Abstract
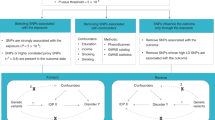
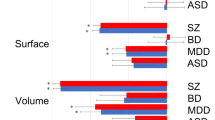
Brain cortical morphology, indexed by its surface area and thickness, is known to be highly heritable. Previous research has suggested a relationship of cortical morphology with several neuropsychiatric phenotypes. However, the multitude of potential confounders makes it difficult to establish causal relationships. Here we employ generalized summary-data-based Mendelian randomization and a series of sensitivity analyses to investigate causal links between 70 cortical morphology measures and 199 neuropsychiatric, behavioral and metabolic phenotypes. We show that total brain cortical surface area (TSA) has significant positive causal effects on 18 phenotypes. The strongest effects include TSA positively influencing cognitive performance, while reverse analyses reveal small effects of cognitive performance on TSA. Global mean cortical thickness (MTH) exhibits significant causal effects on five phenotypes, including schizophrenia. MTH reduces schizophrenia risk, and bidirectional causality is found between MTH and smoking initiation. Finally, in regional analyses, we detect positive influences of the transverse temporal surface area on cognitive performance and negative influences of transverse temporal thickness on schizophrenia risk. Overall, our results highlight bidirectional associations between TSA, MTH and neuropsychiatric traits. These insights offer potential avenues for intervention studies aimed at improving brain health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All results of data generated and analyzed during this study are included in Supplementary Information and Supplementary Data Tables 1–11. These files provide the complete dataset necessary to interpret, verify and extend the research presented in the Article. For any additional information or access to specific datasets beyond those provided, reasonable requests can be made to the corresponding author.
Code availability
The code used for data analysis is available via GitHub (https://github.com/Bochao1/Brian_NMH).
References
Schmitt, J. E. et al. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cereb. Cortex 18, 1737–1747 (2008).
Panizzon, M. S. et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb. Cortex 19, 2728–2735 (2009).
Patel, Y. et al. Virtual histology of cortical thickness and shared neurobiology in 6 psychiatric disorders. JAMA Psychiatry 78, 47–63 (2021).
Hettwer, M. D. et al. Coordinated cortical thickness alterations across six neurodevelopmental and psychiatric disorders. Nat. Commun. 13, 6851 (2022).
Hibar, D. P. et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry 23, 932–942 (2018).
Lorenzetti, V., Chye, Y., Silva, P., Solowij, N. & Roberts, C. A. Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur. Arch. Psychiatry Clin. Neurosci. 269, 59–71 (2019).
Momenan, R. et al. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. Neuroimaging 204, 101–111 (2012).
Vita, A., De Peri, L., Deste, G., Barlati, S. & Sacchetti, E. The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A meta-analysis and meta-regression of longitudinal magnetic resonance imaging studies. Biol. Psychiatry 78, 403–412 (2015).
Henderson, D. C. Schizophrenia and comorbid metabolic disorders. J. Clin. Psychiatry 66, 11–20 (2005).
Richmond, R. C. & Davey Smith, G. Mendelian randomization: concepts and scope. Cold Spring Harb. Perspect. Med. 12, a040501 (2022).
Voineskos, A. N. et al. Effects of antipsychotic medication on brain structure in patients with major depressive disorder and psychotic features: neuroimaging findings in the context of a randomized placebo-controlled clinical trial. JAMA Psychiatry 77, 674–683 (2020).
Minzenberg, M. J. & Leuchter, A. F. The effect of psychotropic drugs on cortical excitability and plasticity measured with transcranial magnetic stimulation: implications for psychiatric treatment. J. Affect. Disord. 253, 126–140 (2019).
Guo, J. et al. Mendelian randomization analyses support causal relationships between brain imaging-derived phenotypes and risk of psychiatric disorders. Nat. Neurosci. 25, 1519–1527 (2022).
Mavromatis, L. A. et al. Association between brain structure and alcohol use behaviors in adults: a Mendelian randomization and multiomics study. JAMA Psychiatry 79, 869–878 (2022).
van der Meer, D. & Kaufmann, T. Mapping the genetic architecture of cortical morphology through neuroimaging: progress and perspectives. Transl. Psychiatry 12, 447 (2022).
