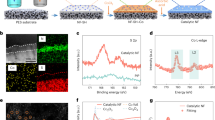
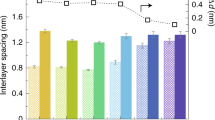
Abstract
Reactive nanofiltration membranes integrate catalytic transformation with molecular separation to remove diverse aqueous contaminants. However, their development is hindered by an incomplete understanding of the interplay between solute mass transport and chemical reactions. Here we introduce key design principles by systematically evaluating their performance using a modelling approach. Efficient oxidant transport is essential for maximizing contaminant degradation. For membranes with surface-loaded catalysts, avoiding mass transport limitations ensures effective catalyst utilization, whereas for membranes with interior-loaded catalysts, optimizing oxidant partitioning enhances oxidant utilization efficiency. In addition, selective solute rejection reduces interference from natural organic matter, facilitating more selective contaminant transformation inside membrane pores. Consequently, contaminant transformation is dominated by surface-catalysed reactions at low permeate water fluxes, while interior-catalysed reactions dominate at high fluxes. However, rejecting both oxidants and contaminants does not enhance surface-catalysed treatment performance under an optimally designed scenario, highlighting the need for strategic design of membrane rejection. Beyond organic contaminant removal, nanofiltration membranes also minimize secondary contamination by rejecting the produced salts during the catalytic reactions. Furthermore, strategic selection of oxidant–catalyst pairs can enhance treatment performance by generating suitable reactive species. By establishing a theoretical framework for designing and optimizing reactive nanofiltration membranes, this study provides critical insights into the development of advanced water treatment technologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The source data for the figures are provided in Excel format (.xlsx) and are publicly available via Figshare at https://doi.org/10.6084/m9.figshare.29333090 (ref. 79).
Code availability
The code for the model described in the paper is available publicly via GitHub at https://github.com/yanghuaduan/Reactive_NF_membrane.
References
Bakker, K. Water security: research challenges and opportunities. Science 337, 914–915 (2012).
Westall, F. & Brack, A. The importance of water for life. Space Sci. Rev. 214, 50 (2018).
Lu, X. & Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: history, current efforts, and future directions. Chem. Soc. Rev. 50, 6290–6307 (2021).
Wu, J., Cao, M., Tong, D., Finkelstein, Z. & Hoek, E. M. V. A critical review of point-of-use drinking water treatment in the United States. npj Clean Water 4, 40 (2021).
Liu, W. et al. Pressure-driven membrane desalination. Nat. Rev. Methods Prim. 4, 10 (2024).
Mauter, M. S. et al. The role of nanotechnology in tackling global water challenges. Nat. Sustain. 1, 166–175 (2018).
Marron, E. L., Mitch, W. A., von Gunten, U. & Sedlak, D. L. A tale of two treatments: the multiple barrier approach to removing chemical contaminants during potable water reuse. Acc. Chem. Res. 52, 615–622 (2019).
Castaño Osorio, S., Biesheuvel, P. M., Spruijt, E., Dykstra, J. E. & van der Wal, A. Modeling micropollutant removal by nanofiltration and reverse osmosis membranes: considerations and challenges. Water Res. 225, 119130 (2022).
Alvarez, P. J. J., Chan, C. K., Elimelech, M., Halas, N. J. & Villagrán, D. Emerging opportunities for nanotechnology to enhance water security. Nat. Nanotechnol. 13, 634–641 (2018).
Miklos, D. B. et al. Evaluation of advanced oxidation processes for water and wastewater treatment—a critical review. Water Res. 139, 118–131 (2018).
Chen, F. et al. Embedding electronic perpetual motion into single-atom catalysts for persistent Fenton-like reactions. Proc. Natl Acad. Sci. USA 121, e2314396121 (2024).
Sedlak, D. et al. National Alliance for Water Innovation (NAWI) Master Technology Roadmap (National Renewable Energy Lab, 2021).
Zhang, S., Hedtke, T., Zhou, X., Elimelech, M. & Kim, J.-H. Environmental applications of engineered materials with nanoconfinement. ACS EST Eng. 1, 706–724 (2021).
Zhang, Y. et al. CoII–NC catalytic membrane for advanced oxidation and mineralization of organic pollutants. ACS EST Eng. 4, 2711–2720 (2024).
Baker, R. W. Membrane Technology and Applications (Wiley, 2023).
