Abstract
Brain capital, broadly defined as a form of capital that prioritizes brain skills and brain health, is urgently required. Integrating social, emotional and cognitive brain resources is a great asset for a wealthy and healthy society. Nevertheless, there is little investment in women’s brain health on a global scale. Women, on average, spend nine additional years in poor health compared with men, which hinders their participation in education, the workforce and society at large. This Perspective highlights the crucial intersection between investing in women’s brain health and the concept of ‘brain capital.’ Here we argue that addressing the women’s health gap could potentially increase the global economy by US $1 trillion in annual incremental gross domestic product. Furthermore, we hope this article will serve as a springboard to stimulate discussion and concrete stakeholder actions toward closing the women’s brain health gap and will add to the growing discourse on sex- and gender-specific healthcare and its impact on global community well-being.
Similar content being viewed by others
Main
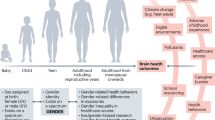
The world aspires to a future in which women can realize their cognitive, social and emotional potential and be their full selves at work, with their families and in the community, thriving with their brains and mental health. Investment in brain health remains critically underfunded despite its importance and potential. This shortfall is even more pronounced for women’s health, for which the lack of resources and attention worsens health disparities. That potential can fall short without a comprehensive understanding of the female brain and equitable investments in women’s brain health. Despite women living longer than men, they spend 25% more time in poor health on average. According to global data in 2019, a 60-year-old woman was expected to live 22.7 additional years, with only 73.1% of those years being in good health, compared with a man of the same age, who is expected to live 19.5 additional years, with 75.9% in good health1. Female individuals also experience higher rates of neurological and psychiatric disorders2 (Box 1) and are more likely to become caregivers3,4.
At the time of writing, none of the targets of the gender equality goal (Sustainable Development Goal 5) of the United Nations 2030 Agenda for Sustainable Development have been attained1,5,6,7. The gender equality goal entails achieving gender equality and empowering all women and girls by addressing barriers such as gender-based discrimination, violence and unequal access to healthcare, education and socioeconomic decision-making processes, which are critical for improving health outcomes. Overcoming the gap in women’s health would improve the quality of women’s lives, increase their workforce participation and create positive ripple effects for families and communities8. The economic impact resulting from expanded participation in the workforce, fewer early deaths, fewer health conditions and increases in productivity could generate at least US $1 trillion annual incremental gross domestic product (GDP) by 2040. Of this opportunity, brain health, including neurological and psychiatric disorders (International Classification of Diseases Tenth Edition (ICD-10)), accounts for nearly 25%, or US $250 billion (ref. 8).
Take, for example, depressive and anxiety disorders, largely prevalent among women of working age. Beyond direct and indirect medical costs, the associated reduced work engagement, absenteeism, presenteeism and high turnover suggest that individuals affected by depressive and anxiety disorders are not contributing effectively to the economy9. It has been estimated that reducing the burden of depressive disorders alone could contribute up to US $100 billion in GDP growth due to an increase in productivity, while improvement in anxiety disorders could add nearly US $50 billion (ref. 8) (Fig. 1).
The opportunity for brain health, encompassing psychiatric and neurological disorders, makes up nearly a quarter of the total GDP opportunity. Analysis made by building on the report published in 2024 by the World Economic Forum in collaboration with the McKinsey Health Institute. Analysis is based on an assessment of neurological disorders and psychiatric diseases burden, measured in disability adjusted life years in women in the USA, as estimated in the Global Burden of Disease dataset published by the Institute of Health Metrics and Evaluation (http://ghdx.healthdata.org/gbd-2021). Conditions were chosen as part of the 64 conditions from the original report on the basis of a ranking of conditions contributing the most to the female health burden globally (as measured in disability adjusted life years). To size the economic gap, we estimated the benefits of having a larger, healthier and more productive female labor force, which was used to project the annual potential GDP contribution to 2040. Neurological disorders (8%) and psychiatric disorders (16%) are combined into brain health. Brain health, at 25%, is the largest segment of conditions impacting the gender health gap. Note: figures do not sum to 100% because of rounding.
In this Perspective, building on the definition of ‘brain capital,’10,11 we propose the concept of women’s brain capital, focusing on what is economically lost if we overlook women’s brain health, including mental health and neurological disorders. Our aim is to provide evidence surrounding sex-specific and gender-specific health research and health-care investments, recognizing their profound impact on the well-being of communities globally. Beyond educating about this unmet medical need, we hope to stimulate discussions on the compelling business case for investing in women’s brain health across the entire lifespan and mobilizing leaders to action.
Women’s brains face unique challenges
While brain health is measured on a spectrum, diagnoses help communicate effectively about clusters of signs/symptoms that severely impact an individual’s functioning. We focus on research about diagnoses of neurological and psychiatric disorders as these are clearly defined by the ICD. More importantly, approximately half of the world’s population will grapple with a psychiatric disorder at some point in their life12. Similarly, neurological disorders have been reported to affect over 43% of the global population13.