Goldman-Rakic, P. S. Topography of cognition: parallel distributed networks in primate association cortex. Annu. Rev. Neurosci. 11, 137–156 (1988).
Buckner, R. L. & Krienen, F. M. The evolution of distributed association networks in the human brain. Trends Cogn. Sci. 17, 648–665 (2013).
Masand, P. S. et al. Metabolic and endocrine disturbances in psychiatric disorders: a multidisciplinary approach to appropriate atypical antipsychotic utilization. CNS Spectr. 10, 1–16 (2005).
Akhaury, K. & Chaware, S. Relation between diabetes and psychiatric disorders. Cureus 14, e30733 (2022).
Williams, J. A. et al. Inflammation and brain structure in schizophrenia and other neuropsychiatric disorders: a Mendelian randomization study. JAMA Psychiatry 79, 498–507 (2022).
Kang, S. et al. Altered cortical thickness, degree centrality, and functional connectivity in middle-age type 2 diabetes mellitus. Front. Neurol. 13, 939318 (2022).
Veit, R. et al. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin. 6, 307–311 (2014).
Kiltschewskij, D. J., Reay, W. R. & Cairns, M. J. Evidence of genetic overlap and causal relationships between blood-based biochemical traits and human cortical anatomy. Transl. Psychiatry 12, 373 (2022).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383 (2014).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Zhu, Z. et al. Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat. Commun. 9, 224 (2018).
Grasby, K. L. et al. The genetic architecture of the human cerebral cortex. Science 367, eaay6690 (2020).
Thompson, P. M. et al. ENIGMA and global neuroscience: a decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry 10, 100 (2020).
Lee, J. J. et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat. Genet. 50, 1112–1121 (2018).
Okbay, A. et al. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat. Genet. 54, 437–449 (2022).
Wood, A. R. et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat. Genet. 46, 1173–1186 (2014).
Lee, P. H. et al. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 179, 1469–1482.e11 (2019).
Saunders, G. R. B. et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature 612, 720–724 (2022).
Graham, S. E. et al. The power of genetic diversity in genome-wide association studies of lipids. Nature 600, 675–679 (2021).
Stahl, E. A. et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 51, 793–803 (2019).
Liu, M. et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 51, 237–244 (2019).
Wightman, D. P. et al. A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nat. Genet. 53, 1276–1282 (2021).
Demontis, D. et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51, 63–75 (2019).
Cai, L. et al. Genome-wide association analysis of type 2 diabetes in the EPIC-InterAct study. Sci. Data 7, 393 (2020).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Pardiñas, A. F. et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 50, 381–389 (2018).
Ruderfer, D. M. et al. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 173, 1705–1715.e16 (2018).
Kettunen, J. et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat. Commun. 7, 11122 (2016).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206 (2015).
Slob, E. A. W. & Burgess, S. A comparison of robust Mendelian randomization methods using summary data. Genet. Epidemiol. 44, 313–329 (2020).
Xue, A. et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 9, 2941 (2018).
Pettersson, E. et al. Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls. Psychol. Med. 49, 1166–1173 (2019).
Hofer, E. et al. Genetic correlations and genome-wide associations of cortical structure in general population samples of 22,824 adults. Nat. Commun. 11, 4796 (2020).
Rakic, P. Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci. 10, 724–735 (2009).
Winkler, A. M. et al. Measuring and comparing brain cortical surface area and other areal quantities. Neuroimage 61, 1428–1443 (2012).
de Moraes, F. H. P., Mello, V. B. B., Tovar-Moll, F. & Mota, B. Establishing a baseline for human cortical folding morphological variables: a multisite study. Front. Neurosci. 16, 897226 (2022).
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
Elvsåshagen, T. et al. Bipolar II disorder is associated with thinning of prefrontal and temporal cortices involved in affect regulation. Bipolar Disord. 15, 855–864 (2013).
