels in these mice. Red bars represent MSO treatment. Both sexes were analyzed. (c) Gln-to-Glu ratio (GGR) was calculated (n = 7-8 mice per group; statistical significance was assessed with two-tailed t-test, with P values displayed). (d) From left to right: H&E staining of paraffin-embedded serial sections obtained from the liver of these animals (left panel). Scale bar, 200 mm. Liver triglyceride (TG) content, plasma AST and ALT and urea levels (right panel) (n = 7-8 mice per group, except AST and ALT levels, for which some measurement failed; P values displayed; two-tailed t test). (e) Plasma GGR, Gln and Glu levels and (f) cholesterol and TG were also determined in these mice at the end of the study period (n = 7-8 mice per group; statistical significance was assessed with two-tailed t test, with P values displayed). (g) Glucose tolerance test was performed on saline- or MSO- treated atherogenic diet-fed Apoe−/− mice. Blood glucose concentrations were measured at the indicated time points. The glucose tolerance index was calculated as the product of areas under glucose curves (n = 7-8 mice per group; P values displayed; two-tailed t test). (h) Representative H&E staining of paraffin-embedded serial sections obtained from proximal aortas (left panel) and quantification of proximal aortic root lesion area by morphometric analysis (right panel) from these mice (n = 7-8 mice per group; P = 0.95; two-tailed t test). Scale bar, 200 mm. (i) Oil Red O-stained descending aortas from representative atherogenic diet-fed Apoe−/− mice treated or not with MSO. (j) Atherosclerotic lesion severity in the aortic root of saline or MSO-treated atherogenic diet-fed Apoe−/− mice. Data from individual mice are shown and values are given as the mean ± s.e.m. of one independent experiment. Two-tailed Student’s t-tests were used.
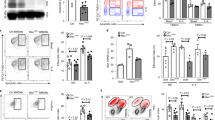
Extended Data Fig. 2 Effects of defective hepatic glutaminolysis on Gln-to-Glu cycle in atherosclerosis.
(a) Experimental outline. Apoe−/− mice were infected with the AAV-Gls2shRNA (Apoe−/−Gls2shRNA mice) or AAV-scrambled shRNA controls and fed an atherogenic diet for 12 weeks (left panel). mRNA expression of Gls2 in indicated tissues (values were normalized to m36B4 and expressed as arbitrary unit) (n = 8 mice per group; P values displayed; two-tailed t test) and Western blot of GLS2 protein in the liver of these mice (middle panel). GLS2 was also visualized by immunofluorescent staining in the hepatic periportal vein (right panel). Scale bar, 200 mm. (b,c) Tissue and (d) plasma Gln and Glu levels and GGR in these mice (n = 9-10 mice per group except liver tissue due to outlier analysis (Grubb’s test); P values displayed; two-tailed t test). (e) Overall survival curves of atherogenic diet-fed Apoe−/−Gls2shRNA and control mice after restraint stress exposure and expressed as the percentage of mice surviving at the times indicated. (f) Experimental outline. Ldlr−/−Gls2−/− and Ldlr−/− mice were fed an atherogenic diet for 12 weeks (left panel). Western blot of GLS2 protein in the liver of these mice (right panel). (g) Tissue (n = 8-13 mice per group due to failure of some measurements; P values displayed; two-tailed t test) and (h) plasma (n = 13-20 mice per group from two separate experiments; P values displayed; two-tailed t test) Gln and Glu levels and GGR. Data from individual mice are shown and values are presented as the mean ± s.e.m. of at least one independent experiment unless otherwise stated on the corresponding panel legend. Two-tailed Student’s t-tests were used.
Extended Data Fig. 3 Lesional monocyte infiltration in Gls2-deficient mice independent of systemic inflammation.
