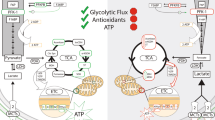
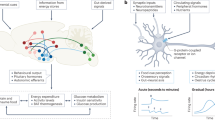
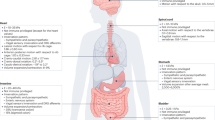
Abstract
Brain function is critically dependent on energy metabolism. Research over the past half century has provided many insights into energy production and utilization in the brain, and identified ways that it can be improved in brain disorders. A parallel literature indicates that major aberrations exist in brain bioenergetics in neuropsychiatric disorders. Targeting bioenergetic abnormalities may be an efficacious approach to treatment for these conditions. In this Perspective, we provide a survey of the relevant literature on these topics and sketch the outlines of a research agenda to maximize the impact of future research and treatment interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$79.00 per year
only $6.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Paulus, M. P. & Thompson, W. K. The challenges and opportunities of small effects: the new normal in academic psychiatry. JAMA Psychiatry 76, 353–354 (2019).
Abi-Dargham, A. et al. Candidate biomarkers in psychiatric disorders: state of the field. World Psychiatry 22, 236–262 (2023).
Freyberg, Z., Aslanoglou, D., Shah, R. & Ballon, J. S. Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front. Neurosci. 11, 432 (2017).
Pillinger, T., D'Ambrosio, E., McCutcheon, R. & Howes, O. D. Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol. Psychiatry 24, 776–794 (2019).
Kaul, I. et al. Efficacy and safety of xanomeline-trospium chloride in schizophrenia: a randomized clinical trial. JAMA Psychiatry 81, 749–756 (2024).
Bogenschutz, M. P. et al. Percentage of heavy drinking days following psilocybin-assisted psychotherapy vs placebo in the treatment of adult patients With alcohol use disorder: a randomized clinical trial. JAMA Psychiatry 79, 953–962 (2022).
Needham, N. et al. Pilot study of a ketogenic diet in bipolar disorder. BJPsych Open 9, e176 (2023).
Sethi, S. et al. Ketogenic diet intervention on metabolic and psychiatric health in bipolar and schizophrenia: a pilot trial. Psychiatry Res. 335, 115866 (2024).
Picard, M., McEwen, B. S., Epel, E. S. & Sandi, C. An energetic view of stress: focus on mitochondria. Front. Neuroendocrinol. 49, 72–85 (2018).
Shaulson, E. D., Cohen, A. A. & Picard, M. The brain–body energy conservation model of aging. Nat. Aging 4, 1354–1371 (2024).
Shulman, R. G., Hyder, F. & Rothman, D. L. Biophysical basis of brain activity: implications for neuroimaging. Q. Rev. Biophys. 35, 287–325 (2002).
Thulborn, K. R., Waterton, J. C., Matthews, P. M. & Radda, G. K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim. Biophys. Acta 714, 265–270 (1982).
Bobba-Alves, N., Juster, R. P. & Picard, M. The energetic cost of allostasis and allostatic load. Psychoneuroendocrinology 146, 105951 (2022).
Stein, A., Zhu, C., Du, F. & Ongur, D. Magnetic resonance spectroscopy studies of brain energy metabolism in schizophrenia: progression from prodrome to chronic psychosis. Curr. Psychiatry Rep. 25, 659–669 (2023).
Henkel, N. D. et al. Schizophrenia: a disorder of broken brain bioenergetics. Mol. Psychiatry 27, 2393–2404 (2022).
Goyal, M. S. & Raichle, M. E. Glucose requirements of the developing human brain. J. Pediatr. Gastroenterol. Nutr. 66, S46–S49 (2018).
Tomasi, D., Wang, G. J. & Volkow, N. D. Energetic cost of brain functional connectivity. Proc. Natl Acad. Sci. USA 110, 13642–13647 (2013).
Zhang, G. et al. Glycolytic reprogramming in microglia: a potential therapeutic target for ischemic stroke. Cell. Signal. 124, 111466 (2024).
Goyal, M. S. et al. Spatiotemporal relationship between subthreshold amyloid accumulation and aerobic glycolysis in the human brain. Neurobiol. Aging 96, 165–175 (2020).
Sturm, G. et al. OxPhos defects cause hypermetabolism and reduce lifespan in cells and in patients with mitochondrial diseases. Commun. Biol. 6, 22 (2023).
Fitzgerald, P. B., Fountain, S. & Daskalakis, Z. J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 117, 2584–2596 (2006).
Hertz, L. Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem. Int. 45, 285–296 (2004).
Rothman, D. L., Behar, K. L., Hyder, F. & Shulman, R. G. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: implications for brain function. Annu. Rev. Physiol. 65, 401–427 (2003).
Rae, C. D. et al. Brain energy metabolism: a roadmap for future research. J. Neurochem. 168, 910–954 (2024).