Building evidence suggests sex differences in the prevalence and the signs/symptoms of psychiatric and neurological disorders. The definition of sex has been iterative over time, and it is typically a biological distinction between male and female individuals in humans and animals14. It is crucial to acknowledge the distinction between sex and gender definitions as they are not synonymous and are frequently misused15. Sex, typically determined by genetics (for example, females having XX chromosomes and males having XY chromosomes), and gender, a complex interplay of behavioral, social and personal identity factors, can serve as pivotal, independent or interacting determinants. In fact, as it is understood today, gender is multidimensional and includes cisgender and transgender identities, as well as non-binary identities. Cisgender refers to individuals whose gender identity aligns with their sex assigned at birth, whereas transgender describes individuals whose gender identity differs from their sex assigned at birth. Non-binary identities, which encompass a range of gender experiences beyond the male–female binary, include agender, bigender and gender fluid, among others. These distinctions emphasize the diversity and complexity of gender as a construct16.
Female individuals are more likely to experience migraines, multiple sclerosis, Alzheimer disease, eating disorders, depression and anxiety17. Furthermore, while autism spectrum disorders and attention deficit hyperactivity disorder are more prevalent among male individuals, female individuals present with different symptoms, which has historically led to underdiagnosis18,19. It is only recently that researchers have begun to dissect the underlying sex differences in psychiatric and neurological disorders20,21,22. Evidence has shown that fluctuations in sex hormones during menstruation are the major biological factors driving sex differences in anxiety and depression risk,23 with further influence on many brain disorders24,25,26,27,28. We argue that sex and gender are highly intertwined and can individually, in parallel or subsequently influence health outcomes,29,30 although the implications are beyond the scope of this Perspective. To ensure clarity, we strive to incorporate definitions of sex and gender and their use in this paper (Box 2).
Studies have also found sex differences in brain regions such as the amygdala, hippocampus and insula, known to be implicated in neurological disorders31,32. Sex-biased gene expression33 has an impact on cortical brain development, potentially leading to novel sex-specific underlying mechanisms. In fact, one study has shown causal genes with sex-differentiated or sex-biased protein expression34. Sex-specific mechanistic studies are essential for understanding underlying mechanisms, and future advancements in brain health depend on successful sex-specific research.
The women’s brain health gap is a multifaceted domain that extends beyond biological factors, with sex/gender having an essential role in shaping environments and experiences. For example, gender has a substantial role in children’s educational attainment. Girls and women from the most disadvantaged rural areas tend to have the lowest levels of educational attainment1. A low educational attainment increases the risk of neurological disorders such as Alzheimer disease and leads to a pervasive cycle of greater lifetime risk predisposition for dementia35. Furthermore, this pervasive cycle can, in turn, lead to reduced access to health-care systems later in life and heightened chances of negative health outcomes30, as well as reduced employment, lower income and increased caregiver burden3,4.
Finding ways to advance women’s brain health is crucial for a better future for everyone. By addressing women-specific health factors, we can reduce misdiagnosis or late diagnosis generated by biases permeating medicine and therefore enhance women’s brain health, as well as life and career trajectories. This strengthens families, communities and society by reducing health burdens and increasing economic participation, among other benefits.
However, one of the longstanding challenges is the persistent gap in data in which an individual’s sex juxtaposes with their gender at birth or in which data regarding sex at birth have been altered to align with a gender identity and sex at birth is no longer disclosed. At the time of writing, there is limited evidence disentangling the individual or synergistic effects of sex and gender. This includes evaluating the intersection of gender and brain health, particularly for non-cisgender individuals, including transgender and non-binary identities.
Women’s brain health gap
Although there is much to rejoice about regarding women’s resilience (greater longevity, even in the face of disease, lower prevalence of cancer), the neglect of women’s health has led to the current translational gap in women’s brain health. Here we briefly highlight four core global reasons. First, there is a limited understanding of sex-based differences in brain health. Historically, the study of human biology used the male body,36,37 which created a gap in knowledge about mechanisms of disease development in the female body and, therefore, resulted in fewer and less effective treatments available for female individuals. For example, women make up 75% of the population affected by migraines, and evidence suggests differences attributed to sex or gender;38 however, there has been a notable lack of research into understanding the nature of these differences and their clinical implications39. Migraines are likely influenced by gonadal hormones specific to the female sex38,40,41. In fact, migraines tend to appear around puberty, change during pregnancy, coincide with menstruation in more than 50% of women and worsen often during perimenopause42,43. Findings of magnetic resonance imaging studies show that among individuals who suffer from migraines, structural and functional differences between the male and female brain exist, including differences in cortical thickness and connectivity of regions involved in pain perception, interoception and emotional processing. This evidence has been interpreted as indication of a greater association of migraines with pain and disability among women40,41,44. Therapeutics such as combination therapy for migraines with triptan plus acetylsalicylic acid and non-steroidal anti-inflammatory drugs show 22% lower effectiveness in women than in men45. Exploring sex- and gender-specific solutions for migraines could potentially reduce or eliminate suffering, providing a profound advancement in precision medicine for brain health.
