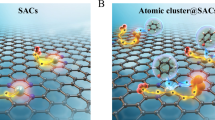
Abstract
Non-radical oxidation of pollutants by direct electron transfer has gained heightened interest in water purification for its higher selectivity and efficiency and lower tendency for byproduct formation than traditional advanced oxidation processes. Engineering of catalysts for efficient activation of two substrates (that is, pollutant and oxidant) is essential to trigger the direct electron transfer reactions but is often hindered by the distinct properties of the co-present substrates. We investigated the individual interaction between the catalyst and each substrate and proposed a dual-substrate synergistic catalysis strategy to achieve separate optimization of each substrate activation process. Experimental and theoretical analyses reveal a strong synergistic effect between the two catalysts that preferentially activate the substrates and have smaller resistance for interfacial electron transfer, thus drastically improving the decontamination efficiency. The dual-substrate synergistic catalysis system offers a conceptual advancement in achieving green and efficient water purification by substrate-specific activation, facilitating flexible design and mechanistic exploration of complex heterogeneous catalytic processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data for this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Gligorovski, S. et al. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 115, 13051–13092 (2015).
Hodges, B. C. et al. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018).
Yang, X. et al. Multiple roles of dissolved organic matter in advanced oxidation processes. Environ. Sci. Technol. 56, 11111–11131 (2022).
Yang, Z. et al. Toward selective oxidation of contaminants in aqueous systems. Environ. Sci. Technol. 55, 14494–14514 (2021).
Ghanbari, F. & Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem. Eng. J. 310, 41–62 (2017).
von Gunten, U. Oxidation processes in water treatment: are we on track? Environ. Sci. Technol. 52, 5062–5075 (2018).
Zhu, L. et al. Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control. Angew. Chem. Int. Ed. 59, 13968–13976 (2020).
Krasner, S. W. et al. Formation, precursors, control, and occurrence of nitrosamines in drinking water: a review. Water Res. 47, 4433–4450 (2013).
Sgroi, M. et al. N-nitrosodimethylamine (NDMA) and its precursors in water and wastewater: a review on formation and removal. Chemosphere 191, 685–703 (2018).
Farré, M. J. et al. Assessment of degradation byproducts and NDMA formation potential during UV and UV/H2O2 treatment of doxylamine in the presence of monochloramine. Environ. Sci. Technol. 46, 12904–12912 (2012).
Zhang, T. et al. Efficient peroxydisulfate activation process not relying on sulfate radical generation for water pollutant degradation. Environ. Sci. Technol. 48, 5868–5875 (2014).
Dou, J. et al. Neglected but efficient electron utilization driven by biochar-coactivated phenols and peroxydisulfate: polyphenol accumulation rather than mineralization. Environ. Sci. Technol. 57, 5703–5713 (2023).
Ren, W. et al. Origins of electron-transfer regime in persulfate-based nonradical oxidation processes. Environ. Sci. Technol. 56, 78–97 (2021).
Yao, C. et al. Insights into the mechanism of non-radical activation of persulfate via activated carbon for the degradation of p-chloroaniline. Chem. Eng. J. 362, 262–268 (2019).
Guan, C. et al. Transformation of iodide by carbon nanotube activated peroxydisulfate and formation of iodoorganic compounds in the presence of natural organic matter. Environ. Sci. Technol. 51, 479–487 (2016).
Hu, P. et al. Selective degradation of organic pollutants using an efficient metal-free catalyst derived from carbonized polypyrrole via peroxymonosulfate activation. Environ. Sci. Technol. 51, 11288–11296 (2017).
Duan, X. et al. Nonradical reactions in environmental remediation processes: uncertainty and challenges. Appl. Catal. B 224, 973–982 (2018).
Peng, Y. et al. Thermodynamic and kinetic behaviors of persulfate-based electron-transfer regime in carbocatalysis. Environ. Sci. Technol. 57, 19012–19022 (2023).
Yu, Z. et al. Decoupled oxidation process enabled by atomically dispersed copper electrodes for in-situ chemical water treatment. Nat. Commun. 15, 1186 (2024).
Zhang, Y.-J. et al. Simultaneous nanocatalytic surface activation of pollutants and oxidants for highly efficient water decontamination. Nat. Commun. 13, 3005 (2022).
Huang, K. Z. & Zhang, H. Direct electron-transfer-based peroxymonosulfate activation by iron-doped manganese oxide (δ-MnO2) and the development of galvanic oxidation processes (GOPs). Environ. Sci. Technol. 53, 12610–12620 (2019).
Liu, X. et al. Selective removal of organic pollutants in groundwater and surface water by persulfate-assisted advanced oxidation: the role of electron-donating capacity. Environ. Sci. Technol. 57, 13710–13720 (2023).
Huang, K. Z. & Zhang, H. Galvanic oxidation processes (GOPs): an effective direct electron transfer approach for organic contaminant oxidation. Sci. Total Environ. 743, 140828–140837 (2020).