(a) Leukocyte population gating strategy and (b) percentage measured by flow cytometry from the blood of atherogenic diet-fed Apoe−/−Gls2shRNA compared to controls (n = 9-10 mice per group; P values displayed; two-tailed t test) or Ldlr−/−Gls2−/− mice compared to respective controls ( = 13-18 mice per group; P values displayed; two-tailed t test). (c) Tracking recruitment of Ly6Chi monocytes to atherosclerotic plaques two days after monocyte labeling with latex beads in atherogenic diet-fed Apoe−/−Gls2shRNA and control mice. Representative flow cytometry plots (left panel) and quantification (right panel) of latex+ monocytes that have infiltrated the aortic arch of atherogenic diet-fed Apoe−/−Gls2shRNA and control mice. (d) Representative pictures (left panel) and quantification of latex beads (indicated by arrows) localized within atherosclerotic lesions and expressed as the number of beads per cross section (right panel) (n = 6 mice per group; P values displayed; two-tailed t test). Scale bar, 200 mm. (e) Venn diagram highlighting the common and specific Gls2-dependent regulated genes between the Ldlr−/− and Apoe−/− atherogenic strains. (f) Differentially expressed genes between Gls2-deficient mice and controls on atherogenic Apoe−/− background were subjected to gene set enrichment analysis (GSEA). The top 4 up- and downregulated pathways are displayed (n = 3-4 mice per group; log2 fold change was used to assess statistical significance with two-tailed t test, with P < 0.05). (g) RNAseq analysis focusing on the previously identified hepatic energy-sensing signature governing monocyte homeostasis in Gls2-deficient mice and controls on atherogenic Ldlr−/− and Apoe−/− background. (h) Quantification of myeloid cells in the bone marrow and spleen and Kupffer cells (KCs) at the end of the study period in atherogenic diet-fed Apoe−/−Gls2shRNA and control mice (n = 4–5 mice per group; P values displayed; two-tailed t test). (i) Expression of chemokines and interleukins in the liver (left panel), plasma (middle panel) and BM fluid (right panel), assessed by multiplex assays. Data from individual mice are shown and values are given as the mean ± s.e.m. of at least one independent experiment. Two-tailed Student’s t-tests were used.
Extended Data Fig. 4 Effects of defective hepatic glutaminolysis on whole-body and hepatic metabolism.
(a) Association of Gls2 variants with several Metabolic Syndrome traits from publicly available GWAS HuGEAMP datasets. (b) H&E staining of paraffin-embedded serial sections (left panel, scale bar, 200 mm) and triglyceride content (right panel) of the liver of Apoe−/−Gls2shRNA mice compared to controls (n = 9-10 mice per group; P values displayed; two-tailed t test) or Ldlr−/−Gls2−/− mice compared to respective controls (n = 8-13 mice per group; P values displayed; two-tailed t test). (c) Plasma ALT, (d) plasma urea and (e) plasma triglyceride levels in Apoe−/−Gls2shRNA mice and controls (n = 9-10 mice per group, except ALT levels, for which some measurement failed; P values displayed; two-tailed t test) and Ldlr−/−Gls2−/− mice compared to controls (n = 8-13 mice per group; P values displayed; two-tailed t test). (f) Glucose tolerance test was performed on atherogenic diet-fed Apoe−/−Gls2shRNA and control mice. Blood glucose concentrations were measured at the indicate time points. The glucose tolerance index was calculated as the product of areas under glucose curves (n = 9 mice per group; P = 0.86; two-tailed t test). (g) Plasma insulin levels were measured in atherogenic diet-fed Apoe−/−Gls2shRNA and control mice (left panel) (n = 9 mice per group; P values displayed; two-tailed t test). Paraffin-embedded serial sections obtained from the adipose tissue of these mice (right panel). Representative H&E staining revealed similar cellularity. (h) Respiratory quotient (RQ) measured by indirect calorimetry in atherogenic diet-fed Apoe−/−Gls2shRNA and control mice (n = 4 mice per group; no statistical difference was regardless of the light-on or light-off phase; two-tailed t test). (i) Plasma triglyceride levels were determined at 0, 1, 2 and 4 hours after the olive oil gavage in these mice (right panel represents area under the curve) (n = 10 mice per group; P = 0.79; two-tailed t test). All values are mean ± s.e.m. and are representative of at least one independent experiment. P values were determined by two-tailed Student’s t-test. (j) Schematic representation of glycolysis and neoglucogenesis pathways (upper left panel), triglyceride synthesis pathway (upper right panel) and VLDL production (lower panel). (k) RNAseq analysis with focus on enzymatic regulators expressed in the liver of Ldlr−/−Gls2−/− and Apoe−/−Gls2−/− mice and their respective controls. (l) RNAseq analysis with focus on of genes involved in VLDL production and hydrolysis depicted in the upper schematic representation.