Ma, W. et al. Catalytic membrane with copper single-atom catalysts for effective hydrogen peroxide activation and pollutant destruction. Environ. Sci. Technol. 56, 8733–8745 (2022).
Wang, T., de Grooth, J. & de Vos, W. M. Effects of concentration polarization and membrane orientation on the treatment of Naproxen by sulfate radical-based advanced oxidation processes within nanofiltration membranes with a catalytic support. Ind. Eng. Chem. Res. 62, 7622–7634 (2023).
Yang, Z., Qian, J., Yu, A. & Pan, B. Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc. Natl Acad. Sci. USA 116, 6659–6664 (2019).
Meng, Y. et al. Nanoconfinement steers nonradical pathway transition in single atom fenton-like catalysis for improving oxidant utilization. Nat. Commun. 15, 5314 (2024).
Zhang, S. et al. Membrane-confined iron oxychloride nanocatalysts for highly efficient heterogeneous Fenton water treatment. Environ. Sci. Technol. 55, 9266–9275 (2021).
Wu, X. et al. Single-atom cobalt incorporated in a 2D graphene oxide membrane for catalytic pollutant degradation. Environ. Sci. Technol. 56, 1341–1351 (2022).
Meng, C. et al. Angstrom-confined catalytic water purification within Co-TiOx laminar membrane nanochannels. Nat. Commun. 13, 4010 (2022).
Zheng, L. et al. Covalent organic framework membrane reactor for boosting catalytic performance. Nat. Commun. 15, 6837 (2024).
Zhang, Y. et al. Monolithic ceramic CoTiO3/TiO2 membrane balancing catalytic efficiency and durability in advanced oxidation processes. Environ. Sci. Technol. 59, 6863–6871 (2025).
Hedtke, T. et al. Copper single-atom catalyst on nanoconfined ceramic membranes for Fenton-like removal of organic contaminants. ACS EST Eng. 5, 1171–1179 (2025).
Jiang, W. et al. Metal-free electrified membranes for contaminants oxidation: synergy effect between membrane rejection and nanoconfinement. Water Res. 248, 120862 (2024).
Heredia Deba, S. A., Wols, B. A., Yntema, D. R. & Lammertink, R. G. H. Transport and surface reaction model of a photocatalytic membrane during the radical filtration of methylene blue. Chem. Eng. Sci. 254, 117617 (2022).
Wu, X. & Kim, J.-H. Outlook on single atom catalysts for persulfate-based advanced oxidation. ACS EST Eng. 2, 1776–1796 (2022).
Duan, Y., Jiang, W. & Sedlak, D. L. Surface processes control the fate of reactive oxidants generated by electrochemical activation of hydrogen peroxide on stainless-steel electrodes. Environ. Sci. Technol. 57, 18680–18689 (2023).
Duan, Y. & Sedlak, D. L. Electrochemical hydrogen peroxide generation and activation using a dual-cathode flow-through treatment system: enhanced selectivity for contaminant removal by electrostatic repulsion. Environ. Sci. Technol. 58, 14042–14051 (2024).
Lead, J. R., Balnois, E., Hosse, M., Menghetti, R. & Wilkinson, K. J. Characterization of Norwegian natural organic matter: size, diffusion coefficients, and electrophoretic mobilities. Environ. Int. 25, 245–258 (1999).
Zhang, Z. et al. Pilot-scale evaluation of oxidant speciation, 1,4-dioxane degradation and disinfection byproduct formation during UV/hydrogen peroxide, UV/free chlorine and UV/chloramines advanced oxidation process treatment for potable reuse. Water Res. 164, 114939 (2019).
Secondary drinking water standards: guidance for nuisance chemicals. United States Environmental Protection Agency https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals (2025).
Deen, W. M. Hindered transport of large molecules in liquid-filled pores. AIChE J. 33, 1409–1425 (1987).
Zhang, S. et al. Mechanism of heterogeneous Fenton reaction kinetics enhancement under nanoscale spatial confinement. Environ. Sci. Technol. 54, 10868–10875 (2020).
Takeuchi, K. et al. Salt rejection behavior of carbon nanotube-polyamide nanocomposite reverse osmosis membranes in several salt solutions. Desalination 443, 165–171 (2018).
Henkens, W. C. M. & Smit, J. A. M. Salt rejection and flux in reverse osmosis with compactible membranes. Desalination 28, 65–85 (1979).