Lee, J. et al. Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 54, 3064–3081 (2020).
Yun, E. T. et al. Identifying the nonradical mechanism in the peroxymonosulfate activation process: singlet oxygenation versus mediated electron transfer. Environ. Sci. Technol. 52, 7032–7042 (2018).
Si, Y. et al. Reusing sulfur-poisoned palladium waste as a highly active, nonradical Fenton-like catalyst for selective degradation of phenolic pollutants. Environ. Sci. Technol. 56, 564–574 (2021).
Huang, G. X. et al. Degradation of bisphenol a by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: synergism between Mn and Fe. Environ. Sci. Technol. 51, 12611–12618 (2017).
Choueiri, R. M. et al. Surface patterning of nanoparticles with polymer patches. Nature 538, 79–83 (2016).
Itta, A. K. et al. Fabrication and characterization of PPO/PVP blend carbon molecular sieve membranes for H2/N2 and H2/CH4 separation. J. Membr. Sci. 372, 387–395 (2011).
Gupta, S. et al. Mechanistic studies for the polymerization of 2,6-dimethylphenol to poly (2,6-dimethyl-1,4-phenylene ether): LC-MS analyses showing rearrangement and redistribution products. Appl. Catal. A 319, 163–170 (2007).
Maeno, Z. et al. Selective C–C coupling reaction of dimethylphenol to tetramethyldiphenoquinone using molecular oxygen catalyzed by Cu complexes immobilized in nanospaces of structurally-ordered materials. Molecules 20, 3089–3106 (2015).
Maeno, Z. et al. Regioselective oxidative coupling of 2,6-dimethylphenol to tetramethyldiphenoquinone using polyamine dendrimer-encapsulated Cu catalysts. RSC Adv. 3, 9662–9665 (2013).
Zhao, Y. et al. The effect of ligand molecular weight on copper salt catalyzed oxidative coupling polymerization of 2,6-dimethylphenol. J. Appl. Polym. Sci. 117, 3473–3481 (2010).
Samec, Z. et al. Reduction of peroxodisulfate on gold(111) covered by surface oxides: inhibition and coupling between two oxide reduction processes. J. Electroanal. Chem. 499, 129–135 (2001).
Ren, W. et al. Activation of peroxydisulfate on carbon nanotubes: electron-transfer mechanism. Environ. Sci. Technol. 53, 14595–14603 (2019).
Yang, Q. et al. Unzipping carbon nanotubes to nanoribbons for revealing the mechanism of nonradical oxidation by carbocatalysis. Appl. Catal. B 276, 119146–119155 (2020).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2017).
Qi, Y. et al. Insights into the activity of nickel boride/nickel heterostructures for efficient methanol electrooxidation. Nat. Commun. 13, 4602 (2022).
Zhou, P. et al. Heterogeneous-interface-enhanced adsorption of organic and hydroxyl for biomass electrooxidation. Adv. Mater. 34, 2204089–2204097 (2022).
Wang, H.-Y. et al. In operando identification of geometrical-site-dependent water oxidation activity of spinel Co3O4. J. Am. Chem. Soc. 138, 36–39 (2015).
Xiao, Z. et al. Operando identification of the dynamic behavior of oxygen vacancy-rich Co3O4 for oxygen evolution reaction. J. Am. Chem. Soc. 142, 12087–12095 (2020).
Park, K. et al. Mitigation strategies of hydrogen sulphide emission in sewer networks—a review. Int. Biodeterior. Biodegrad. 95, 251–261 (2014).
Liu, L. L. et al. Edge electronic vacancy on ultrathin carbon nitride nanosheets anchoring O2 to boost H2O2 photoproduction. Appl. Catal. B 302, 120845–120854 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (51821006 (H.-Q.Y.)), 52293443 (G.-X.H.), 52192684 (H.-Q.Y.), 52027815 (H.-Q.Y.) and 22376191 (G.-X.H.)). The DFT calculations in this work were conducted on the supercomputing system in the Supercomputing Center of the University of Science and Technology of China.
Author information
Authors and Affiliations
Contributions
G.-X.H. and H.-Q.Y. came up with the original idea; L.-J.S. and G.-X.H. designed and conducted the experiments; L.-J.S. performed the DFT calculations; J.-J.C. provided guidance on the DFT calculations; Z.-H.W., Y.-J.Z., W.-W.L., H.-Q.Y., Y.D. and M.E. helped the data interpretations; L.-J.S., G.-X.H., Y.D. and M.E. wrote the paper; all authors commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Shun Mao, Liang Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods 1–4, Figs. 1–25, Tables 1–12 and references.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, LJ., Huang, GX., Wang, ZH. et al. Dual-substrate synergistic catalysis for highly efficient water purification. Nat Water 3, 345–353 (2025). https://doi.org/10.1038/s44221-025-00400-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00400-3