Extended Data Fig. 5 Hepatic GLS2 links GGR to CVD traits.
(a) Experimental outline. Apoe−/−Gls2shRNA and control mice were fed an atherogenic diet for six weeks and divided into three groups: 1) mice continued on an atherogenic diet for an additional two weeks; 2) mice were switched to chow for two weeks, and 3) mice were switched to a chow diet and orally supplemented with Glu (3 g/dL of drinking water) for two weeks. (b) Plasma cholesterol levels in Apoe−/−Gls2−/− and Apoe−/− control mice fed an atherogenic diet for eight weeks or switched for the last two weeks to chow diet (-Gln) or chow diet orally supplemented with Gln ( + Gln) (left panel) (n = 8-12 mice per group; P values displayed; two-tailed t test) or in Apoe−/−Gls2shRNA and control mice fed an atherogenic diet for eight weeks or switched for the last two weeks to chow diet (-Glu) or chow diet orally supplemented with Glu ( + Glu) (right panel) (n = 5−8 mice per group; P values displayed; two-tailed t test). (c) Plasma Gln and Glu levels (left panel) and correlation between plasma GGR and atherosclerotic lesion size (right panel) in Apoe−/−Gls2−/− and Apoe−/− control mice with or without Gln supplementation (n = 8-12 mice per group; P values displayed; two-tailed t test and Pearson correlation coefficient two-tailed t test, respectively). (d) Plasma Gln and Glu levels in Apoe−/−Gls2shRNA and control mice with or without Glu supplementation (n = 5−8 mice per group; P values displayed; two-tailed t test). (e) Correlation between plasma GGR and aorta GGR in Apoe−/−Gls2−/− and Apoe−/− control mice with or without Gln supplementation (n = 8–9 mice per group; P < 0.0001; Pearson correlation coefficient two-tailed t test, respectively). (f) Representative H&E staining of paraffin-embedded serial sections obtained from proximal aortas (left panel), quantification of atherosclerotic lesion area in aortic sinuses (middle panel) and correlation between plasma GGR and atherosclerotic lesion size (right panel) in Apoe−/−Gls2shRNA and control mice were fed an atherogenic diet and supplemented or not with Glu (n = 4−7 mice per group; P values displayed; two-tailed t test and Pearson correlation coefficient two-tailed t test, respectively). Data from individual mice are shown and all values are mean ± s.e.m. of one independent experiment. Two-tailed Student’s t-tests were used.
Extended Data Fig. 6 Effects of modulating GLS activities on metabolic risk factors and architectural regulation of the ECM.
(a) Liver and (b) plasma Gln (left panel) and Glu (right panel) levels and (c) muscle GGR in Apoe−/− mice infected with the AAV-Gls2Ovex or AAV-empty controls and fed an atherogenic diet for 12 weeks (n = 8–9 mice per group; P values displayed; two-tailed t test). (d) From left to right: plasma cholesterol, TG, ALT, urea levels and blood monocyte counts in these mice (n = 8–9 mice per group; P values displayed; two-tailed t test). (e) Aortic GGR in a subgroup of these mice (n = 5 mice per group; P = 0.05; two-tailed t test). (f) Aortic GGR in Ldlr−/−Gls2−/− and Ldlr−/− control mice (n = 7–9 mice per group; P < 0.0001; two-tailed t test). (g) Volcano plot of differentially expressed genes (up, red and down, blue) between Ldlr−/−Gls2−/− and Ldlr−/− aortas. (h) Representative high-resolution Fourier transform infrared (FTIR) images based on the aliphatic chain hydrocarbon (ACH) contribution by FTIR spectroscopy (left panel) or hyperspectral images represented as three color (RGB) composite images (middle panel) recorded in the aortic sinus of atherogenic diet-fed Ldlr−/−Gls2−/− and Ldlr−/− mice. Scale bar, 200 mm. Quantification of the lipid from aliphatic chain hydrocarbon band (represented in red) and the protein from Amide I band (represented in green) in the aortic sinus of these mice (right panel) (n = 3 mice with up to 3 measures per images; P = 0.002; two-tailed t test). (i) Surface plot reconstitution of spectral density plot from images created using factor analysis. (j) Representative infrared images after attenuated total reflectance (ATR) showing the absorbance band centered at 1648 cm-1 (Amide I vibrations) and 1000-1200 cm-1 (proteoglycan vibrations) in the aortic media of these animals. Scale bar, 50 mm. (k) Representative atomic force microscopy (AFM) 3D pictures of the aorta of these mice using a Peak Force QNM mode. (l) Verhoeff-Van Gieson (VVG) staining (scale bar, 200 mm) and quantification of elastic lamina area (expressed as arbitrary unit, a.u) in the proximal aorta of atherogenic diet-fed Apoe−/−Gls2shRNA and control mice (n = 5 mice per group; P = 0.02; two-tailed t test). Data from individual mice are shown and values are given as the mean ± s.e.m. of one independent experiment. Two-tailed Student’s t-tests were used.