Second, data gaps can lead to the underestimation of women’s health burden. In addition to the case of Alzheimer disease (for details, see refs. 46,47,48,49,50), two examples of such data gaps are the neglected investigations of hormonal transitioning phases and the X chromosome transcriptome in brain health studies. Although recent studies pointed out changes in whole-brain dynamics across the menstrual cycle and women’s lifespan51,52,53 and sex steroid hormones as powerful modulators of learning and memory54, less than 0.5% of the neuroimaging literature considers hormonal transition phases, such as the influence of hormonal contraceptives, pregnancy and menopause55,56.
Another example of under-collected data is the X chromosome transcriptome, which makes up a meaningful part of the genome in both males and females but is frequently excluded from genome-wide association studies and DNA methylation arrays57 because of the complex statistical analysis in bioinformatics pipelines58. Less than half of the genes associated with human pathology on the X chromosome are currently known, and many more are yet to be clinically characterized59. Therefore, limited understanding exists regarding the role of sex chromosomes in brain health and beyond37. The under-collection of data on these variables that are crucial to women’s health can lead to overlooking effective starting points for treatments tailored to women, for example, hormone replacement therapy for menopausal women experiencing migraines60.
The third reason is the sex-/gender-based barriers to brain health-care delivery and equitable healthcare. Anyone seeking treatment for brain disorders may face discrimination, limited access or biased care. Women, however, historically encounter additional sex- and gender-specific obstacles. The outdated concept of hysteria, rooted in ancient misconceptions about a ‘wandering uterus,’ perpetuated stereotypes and dismissed women’s signs/symptoms, labeling them as hysterical61. Post-partum depression remains stigmatized, often overshadowed by societal expectations of motherhood. Anxiety disorders, too, are subject to gendered stereotypes, with women unfairly labeled as naturally fearful, undermining the recognition of anxiety as a legitimate and treatable condition. Urgency in addressing these issues is reflected in reported statistics showing that 71% of worldwide anxiety disorders could be avoided with effective prevention and optimal treatment, underlining the need for urgent action13,17. Furthermore, different dimensions of prenatal maternal distress potentially contribute in a cross-generational way, shaping infant brain and behavior62,63. This underscores the critical need to confront bias to ensure equitable access to brain healthcare for women across the lifespan.
In addition, socioeconomic barriers such as limited finances, lack of insurance and caregiving duties can impede women’s access to healthcare. Women may be especially affected during their childbearing years as many treatments for brain disorders are not fully compatible with pregnancy and breastfeeding64,65. Reproductive psychiatrists, who focus on mental health of women during reproductive years, remain inaccessible to many. Last, sex bias in diagnostics may overlook women’s signs/symptoms, frequently attributing them to hormones and therefore dismissing their seriousness. While hormones and hormonal fluctuations influence brain health and are, for example, a major biological driver for migraines66, this should not justify ineffective treatment. Women in historically underrepresented or vulnerable populations may face an additional challenge in accessing the health-care system, demonstrating the intersectional nature of brain health equity. For example, studies indicate that Black women are less likely than their white counterparts to seek treatment for psychiatric disorders67,68. Investing in women’s brain capital requires developing the systems and foundation to support all women in getting access to equitable and comprehensive care.
Fourth, low investment in women’s health limits the scale of innovation. For example, although women are two to three times more likely than men to be affected by migraines, just 37% of US National Institutes of Health (NIH) funding in migraine research in 2019 was directed toward understanding sex-specific differences69. Moreover, research consistently highlights the profound impact of investing in brain health, revealing a remarkable return of US $4 for every $1 invested globally69. Improved brain health not only enhances health and productivity but also alleviates burdens on both individuals and society. To capture brain health opportunities, we must re-evaluate our investment strategies, which involves not only allocating adequate resources but also incentivizing and de-risking investments specifically targeted toward women’s brain health. The NIH mandates that sex must be considered as a biological variable in all research grants. While it is essential to recognize areas for improvement, we also commend the strides the NIH has made in addressing these inequalities. The next step is to create an incentive system to report sex- and gender-stratified results of such funded research.
By fostering partnerships among government entities, academia, non-profits and the private sector, we can leverage our collective expertise and resources to maximize the impact of brain health investments. Creating investment funds that are specifically designed to achieve measurable social and health outcomes related to women’s brain health will help ensure accountability and allow us to track progress effectively. Standardized metrics will enable investors to assess the societal and health impacts of their investments, empowering them to make informed decisions and drive meaningful change. Most importantly, addressing the limited understanding of sex differences and the complex interplay of gender differences, as well as closing data gaps that undercount women’s health burdens, combating bias in health-care delivery and increasing investments, is essential to realizing the potential lost in the women’s brain health gap.
Global actions
Understanding the importance of brain capital throughout a woman’s life sheds light on the profound impact of sex-/gender-specific health factors. However, understanding and awareness are only the first steps to change. Every stakeholder in the health-care ecosystem has the opportunity to contribute to closing the gap and empowering women to optimize their brain health at all stages of life, reducing their burden of mental health and neurological disorders (Table 1). While it is essential to recognize areas for improvement, we acknowledge the needs of different countries, for which addressing educational inequalities and access to basic health-care rights is of monumental importance but also sits within an extremely complicated context of cultural norms and societal structures, which are usually highly gendered30. Therefore, global actions should be iterative and adapted to regional settings.