Dienel, G. A. Brain glucose metabolism: integration of energetics with function. Physiol. Rev. 99, 949–1045 (2019).
Harris, J. J., Jolivet, R. & Attwell, D. Synaptic energy use and supply. Neuron 75, 762–777 (2012).
Raichle, M. E. The restless brain: how intrinsic activity organizes brain function. Phil. Trans. R. Soc. Lond. B 370, 20140172 (2015).
Raichle, M. E. & Gusnard, D. A. Appraising the brain's energy budget. Proc. Natl Acad. Sci. USA 99, 10237–10239 (2002).
Cho, Z. H. et al. Substructural hippocampal glucose metabolism observed on PET/MRI. J. Nucl. Med. 51, 1545–1548 (2010).
Shao, J. et al. Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain Imaging Behav. 12, 1708–1719 (2018).
Herculano-Houzel, S. & Rothman, D. L. From a demand-based to a supply-limited framework of brain metabolism. Front. Integr. Neurosci. 16, 818685 (2022).
DiNuzzo, M. et al. Neurovascular coupling is optimized to compensate for the increase in proton production from nonoxidative glycolysis and glycogenolysis during brain activation and maintain homeostasis of pH, \(p_{{\mathrm{CO}}_2}\), and \(p_{{\mathrm{O}}_2}\). J. Neurochem. 168, 632–662 (2024).
Bouzat, P. & Oddo, M. Lactate and the injured brain: friend or foe? Curr. Opin. Crit. Care 20, 133–140 (2014).
Trigo, D., Avelar, C., Fernandes, M., Sa, J. & da Cruz, E. S. O. Mitochondria, energy, and metabolism in neuronal health and disease. FEBS Lett. 596, 1095–1110 (2022).
Peters, A. The selfish brain: competition for energy resources. Am. J. Hum. Biol. 23, 29–34 (2011).
Wang, Z. et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 92, 1369–1377 (2010).
Steullet, P. et al. Redox dysregulation, neuroinflammation, and NMDA receptor hypofunction: a "central hub" in schizophrenia pathophysiology? Schizophr. Res. 176, 41–51 (2016).
Turkheimer, F. E. et al. Corrigendum to: normalizing the abnormal: do antipsychotic drugs push the cortex into an unsustainable metabolic envelope? Schizophr. Bull. 48, 721 (2022).
Kim, Y. et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid. Redox Signal. 31, 275–317 (2019).
Roberts, R. C. Mitochondrial dysfunction in schizophrenia: with a focus on postmortem studies. Mitochondrion 56, 91–101 (2021).
Sullivan, C. R., O'Donovan, S. M., McCullumsmith, R. E. & Ramsey, A. Defects in bioenergetic coupling in schizophrenia. Biol. Psychiatry 83, 739–750 (2018).
Bergman, O. & Ben-Shachar, D. Mitochondrial oxidative phosphorylation system (OXPHOS) deficits in schizophrenia: possible interactions with cellular processes. Can. J. Psychiatry 61, 457–469 (2016).
Trumpff, C. et al. Psychosocial experiences are associated with human brain mitochondrial biology. Proc. Natl Acad. Sci. USA 121, e2317673121 (2024).
Li, J. et al. Association of mitochondrial biogenesis with variable penetrance of schizophrenia. JAMA Psychiatry 78, 911–921 (2021).
Glausier, J. R., Enwright, J. F. 3rd & Lewis, D. A. Diagnosis- and cell type-specific mitochondrial functional pathway signatures in schizophrenia and bipolar disorder. Am. J. Psychiatry 177, 1140–1150 (2020).
Townsend, L. et al. Brain glucose metabolism in schizophrenia: a systematic review and meta-analysis of 18FDG-PET studies in schizophrenia. Psychol. Med. 53, 4880–4897 (2023).
Manosalva, C. et al. Role of lactate in inflammatory processes: friend or foe. Front. Immunol. 12, 808799 (2021).
Dwir, D. et al. Redox and immune signaling in schizophrenia: new therapeutic potential. Int. J. Neuropsychopharmacol. 26, 309–321 (2023).
Gimenez-Palomo, A. et al. The role of mitochondria in mood disorders: from physiology to pathophysiology and to treatment. Front. Psychiatry 12, 546801 (2021).
Abdallah, C. G. et al. Glutamate metabolism in major depressive disorder. Am. J. Psychiatry 171, 1320–1327 (2014).
Yuksel, C. et al. Abnormal high-energy phosphate molecule metabolism during regional brain activation in patients with bipolar disorder. Mol. Psychiatry 20, 1079–1084 (2015).
Kong, D. et al. Ketogenic diet: a potential adjunctive treatment for substance use disorders. Front. Nutr. 10, 1191903 (2023).