Extended Data Fig. 7 Hepatic GLS2 deficiency controls post-transcriptional ECM remodeling.
(a) Schematic representation of potential mechanisms involving Gln- and Glu-dependent extracellular matrix remodeling. (b) RNAseq analysis with focus on genes involved in extracellular matrix composition. (c) Schematic representation of glutamine incorporation into metabolic cycles driving glycosaminoglycan synthesis and hexosamine biosynthetic pathway (left panel) and RNAseq analysis with focus on genes involved in KEGG glycan and glycosaminoglycan metabolism (right panel) (n = 5 mice per group from an independent experimental group; log2 fold change was used to assess statistical significance with two-tailed t test). (d) Alcian blue staining (scale bar, 200 mm) and quantification of proteoglycan content normalized to plaque size in the proximal aorta of atherogenic diet-fed Ldlr−/−Gls2−/− and Ldlr−/− mice (left panel) or Apoe−/−Gls2shRNA and control mice (right panel) (n = 5 mice per group; P = 0.10 and P = 0.11, respectively; two-tailed t test). (e) RNAseq analysis with focus on genes involved in proline (Pro), transglutaminase and glutamylation pathways in the aorta of atherogenic diet-fed Ldlr−/−Gls2−/− and Ldlr−/− mice (n = 5 mice per group; statistical significance in blue; two-tailed t test). (f) Picro Sirius Red staining of aortic root sections under polarized light allows for visualization of collagen I in yellow, orange and red in order of increasing thickness in atherogenic diet-fed Ldlr−/−Gls2−/− and Ldlr−/− mice; PL, polarized light. Picro sirius red staining was quantified and expressed as arbitrary unit (a.u) (n = 5 mice per group; P = 0.0004; two-tailed t test). (g) Immunofluorescent staining (scale bar, 200 mm) and quantification of collagen IV (CollV) in the atherosclerotic lesions of these mice (n = 5 mice per group; P = 0.06; two-tailed t test. Immunofluorescent staining and quantification of (h) polyglutamylation (GT335) and (i) cross-linked proteins by Nε-(γ-L-glutamyl)-L-lysine (81D4) in the atherosclerotic lesions of atherogenic diet-fed Apoe−/−Gls2shRNA and control mice (n = 7-8 mice per group; P = 0.04 and P = 0.05, respectively; two-tailed t test). Data from individual mice are shown and values are given as the mean ± s.e.m. of independent experiment. Two-tailed Student’s t-tests were used.
Extended Data Fig. 8 Cellular GGR controls SMC switching.
(a) Experimental outline. Mouse smooth muscle cell line (MOVAS) was treated for 24 hours with glutaminase inhibitors in presence or absence of glutamate supplementation to set up GGRhigh and GGRlow conditions (upper panel). Venn diagram highlights genes specifically regulated by GGRhigh and GGRlow conditions and those oppositely regulated between these conditions. (b) RNA-seq results on genes belonging to Gln- or Glu-dependent metabolism (upper panel) (n = 3 independent cultures; log2 fold change was used to assess statistical significance with two-tailed t test, with P < 0.05 indicated by an asterisk). Schematic representation of upregulated (red) and downregulated (blue) Gln- and Glu-dependent transcripts in SMC in GGRhigh and GGRlow conditions. (c) Differentially expressed genes between these cell culture conditions were subjected to gene set enrichment analysis (GSEA). The top 4 downregulated or oppositely regulated pathways are shown. (d) Immunoblot and quantification of Glu-tubulin cultured on soft (1 kPa) or stiff (50 kPa) conditions with the indicated conditions (n = 3 independent cultures, P values displayed; two-tailed t test). (e) Representative images (upper panel) and quantification of gel contraction (lower right panel) (n = 12 replicates with four independent cultures, P values displayed; two-tailed t test). Quantification of force magnitude using traction force microscopy (lower right panel) (n = 9 replicates with three independent cultures, P values displayed; two-tailed t test). Data from individual cultures and results are means ± s.e.m. Two-tailed Student’s t-tests were used.