To close the women’s brain health gap, we propose four opportunity areas. The first is to destigmatize and raise awareness of psychiatric and neurological disorders across the lifespan. This involves increasing societal awareness of stigmas and women-specific challenges related to psychiatric and neurological disorders. This could include targeted education campaigns at schools and workplaces and among decision makers. It also means specifically destigmatizing the notion that women’s hormonal changes throughout their lives are legitimate health risks. This can be tackled by promoting a holistic understanding of the impact of hormone cycles and hormone transitions on brain health. Furthermore, it is crucial to advocate the inclusion of sex-/gender-specific brain health topics in medical school curricula to ensure health-care providers are equipped to address the unique needs of women.
The second opportunity area is to de-bias the brain health-care delivery system at large and for women. This could be aided by implementing training for health-care providers to recognize and address gender biases in diagnosis, treatment and referral practices. It is also important to develop clinical guidelines that consider sex/gender differences in psychiatric and neurological disorders, ensuring equitable access to diagnosis and treatment for women. In addition, increasing the representation of women in clinical trials and research studies ensures that findings are applicable to diverse populations of women.
The third opportunity area is to implement policies that advance women’s brain health. This could include advocating the integration of comprehensive coverage for brain disorders within health-care plans, encompassing regular screenings and interventions. It is important to emphasize the inclusion of treatments compatible with different stages of a woman’s life, including pregnancy and breastfeeding. Employers should be encouraged to create workplace policies that support individuals impacted by psychiatric and neurological disorders or those caring for affected loved ones. Promoting flexible work arrangements can alleviate employees’ psychological stress, which women have indicated is a top priority70. Allocating government funds for sex-/gender-specific research on brain health and amplifying current academic policies that include sex as a biological variable in preclinical and clinical brain health studies is crucial.
The fourth opportunity area is to invest in women’s brain health. This includes, but is not limited to, offering national and region-specific funding for understanding sex differences in preclinical research, emphasizing a lifespan approach. It is also beneficial to foster an ecosystem of public and private investors interested in funding women’s brain health to share knowledge, promote collaborations and provide information about the progress and return on investments in women’s brain health. Providing specific grants and accelerator programs for start-ups focusing on developing diagnostic tools, protocols and therapies tailored to women’s brain health is another important consideration.
How can investments in women’s brain capital be implemented across the lifespan?
Building on the economic benefits gained from reducing the burden of psychiatric and neurological disorders among women, we encourage a broader perspective on women’s brain health across the lifespan. We seek to place women’s brain capital at the center of a new narrative that includes young and late-life brain capital71,72. We have laid out initial global recommendations on how investments in women’s brain capital can be implemented across the life course (Table 2). Engaging with local communities to ensure that the proposed solutions and indicators are culturally sensitive and feasible within the local context is warranted.
Furthermore, we envision that women’s brain capital over the course of a lifetime can be tailored for each psychiatric and neurological disorder. For example, given the high risk of depression associated with a family history of depression, it may be possible to determine which young girls are at elevated risk and study their psychobiological functioning73. Thus, by identifying known sex-/gender-specific risks of depression, there is an opportunity to intervene earlier in the life course. Investing in a young girl’s brain health through a multi-pronged strategy to prevent depression could improve brain capital in her life and in generations to follow. More concretely, this could include increasing depression screening during the pregnancy period and developing personalized detection, prevention and intervention programs for girls at higher risk of depression. In addition, brain capital emphasizes a focus on the brain in society, recognizing that brain health is not improved merely through clinical interventions. It is important to develop strategies to reduce depression in women through education and community systems.
Another brain disorder that will benefit from being considered from a lifespan perspective is dementia. While it contributes to a relatively small percentage of the calculated GDP women’s health gap, in 2021, 64% of the 51.6 million people affected by dementia globally were women74. Similar to other psychiatric and neurological disorders, sex accounts for notable heterogeneity of dementia signs/symptoms, and biological sex differences such as gonadal hormones, hormone cycles and sex chromosomes may be the underlying cause2,20,37,75. Many have extensively examined the effects of menopausal hormone therapy and its implications for dementia76. However, we recognize that the relationship between gonadal hormones, hormone cycles and dementia remains a topic of debate, with only weak evidence linking endogenous circulating estrogens to Alzheimer disease biomarkers, which is beyond the scope of this Perspective. Still, roughly 4% of the NIH budget dedicated to Alzheimer disease research was allocated to women-specific research in 2019 (ref. 77). Dementia is known for its strong impact on the affected individual’s family: in the United States, nearly half (48%) of all informal caregivers of older adults care for someone with dementia, for an average of 31 hours per week. One out of four dementia caregivers is a ‘sandwich generation’ caregiver, caring for both an aging parent and at least one child, and 41% have a household income of US $50,000 or less74. A meta-analysis showed that dementia caregivers are at higher risk of depression and anxiety compared with non-caregivers78 and experience more depressive signs/symptoms than non-dementia caregivers79. Recent research suggests that the caregiver burden is further amplified for women80,81,82,83,84, with additional variables (for example, sociodemographic variables, culture and ethnicity), requiring further study. Addressing the women’s health gap for dementia would substantially influence the lives of patients and informal caregivers, of whom nearly two thirds are women, who are currently reducing their working hours or quitting work completely to be able to care for affected family members.