Mahapatra, G. et al. Blood-based bioenergetic profiling reveals differences in mitochondrial function associated with cognitive performance and Alzheimer's disease. Alzheimers Dement. 19, 1466–1478 (2023).
Goyal, M. S. et al. Brain aerobic glycolysis and resilience in Alzheimer disease. Proc. Natl Acad. Sci. USA 120, e2212256120 (2023).
Gabuzyan, R., Lee, C. & Nygaard, H. B. Ketogenic approaches for the treatment of Alzheimer's disease. J. Alzheimers Dis. 101, S443–S453 (2024).
Ongur, D. & Paulus, M. P. Embracing complexity in psychiatry—from reductionistic to systems approaches. Lancet Psychiatry 12, 220–227 (2025).
Emmerzaal, T. L. et al. Effect of neuropsychiatric medications on mitochondrial function: for better or for worse. Neurosci. Biobehav. Rev. 127, 555–571 (2021).
Ryan, M. C., Collins, P. & Thakore, J. H. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am. J. Psychiatry 160, 284–289 (2003).
Maudsley, H. An address on medical psychology. BMJ 2, 163–167 (1872).
Kasanin, J. The blood sugar curve in mental disease: II. The schizophrenic (dementia praecox) groups. Arch. Neurol. Psychiatry 16, 414–419 (1926).
Pillinger, T. et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 74, 261–269 (2017).
Milaneschi, Y., Lamers, F., Berk, M. & Penninx, B. Depression heterogeneity and its biological underpinnings: toward immunometabolic depression. Biol. Psychiatry 88, 369–380 (2020).
Rashidian, H. et al. Insulin resistance is associated with deficits in hedonic, self-reported cognitive, and psychosocial functional response to antidepressant treatment in individuals with major depressive disorder. J. Affect. Disord. 282, 448–453 (2021).
Gruber, J. et al. Impact of insulin and insulin resistance on brain dopamine signalling and reward processing—an underexplored mechanism in the pathophysiology of depression? Neurosci. Biobehav. Rev. 149, 105179 (2023).
Roy, T. & Lloyd, C. E. Epidemiology of depression and diabetes: a systematic review. J. Affect. Disord. 142, S8–S21 (2012).
Sarnyai, Z. & Palmer, C. M. Ketogenic therapy in serious mental illness: emerging evidence. Int. J. Neuropsychopharmacol. 23, 434–439 (2020).
Mujica-Parodi, L. R. & Strey, H. H. Making sense of computational psychiatry. Int. J. Neuropsychopharmacol. 23, 339–347 (2020).
McEwen, B. S. Allostasis and the epigenetics of brain and body health over the life course: the brain on stress. JAMA Psychiatry 74, 551–552 (2017).
Castrillon, G. et al. An energy costly architecture of neuromodulators for human brain evolution and cognition. Sci. Adv. 9, eadi7632 (2023).
Calkin, C. V. et al. Treating insulin resistance with metformin as a strategy to improve clinical outcomes in treatment-resistant bipolar depression (the TRIO-BD Study): a randomized, quadruple-masked, placebo-controlled clinical trial. J. Clin. Psychiatry 83, 21m14022 (2022).
Yammine, L. et al. Exenatide once weekly for smoking cessation: study protocol for a randomized clinical trial. Medicine 97, e9567 (2018).
Ehrenreich, H. et al. Exploiting moderate hypoxia to benefit patients with brain disease: molecular mechanisms and translational research in progress. Neuroprotection 1, 9–19 (2023).
Falkai, P. et al. Aerobic exercise in severe mental illness: requirements from the perspective of sports medicine. Eur. Arch. Psychiatry Clin. Neurosci. 272, 643–677 (2022).
Zapata, R. C. et al. Antipsychotic-induced weight gain and metabolic effects show diurnal dependence and are reversible with time restricted feeding. Schizophrenia 8, 70 (2022).
Kim, D. J. et al. Altered physical pain processing in different psychiatric conditions. Neurosci. Biobehav. Rev. 133, 104510 (2022).
Forester, B. P. et al. Coenzyme Q10 effects on creatine kinase activity and mood in geriatric bipolar depression. J. Geriatr. Psychiatry Neurol. 25, 43–50 (2012).
Yoshino, J., Baur, J. A. & Imai, S. I. NAD(+) intermediates: the biology and therapeutic potential of NMN and NR. Cell Metab. 27, 513–528 (2018).
Chouinard, V. A. et al. Intranasal insulin increases brain glutathione (GSH) and enhances antioxidant capacity in healthy participants, but not in those with early psychotic disorders. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 10, 286–294 (2025).
Battini, V. et al. The potential effect of metformin on cognitive and other symptom dimensions in patients with schizophrenia and antipsychotic-induced weight gain: a systematic review, meta-analysis, and meta-regression. Front. Psychiatry 14, 1215807 (2023).