Maller, J. J., Thaveenthiran, P., Thomson, R. H., McQueen, S. & Fitzgerald, P. B. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J. Affect. Disord. 169, 118–127 (2014).
Guzman-Parra, J. et al. Clinical and genetic differences between bipolar disorder type 1 and 2 in multiplex families. Transl. Psychiatry 11, 31 (2021).
Van Rheenen, T. E. et al. Increased cortical surface area but not altered cortical thickness or gyrification in bipolar disorder following stabilisation from a first episode of mania. Prog. Neuropsychopharmacol. Biol. Psychiatry 122, 110687 (2023).
Hanford, L. C., Nazarov, A., Hall, G. B. & Sassi, R. B. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 18, 4–18 (2016).
Cheng, W. et al. Genetic association between schizophrenia and cortical brain surface area and thickness. JAMA Psychiatry 78, 1020–1030 (2021).
Westlye, L. T., Alnæs, D., van der Meer, D., Kaufmann, T. & Andreassen, O. A. Population-based mapping of polygenic risk for schizophrenia on the human brain: new opportunities to capture the dimensional aspects of severe mental disorders. Biol. Psychiatry 86, 499–501 (2019).
Alnæs, D. et al. Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry 76, 739–748 (2019).
Neilson, E. et al. Impact of polygenic risk for schizophrenia on cortical structure in UK Biobank. Biol. Psychiatry 86, 536–544 (2019).
Chen, X. et al. Reduced cortical thickness in right Heschl’s gyrus associated with auditory verbal hallucinations severity in first-episode schizophrenia. BMC Psychiatry 15, 152 (2015).
Hubl, D. et al. Structural analysis of Heschl’s gyrus in schizophrenia patients with auditory hallucinations. Neuropsychobiology 61, 1–9 (2010).
Shinn, A. K., Baker, J. T., Cohen, B. M. & Ongür, D. Functional connectivity of left Heschl’s gyrus in vulnerability to auditory hallucinations in schizophrenia. Schizophr. Res. 143, 260–268 (2013).
Seiferth, N. Y. et al. Increased neural response related to neutral faces in individuals at risk for psychosis. Neuroimage 40, 289–297 (2008).
Hoogman, M. et al. Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. Am. J. Psychiatry 176, 531–542 (2019).
Wu, B. S. et al. Cortical structure and the risk for Alzheimer’s disease: a bidirectional Mendelian randomization study. Transl. Psychiatry. 11, 476 (2021).
Dickerson, B. C. et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 19, 497–510 (2009).
Schaer, M., Bach Cuadra, M., Thiran, J. & Eliez, S. Determinants of cortical gray matter volume: hypothesis based on developmental cohorts with normal and abnormal cortical morphology. Infoscience http://infoscience.epfl.ch/record/90990 (2006).
Weissenborn, R. & Duka, T. Acute alcohol effects on cognitive function in social drinkers: their relationship to drinking habits. Psychopharmacology 165, 306–312 (2003).
Courtney, K. E. & Polich, J. Binge drinking in young adults: data, definitions, and determinants. Psychol. Bull. 135, 142–156 (2009).
Menary, K. et al. Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence 41, 597–606 (2013).
Galovic, M. et al. Resective surgery prevents progressive cortical thinning in temporal lobe epilepsy. Brain 143, 3262–3272 (2021).
Shaw, M. E., Abhayaratna, W. P., Sachdev, P. S., Anstey, K. J. & Cherbuin, N. Cortical thinning at midlife: the PATH through life study. Brain Topogr. 29, 875–884 (2016).
Gu, Y. et al. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 85, 1744–1751 (2015).
Bashir, S. et al. Physical exercise and cortical thickness in healthy controls: a pilot study. Eur. Rev. Med. Pharmacol. Sci. 25, 7375–7379 (2021).
Lee, J. S. et al. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci. Rep. 6, 24284 (2016).
Karama, S. et al. Cigarette smoking and thinning of the brain’s cortex. Mol. Psychiatry 20, 778–785 (2015).