A holistic understanding of brain health goes beyond the absence of disease, including efforts to improve overall cognitive clarity, resilience to stress, social skills, a sense of direction and life satisfaction. A recent survey by the McKinsey Health Institute of over 30,000 employees across 30 countries revealed that women are more exhausted and experience poorer mental and spiritual health than men, putting them at higher risk of burnout. Globally, 46% of female and 38% of male participants reported symptoms of exhaustion. Slightly fewer female than male participants reported having good mental health (65% versus 70%, respectively) and good spiritual health (56% versus 61%, respectively)85. Employers can play a crucial role in driving markers of positive health and reducing markers of negative health (for example, burnout). For example, women who have the flexibility to work from their preferred location have 15% better mental health, 19% better spiritual health and 19% less exhaustion85. Employers are advised to view such workplace design changes as a fruitful investment. Employees who reported better overall health also tended to score higher in job performance and innovation85. One example of workplace investment is city-level interventions that take into account the diverse urban contexts to prioritize women’s brain health (Box 3). Investing in women’s brain capital and women’s health is an essential perspective that weaves together both psychiatric and neurological disorders with broader brain health—including burnout and resilience. By investing in women’s brain capital and women’s health, we can holistically strengthen brain skills and well-being (Fig. 1).
Conclusion
For far too long, women’s brain health has been totally neglected. The rising interest in sex- and gender-specific health factors of neurological and psychiatric disorders offers a great potential for improving women’s physical and mental health, discovering tailored ways to diagnose and treat these disorders and enabling women worldwide to increase their participation in workforce and community with notably decreased health burden. By closing the gap in women’s brain health, we unlock a brighter future for everyone. Furthermore, another compelling perspective is to frame women’s brain health as an integral part of women’s health as a whole. This approach not only underscores the holistic nature of health but also creates synergies that could potentially leverage greater investment opportunities. By integrating women’s brain health into the broader context of women’s health, stakeholders can address multiple health determinants simultaneously, thereby driving more comprehensive and impactful health interventions and funding initiatives. We encourage leaders across industries to tap into this potential with focused actions to increase understanding of and investment in women’s brain health (Box 4).
References
Progress on the Sustainable Development Goals: The Gender Snapshot 2023 (UN Women and United Nations Department of Economic and Social Affairs, 2023).
Patwardhan, V. et al. Differences across the lifespan between females and males in the top 20 causes of disease burden globally: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Public Health 9, e282–e294 (2024).
Stall, N. M., Shah, N. R. & Bhushan, D. Unpaid family caregiving—the next frontier of gender equity in a postpandemic future. JAMA Health Forum 4, e231310 (2023).
Sex, gender, and the cost of neurological disorders. Lancet Neurol. 22, 367 (2023).
Measures of Early-Life Brain Health at Population Level: Report of a Technical Meeting (WHO, 2023).
Optimizing Brain Health Across the Life Course: WHO Position Paper (WHO, 2022).
Winter, S. F. et al. National plans and awareness campaigns as priorities for achieving global brain health. Lancet Glob. Health 12, e697–e706 (2024).
Ellingrud, K., Pérez, L., Petersen, A. & Sartori, V. Closing the Women’s Health Gap: A $1 Trillion Opportunity to Improve Lives and Economies (McKinsey & Company, 2024); https://www.mckinsey.com/mhi/our-insights/closing-the-womens-health-gap-a-1-trillion-dollar-opportunity-to-improve-lives-and-economies#/
Deady, M. et al. The impact of depression, anxiety and comorbidity on occupational outcomes. Occup. Med. 72, 17–24 (2022).
Smith, E. et al. A brain capital grand strategy: toward economic reimagination. Mol. Psychiatry 26, 3–22 (2021).
Eyre, H. A., Hynes, W., Ayadi, R., Manes, F. & Swieboda, P. Brain capital is crucial for global sustainable development. Lancet Neurol. 23, 233–235 (2024).
McGrath, J. J. et al. Age of onset and cumulative risk of mental disorders: a cross-national analysis of population surveys from 29 countries. Lancet Psychiatry 10, 668–681 (2023).
Steinmetz, J. D. et al. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 23, 344–381 (2024).
Heidari, S., Babor, T. F., De Castro, P., Tort, S. & Curno, M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 1, 2 (2016).
Madsen, T. E. et al. Sex- and gender-based medicine: the need for precise terminology. Gend. Genome https://doi.org/10.1089/gg.2017.0005 (2017).
Hyde, J. S., Bigler, R. S., Joel, D., Tate, C. C. & van Anders, S. M. The future of sex and gender in psychology: five challenges to the gender binary. Am. Psychol. 74, 171–193 (2019).