Murta, L., Seixas, D., Harada, L., Damiano, R. F. & Zanetti, M. Intermittent fasting as a potential therapeutic instrument for major depression disorder: a systematic review of clinical and preclinical studies. Int. J. Mol. Sci. 24, 15551 (2023).
Johnson, S. L. et al. A randomized controlled trial to compare the effects of time-restricted eating versus Mediterranean diet on symptoms and quality of life in bipolar disorder. BMC Psychiatry 24, 374 (2024).
Lii, T. R. et al. Randomized trial of ketamine masked by surgical anesthesia in patients with depression. Nat. Ment. Health 1, 876–886 (2023).
Szigeti, B. & Heifets, B. D. Expectancy effects in psychedelic trials. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 9, 512–521 (2024).
Manji, H. et al. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 13, 293–307 (2012).
Diez-Arroyo, C. et al. Effect of the ketogenic diet as a treatment for refractory epilepsy in children and adolescents: a systematic review of reviews. Nutr. Rev. 82, 487–502 (2024).
Longhitano, C. et al. The effects of ketogenic metabolic therapy on mental health and metabolic outcomes in schizophrenia and bipolar disorder: a randomized controlled clinical trial protocol. Front. Nutr. 11, 1444483 (2024).
Rasgon, N. L. & McEwen, B. S. Insulin resistance—a missing link no more. Mol. Psychiatry 21, 1648–1652 (2016).
Cuenod, M. et al. Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol. Psychiatry 27, 1886–1897 (2022).
Rasgon, N. L. et al. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. ScientificWorldJournal 10, 321–328 (2010).
Nasca, C. et al. Insulin receptor substrate in brain-enriched exosomes in subjects with major depression: on the path of creation of biosignatures of central insulin resistance. Mol. Psychiatry 26, 5140–5149 (2021).
Saha, S., Chant, D. & McGrath, J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch. Gen. Psychiatry 64, 1123–1131 (2007).
Lkhagvasuren, B. et al. Pancreas–brain crosstalk. Front. Neuroanat. 15, 691777 (2021).
Trelle, S. Exploratory trials in mental health: anything to learn from other disciplines? Evid. Based Ment. Health 20, 21–24 (2017).
Author information
Authors and Affiliations
Contributions
Conception/design of the paper: A.C.A., L.F.B., A.B., D.B.-S., S.B., V.-A.C., K.D., S.E.T., H.E., P.F., J.F., Z.F., J.G.-J., J.R.G., M.S.G., M.H., S.H.-H., D.H., I.-T.K., M.M., R.N.M., R.M., Y.M., A.J.A.M., L.M.-P., D.Ö., M.P., D.P.-R., B.P., M.P., T.P., C.R., D.R., Z.S, J.S., R.U., A.C.V., M.W., C.W. Analysis/interpretation of evidence: A.C.A., L.F.B., A.B., D.B.-S., S.B., V.-A.C., K.D., S.E.T., H.E., P.F., J.F., Z.F., J.G.-J., J.R.G., M.S.G., M.H., S.H.-H., D.H., I.-T.K., M.M., R.N.M., R.M., Y.M., A.J.A.M., L.M.-P., D.Ö., M.P., D.P.-R., B.P., M.P., T.P., C.R., D.R., Z.S., J.S., R.U., A.C.V., M.W., C.W. Writing parts of the work/critical editing for important intellectual content: A.C.A., L.F.B., A.B., D.B.-S., S.B., V.-A.C., K.D., S.E.T., H.E., P.F., J.F., Z.F., J.G.-J., J.R.G., M.S.G., M.H., S.H.-H., D.H., I.-T.K., M.M., R.N.M., R.M., Y.M., A.J.A.M., L.M.-P., D.Ö., M.P., D.P.-R., B.P., M.P., T.P., C.R., D.R., Z.S., J.S., R.U., A.C.V., M.W., C.W.
Corresponding author
Ethics declarations
Competing interests
M.H. has received consultation fees from Merck and Alkermes. Y.M. has received consulting fees from Noema Pharma AG. D.Ö. has received honoraria from Rapport Therapeutics. M.P. is receiving support from KetoSwiss for a project. R.M. conducts reviews for the schizophrenia grant program at Alkermes. Z.S. is the chief scientist at Ally Bio, a brain health supplement start-up. The other authors declare no competing interests.
Peer review
Peer review information
Nature Mental Health thanks Shebani Sethi, Kathleen Watson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andreazza, A.C., Barros, L.F., Behnke, A. et al. Brain and body energy metabolism and potential for treatment of psychiatric disorders. Nat. Mental Health 3, 763–771 (2025). https://doi.org/10.1038/s44220-025-00422-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44220-025-00422-6