Durazzo, T. C., Meyerhoff, D. J. & Yoder, K. K. Cigarette smoking is associated with cortical thinning in anterior frontal regions, insula and regions showing atrophy in early Alzheimer’s Disease. Drug Alcohol Depend. 192, 277–284 (2018).
Gómez-Apo, E., Mondragón-Maya, A., Ferrari-Díaz, M. & Silva-Pereyra, J. Structural brain changes associated with overweight and obesity. J. Obes. 2021, 6613385 (2021).
Sharkey, R. J., Karama, S. & Dagher, A. Overweight is not associated with cortical thickness alterations in children. Front. Neurosci. 9, 24 (2015).
Ruipérez, V., Darios, F. & Davletov, B. Alpha-synuclein, lipids and Parkinson’s disease. Prog. Lipid Res. 49, 420–428 (2010).
Spiga, F. et al. Tools for assessing quality and risk of bias in Mendelian randomization studies: a systematic review. Int. J. Epidemiol. 52, 227–249 (2023).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Br. Med. J. 362, k601 (2018).
Mullins, N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241 (2015).
Lin, B. D. et al. Nongenetic factors associated with psychotic experiences among UK Biobank participants: exposome-wide analysis and Mendelian randomization analysis. JAMA Psychiatry 79, 857–868 (2022).
Choi, K. W. et al. An exposure-wide and Mendelian randomization approach to identifying modifiable factors for the prevention of depression. Am. J. Psychiatry 177, 944–954 (2020).
Yang, J., Lee, S. H., Goddard, M. E. & Visscher, P. M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 88, 76–82 (2011).
Luykx, J. J. & Lin, B. D. Are psychiatric disorders risk factors for COVID-19 susceptibility and severity? A two-sample, bidirectional, univariable, and multivariable Mendelian randomization study. Transl. Psychiatry 11, 210 (2021).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, e34408 (2018).
R Core Team. R: a language and environment for statistical computing v.4.0.4 (R Foundation for Statistical Computing, 2020).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665 (2013).
Burgess, S. & Thompson, S. G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32, 377–389 (2017).
Bowden, J., Smith, G. D. & Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525 (2015).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314 (2016).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698 (2018).
Mowinckel, A.M. & Vidal-Piñeiro, D. Visualization of brain statistics with R packages ggseg and ggseg3d. Adv. Methods Pract. Psychol. Sci. 4, 466–483 (2020).
Acknowledgements
We acknowledge the authors of the original GWAS studies who shared (either publicly or privately) the GWAS summary statistics, encouraging data accessibility and thus scientific collaboration. D.v.d.M. is funded by the Research Council of Norway (no. 324252). Y.L. is funded by the Natural Science Foundation of Gansu Province, China (no. 22JR5RA728). K.L.G. is funded by the National Health and Medical Research Council (APP1173025). S.G., B.P.F.R. and B.D.L. are supported by the YOUTH-GEMs project, funded by the European Union’s Horizon Europe program under grant agreement number 101057182. No funding was provided to carry out this work.
Author information
Authors and Affiliations
Contributions
B.D.L., Y.L. and A.A.G. wrote the first draft. J.J.L. conceived the study and conducted project management. B.D.L. conceptualized the data analysis plan, performed the data analysis and drafted the methods and results sections. Y.L. and A.A.G. contributed to data interpretation and paper drafting. Y.L. wrote sections of the discussion, while A.A.G. wrote sections of the introduction. X.C., K.L.G., S.M., O.A.A., B.P.F.R. and S.G. provided critical feedback on the methodology and statistical analysis. D.v.d.M. and J.J.L., who share senior authorship, oversaw the entire project, contributed to the study design and finalized the paper. All authors reviewed and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Shinichiro Luke Nakajima and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7, Tables 1–4, Methods and Results.
Supplementary Data Tables
Supplementary Data Tables 1–11 and a README sheet to explain the contents.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, B.D., Li, Y., Goula, A.A. et al. Dissecting causal relationships between cortical morphology and neuropsychiatric disorders: a bidirectional Mendelian randomization study. Nat. Mental Health 3, 613–625 (2025). https://doi.org/10.1038/s44220-025-00397-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00397-4