GBD Results (IHME, 2021); https://vizhub.healthdata.org/gbd-results/
Nussbaum, N. L. ADHD and female specific concerns. J. Atten. Disord. 16, 87–100 (2012).
Attoe, D. E. & Climie, E. A. Miss. Diagnosis: a systematic review of ADHD in adult women. J. Atten. Disord. 27, 645–657 (2023).
Ferretti, M. T. et al. Sex differences in Alzheimer disease—the gateway to precision medicine. Nat. Rev. Neurol. 14, 457–469 (2018).
Woolley, C. S. His and hers: sex differences in the brain. Cerebrum 2021, cer-02-21 (2021).
Hentzen, N. B. et al. Mapping of European activities on the integration of sex and gender factors in neurology and neuroscience. Eur. J. Neurol. 29, 2572–2579 (2022).
Kundakovic, M. & Rocks, D. Sex hormone fluctuation and increased female risk for depression and anxiety disorders: from clinical evidence to molecular mechanisms. Front. Neuroendocrinol. 66, 101010 (2022).
Roeder, H. J. & Leira, E. C. Effects of the menstrual cycle on neurological disorders. Curr. Neurol. Neurosci. Rep. 21, 34 (2021).
Handy, A. B., Greenfield, S. F., Yonkers, K. A. & Payne, L. A. Psychiatric symptoms across the menstrual cycle in adult women: a comprehensive review. Harv. Rev. Psychiatry 30, 100–117 (2022).
McCarthy, M. M. Sex differences in the developing brain as a source of inherent risk. Dialogues Clin. Neurosci. 18, 361–372 (2016).
McCarthy, M. M. Multifaceted origins of sex differences in the brain. Phil. Trans. R. Soc. B 371, 20150106 (2016).
McCarthy, M. M., Arnold, A. P., Ball, G. F., Blaustein, J. D. & De Vries, G. J. Sex differences in the brain: the not so inconvenient truth. J. Neurosci. 32, 2241–2247 (2012).
Quintana, G. R. & Pfaus, J. G. Do sex and gender have separate identities? Arch. Sex. Behav. 53, 2957–2975 (2024).
Baez, S., Castro-Aldrete, L., Britton, G., Ibañez, A. & Santuccione-Chadha, A. Enhancing brain health in the Global South through a sex and gender lens. Nat. Ment. Health https://doi.org/10.1038/s44220-024-00339-6 (2024).
Ruigrok, A. N. V. et al. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 39, 34–50 (2014).
Giedd, J. N., Raznahan, A., Mills, K. L. & Lenroot, R. K. Review: magnetic resonance imaging of male/female differences in human adolescent brain anatomy. Biol. Sex Differ. 3, 19 (2012).
Kang, H. J. et al. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489 (2011).
Wingo, A. P. et al. Sex differences in brain protein expression and disease. Nat. Med. 29, 2224–2232 (2023).
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
Mazure, C. M. & Jones, D. P. Twenty years and still counting: including women as participants and studying sex and gender in biomedical research. BMC Womens Health 15, 94 (2015).
Castro-Aldrete, L. et al. Sex and gender considerations in Alzheimer’s disease: the Women’s Brain Project contribution. Front. Aging Neurosci. 15, 1105620 (2023).
Maleki, N. et al. Her versus his migraine: multiple sex differences in brain function and structure. Brain 135, 2546–2559 (2012).
Eisenstein, M. Closing the gender gap in migraine research. Nature 586, S16–S17 (2020).
Liu, J. et al. Gender-related differences in the dysfunctional resting networks of migraine suffers. PLoS ONE 6, e27049 (2011).
Granella, F. et al. Migraine without aura and reproductive life events: a clinical epidemiological study in 1300 women. Headache 33, 385–389 (1993).
Delaruelle, Z. et al. Male and female sex hormones in primary headaches. J. Headache Pain 19, 117 (2018).
Vetvik, K. G., MacGregor, E. A., Lundqvist, C. & Russell, M. B. Prevalence of menstrual migraine: a population-based study. Cephalalgia 34, 280–288 (2014).
Clayton, J. A. Sex influences in neurological disorders: case studies and perspectives. Dialogues Clin. Neurosci. 18, 357–360 (2016).
Petersen, A., Pérez, L., Herbig, B. & Tatwawadi, P. Bridging the Womens Health Gap: A Country-Level Exploration (McKinsey & Company, 2024); https://www.mckinsey.com/mhi/our-insights/bridging-the-womens-health-gap-a-country-level-exploration
Arenaza-Urquijo, E. M. et al. Sex and gender differences in cognitive resilience to aging and Alzheimer’s disease. Alzheimers Dement. 20, 5695–5719 (2024).
Ferretti, M. T. et al. Sex and gender differences in Alzheimer’s disease: current challenges and implications for clinical practice. Eur. J. Neurol. 27, 928–943 (2020).
Rosende-Roca, M. et al. The role of sex and gender in the selection of Alzheimer patients for clinical trial pre-screening. Alzheimers Res. Ther. 13, 95 (2021).
Mielke, M. M. Sex and gender differences in Alzheimer’s disease dementia. Psychiatr. Times 35, 14–17 (2018).