Extended Data Fig. 9 Hepatic GLS2 deficiency disrupts stromal cell homeostasis.
(a) Experimental outline. Ldlr−/− mice were infected with the AAV-Gls2shRNA (ldlr−/−Gls2shRNA mice) or AAV-scrambled shRNA controls and fed an atherogenic diet for 8 weeks before performing single-nuclei transcriptomes of pooled mouse aortas (left panel). Plasma GGR and quantification of atherosclerotic lesion area in aortic sinuses of these mice (middle panel) (n = 4 mice per group; P values displayed; two-tailed t test). Data from individual mice are shown and values are given as the mean ± s.e.m. Atherosclerotic lesion severity was also typified (right panel). (b) Top 2 differentially expressed genes among all detected cell clusters from SnRNA-seq performed in the aortas of atherogenic diet-fed control and Ldlr−/−Gls2shRNA mice. Retrieved clusters were assigned to ten lineages with specific biological identification of each cluster. (c) Enzymatically digested aortas from atherogenic diet-fed Apoe−/−Gls2shRNA and control mice were analyzed by flow cytometry. Representative dot plots showing the gating strategy used to analyze CD45−CD31+CD144+ and CD45-CD31+CD144- endothelial cells (BEC1 and BEC2, respectively), CD45-CD31−CD90+ myofibroblasts, CD45−CD31−aSMA+CD140a+ fibroblasts and CD45−CD31−aSMA+CD140a− smooth muscle cells (left panel). t-SNE plots depicting all stromal cells within CD45− fraction (upper right panel) and quantification of the proportion of stromal cell populations for each group of animals (lower right panel). Violin plots representing the mean expression of (d) SMC phenotypic markers, (e) glycan-modifying enzymes, (f) detoxifying and antioxidant enzymes and (g) EMT signaling pathways in aortic SMC and MyoFib populations from atherogenic diet-fed control and Ldlr−/−Gls2shRNA mice (n = 1946 Ldlr−/−ScblshRNA SMCs, n = 874 Ldlr−/−Gls2shRNA SMCs; n = 23 Ldlr−/−ScblshRNA myofibroblasts, n = 214 Ldlr−/−Gls2shRNA myofibroblasts; gene regulation was statistically evaluated by two-tailed t test on the assumption of equal variances, with P values displayed). Two-tailed Student’s t-tests were used. ND, not detectable.
Supplementary information
Supplementary Data 1
Uncropped immunoblots for Extended Data Figs. 1a, 2a, 2f and 8.
Supplementary Data 2
Statistical source data.
Supplementary Data 3
Statistical source data.
Supplementary Data 4
Statistical source data.
Supplementary Data 5
Statistical source data.
Supplementary Data 6
Statistical source data.
Supplementary Data 7
Statistical source data.
Supplementary Data 8
Statistical source data.
Supplementary Data 9
Statistical source data.
Supplementary Data 10
Statistical source data.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Murcy, F., Borowczyk, C., Gourion-Arsiquaud, S. et al. GLS2 links glutamine metabolism and atherosclerosis by remodeling artery walls. Nat Cardiovasc Res 3, 1454–1467 (2024). https://doi.org/10.1038/s44161-024-00566-1
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44161-024-00566-1
This article is cited by
-
Impacts of aging and fluid shear stress on vascular endothelial metabolism and atherosclerosis development
Journal of Biomedical Science (2025)
-
Preclinical atherosclerosis and prediabetes: a cross-sectional metabolic assessment in apparently healthy population
Cardiovascular Diabetology (2025)
-
Overlapping risk factors and pathogenic mechanisms in lung cancer and cardiovascular disease
Discover Oncology (2025)
-
Slc7a7 licenses macrophage glutaminolysis for restorative functions in atherosclerosis
Nature Metabolism (2025)
-
Glutamine–glutamate imbalance in the pathogenesis of cardiovascular disease
Nature Cardiovascular Research (2024)