Rahman, A. et al. Sex and gender driven modifiers of Alzheimer’s: the role for estrogenic control across age, race, medical, and lifestyle risks. Front. Aging Neurosci. 11, 315 (2019).
Pletzer, B., Harris, T.-A., Scheuringer, A. & Hidalgo-Lopez, E. The cycling brain: menstrual cycle related fluctuations in hippocampal and fronto-striatal activation and connectivity during cognitive tasks. Neuropsychopharmacology 44, 1867–1875 (2019).
Avila-Varela, D. S. et al. Whole-brain dynamics across the menstrual cycle: the role of hormonal fluctuations and age in healthy women. NPJ Womens Health 2, 8 (2024).
Zsido, R. G. et al. Ultra-high-field 7T MRI reveals changes in human medial temporal lobe volume in female adults during menstrual cycle. Nat. Ment. Health 1, 761–771 (2023).
Nerattini, M. et al. Systematic review and meta-analysis of the effects of menopause hormone therapy on risk of Alzheimer’s disease and dementia. Front. Aging Neurosci. 15, 1260427 (2023).
Taylor, C. M., Pritschet, L. & Jacobs, E. G. The scientific body of knowledge—whose body does it serve? A spotlight on oral contraceptives and women’s health factors in neuroimaging. Front. Neuroendocrinol. 60, 100874 (2021).
Jacobs, E. G. Only 0.5% of neuroscience studies look at women’s health. Here’s how to change that. Nature 623, 667 (2023).
Inkster, A. M., Wong, M. T., Matthews, A. M., Brown, C. J. & Robinson, W. P. Who’s afraid of the X? Incorporating the X and Y chromosomes into the analysis of DNA methylation array data. Epigenetics Chromatin 16, 1 (2023).
Wise, A. L., Gyi, L. & Manolio, T. A. eXclusion: toward Integrating the X chromosome in genome-wide association analyses. Am. J. Hum. Genet. 92, 643–647 (2013).
Leitão, E. et al. Systematic analysis and prediction of genes associated with monogenic disorders on human chromosome X. Nat. Commun. 13, 6570 (2022).
MacGregor, E. A. Migraine, menopause and hormone replacement therapy. Post Reprod. Health 24, 11–18 (2018).
Tasca, C., Rapetti, M., Carta, M. G. & Fadda, B. Women and hysteria in the history of mental health. Clinic. Pract. Epidemiol. Ment. Health 8, 110–119 (2012).
Tu, H.-F., Skalkidou, A., Lindskog, M. & Gredebäck, G. Maternal childhood trauma and perinatal distress are related to infants’ focused attention from 6 to 18 months. Sci. Rep. 11, 24190 (2021).
Scheinost, D., Spann, M. N., McDonough, L., Peterson, B. S. & Monk, C. Associations between different dimensions of prenatal distress, neonatal hippocampal connectivity, and infant memory. Neuropsychopharmacology 45, 1272–1279 (2020).
Armstrong, C. ACOG guidelines on psychiatric medication use during pregnancy and lactation. Am. Fam. Physician 78, 772–778 (2008).
Liu, C., Pace, S., Bromley, R. & Dobson, R. Exposure to medication for neurological disease in pregnancy—time to consider the long-term implications? EClinicalMedicine 63, 102157 (2023).
Todd, C., Lagman-Bartolome, A. M. & Lay, C. Women and migraine: the role of hormones. Curr. Neurol. Neurosci. Rep. 18, 42 (2018).
Anglin, D. M., Alberti, P. M., Link, B. G. & Phelan, J. C. Racial differences in beliefs about the effectiveness and necessity of mental health treatment. Am. J. Community Psychol. 42, 17–24 (2008).
Nelson, T., Shahid, N. N. & Cardemil, E. V. Do I really need to go and see somebody? Black women’s perceptions of help-seeking for depression. J. Black Psychol. 46, 263–286 (2020).
Chisholm, D. et al. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry 3, 415–424 (2016).
Krivkovich, A., Field, E., Yee, L., McConnell, M. & Smith, H. Women in the Workplace (McKinsey & Company, 2023); https://www.mckinsey.com/featured-insights/diversity-and-inclusion/women-in-the-workplace
Farina, F. R. et al. Young adult brain capital: a new opportunity for dementia prevention. J. Alzheimers Dis. 94, 415–423 (2023).
Dawson, W. D. et al. Investing in late-life brain capital. Innov. Aging 6, igac016 (2022).
Gotlib, I. H., Joormann, J. & Foland-Ross, L. C. Understanding familial risk for depression: a 25-year perspective. Perspect. Psychol. Sci. 9, 94–108 (2014).
2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 19, 1598–1695 (2023).
Pallier, P. N. et al. Chromosomal and environmental contributions to sex differences in the vulnerability to neurological and neuropsychiatric disorders: implications for therapeutic interventions. Prog. Neurobiol. 219, 102353 (2022).
Vinogradova, Y. et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. Br. Med. J. 374, n2182 (2021).
Report of the Advisory Committee on Research on Women’s Health: Fiscal Years 2019–2020 (Office of Research on Women’s Health, 2021).
Ma, M., Dorstyn, D., Ward, L. & Prentice, S. Alzheimers’ disease and caregiving: a meta-analytic review comparing the mental health of primary carers to controls. Aging Ment. Health 22, 1395–1405 (2018).
Sheehan, O. C. et al. Stress, burden, and well-being in dementia and nondementia caregivers: insights from the caregiving transitions study. Gerontologist 61, 670–679 (2021).
Ibáñez, A. et al. Dementia caregiving across Latin America and the Caribbean and brain health diplomacy. Lancet Healthy Longev. 2, e222–e231 (2021).
Liu, R., Chi, I. & Wu, S. Caregiving burden among caregivers of people with dementia through the lens of intersectionality. Gerontologist 62, 650–661 (2022).
Xiong, C. et al. Sex and gender differences in caregiving burden experienced by family caregivers of persons with dementia: a systematic review. PLoS ONE 15, e0231848 (2020).
Arbel, I., Bingham, K. S. & Dawson, D. R. A scoping review of literature on sex and gender differences among dementia spousal caregivers. Gerontologist 59, e802–e815 (2019).
Abken, E., Ferretti, M. T., Castro-Aldrete, L., Santuccione Chadha, A. & Tartaglia, M. C. The impact of informant-related characteristics including sex/gender on assessment of Alzheimer’s disease symptoms and severity. Front. Glob. Womens Health 5, 1326881 (2024).
Brassey, J., Herbig, B., Jeffery, B. & Ungerman, D. Reframing Employee Health: Moving Beyond Burnout to Holistic Health (McKinsey & Company, 2024); https://www.mckinsey.com/mhi/our-insights/reframing-employee-health-moving-beyond-burnout-to-holistic-health
Integrated Results and Resources Framework (IRRF) of UN-Women Strategic Plan (UN Women, 2022); https://www.unwomen.org/sites/default/files/Headquarters/Attachments/Sections/Library/Publications/2021/UN-Women-Strategic-Plan-2022-2025-Annex-01-Integrated-results-and-resources-framework-en.pdf
International Classification of Diseases Eleventh Revision (ICD-11) (WHO, 2022).
Owolabi, M. O. et al. Global synergistic actions to improve brain health for human development. Nat. Rev. Neurol. 19, 371–383 (2023).
Ibanez, A. & Zimmer, E. R. Time to synergize mental health with brain health. Nat. Ment. Health 1, 441–443 (2023).
McKinsey Health Institute. Brain Health https://www.mckinsey.com/mhi/focus-areas/brain-health
Eyre, H. A. et al. Building brain capital. Neuron 109, 1430–1432 (2021).
Crenshaw, K. Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanford Law Rev. 43, 1241 (1991).
Lock, S. L., Chura, L. R., Dilworth-Anderson, P. & Peterson, J. Equity across the life course matters for brain health. Nat. Aging 3, 466–468 (2023).
Ungerman, D. et al. How to Achieve Great Health for All? Start in Your City (McKinsey & Company, 2024); https://www.mckinsey.com/mhi/our-insights/how-to-achieve-great-health-for-all-start-in-your-city
Lange, K. W. Task sharing in psychotherapy as a viable global mental health approach in resource-poor countries and also in high-resource settings. Glob. Health J. https://doi.org/10.1016/j.glohj.2021.07.001 (2021).
Raviola, G., Naslund, J. A., Smith, S. L. & Patel, V. Innovative models in mental health delivery systems: task sharing care with non-specialist providers to close the mental health treatment gap. Curr. Psychiatry Rep. https://doi.org/10.1007/s11920-019-1028-x (2019).
Acknowledgments
We thank N. Camargo, E. Coe, K. Enomoto, L. Hartenstein, A. Kourti, K. Midden, A. P. Ternent and D. Sandill for insightful discussions on this Perspective.
Author information
Authors and Affiliations
Contributions
L.C.-A. conceived the paper. L.C.-A., M.G., L.P., E.S. and H.A.E. collected data for the article. L.C.-A. consolidated the first and final draft. L.C.-A., M.G., L.P. and E.S. contributed with substantial writing and discussion of all sections. M.B. contributed substantially to the finalization of the revised manuscript. H.A.E. and A.S.C. reviewed the manuscript before submission. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.C.-A. was the scientific project manager of the Women’s Brain Foundation. M.B. is the scientific lead of the Women’s Brain Foundation. H.A.E. is an employee of Rice University’s Baker Institute for Public Policy; receives consulting fees from Meadows Mental Health Policy Institute and Kooth; has received speaking fees from Novo Nordisk, Roche and Lundbeck; and has received consulting fees from Novo Nordisk and ALTOIDA. A.S.C. is the co-founder and pro bono CEO of the Women’s Brain Foundation, and is also the pro bono Euresearch vice president.
Peer review
Peer review information
Nature Mental Health thanks Rachel Buckley, Andrea Winkler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castro-Aldrete, L., Greenfield, M., Smith, E. et al. Women’s brain health and brain capital. Nat. Mental Health 3, 488–497 (2025). https://doi.org/10.1038/s44220-025-00406-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00406-6