Epsztein, R., DuChanois, R. M., Ritt, C. L., Noy, A. & Elimelech, M. Towards single-species selectivity of membranes with subnanometre pores. Nat. Nanotechnol. 15, 426–436 (2020).
Epsztein, R., Shaulsky, E., Dizge, N., Warsinger, D. M. & Elimelech, M. Role of ionic charge density in donnan exclusion of monovalent anions by nanofiltration. Environ. Sci. Technol. 52, 4108–4116 (2018).
Cheng, W. et al. Selective removal of divalent cations by polyelectrolyte multilayer nanofiltration membrane: role of polyelectrolyte charge, ion size, and ionic strength. J. Membr. Sci. 559, 98–106 (2018).
Zhang, T., Zhu, H. & Croué, J.-P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: efficiency, stability, and mechanism. Environ. Sci. Technol. 47, 2784–2791 (2013).
Hu, J., Dong, H., Qu, J. & Qiang, Z. Enhanced degradation of iopamidol by peroxymonosulfate catalyzed by two pipe corrosion products (CuO and δ-MnO2). Water Res. 112, 1–8 (2017).
Hu, J. et al. Transformation of iopamidol and atrazine by peroxymonosulfate under catalysis of a composite iron corrosion product (Fe/Fe3O4): electron transfer, active species and reaction pathways. J. Hazard. Mater. 403, 123553 (2021).
Pham, A. L.-T., Lee, C., Doyle, F. M. & Sedlak, D. L. A silica-supported iron oxide catalyst capable of activating hydrogen peroxide at neutral pH values. Environ. Sci. Technol. 43, 8930–8935 (2009).
Pham, A. L.-T., Doyle, F. M. & Sedlak, D. L. Kinetics and efficiency of H2O2 activation by iron-containing minerals and aquifer materials. Water Res. 46, 6454–6462 (2012).
Liu, H. et al. Oxidation of benzene by persulfate in the presence of Fe(III)- and Mn(IV)-containing oxides: stoichiometric efficiency and transformation products. Environ. Sci. Technol. 50, 890–898 (2016).
Yang, X. et al. Impact of peroxymonocarbonate on the transformation of organic contaminants during hydrogen peroxide in situ chemical oxidation. Environ. Sci. Technol. Lett. 6, 781–786 (2019).
Kearns, D. R. Physical and chemical properties of singlet molecular oxygen. Chem. Rev. 71, 395–427 (1971).
Frimer, A. A. The reaction of singlet oxygen with olefins: the question of mechanism. Chem. Rev. 79, 359–387 (1979).
Scully, F. E. & Hoigné, J. Rate constants for reactions of singlet oxygen with phenols and other compounds in water. Chemosphere 16, 681–694 (1987).
Cheng, X., Guo, H., Zhang, Y., Wu, X. & Liu, Y. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 113, 80–88 (2017).
Liang, P. et al. An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: formation mechanism and generation of singlet oxygen from peroxymonosulfate. Environ. Sci. Nano 4, 315–324 (2017).
Liang, P. et al. N-doped graphene from metal–organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism. ACS Sustain. Chem. Eng. 5, 2693–2701 (2017).
Zhu, S., Li, X., Kang, J., Duan, X. & Wang, S. Persulfate activation on crystallographic manganese oxides: mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants. Environ. Sci. Technol. 53, 307–315 (2019).
Wang, Y., Cao, D., Liu, M. & Zhao, X. Insights into heterogeneous catalytic activation of peroxymonosulfate by Pd/g-C3N4: the role of superoxide radical and singlet oxygen. Catal. Commun. 102, 85–88 (2017).
Tian, X. et al. A novel singlet oxygen involved peroxymonosulfate activation mechanism for degradation of ofloxacin and phenol in water. Chem. Commun. 53, 6589–6592 (2017).
Gao, P. et al. Promoted peroxymonosulfate activation into singlet oxygen over perovskite for ofloxacin degradation by controlling the oxygen defect concentration. Chem. Eng. J. 359, 828–839 (2019).
Heredia Deba, S. A., Wols, B. A., Yntema, D. R. & Lammertink, R. G. H. Advanced ceramics in radical filtration: TiO2 layer thickness effect on the photocatalytic membrane performance. J. Membr. Sci. 672, 121423 (2023).
Bellardita, M., Camera-Roda, G., Loddo, V., Parrino, F. & Palmisano, L. Coupling of membrane and photocatalytic technologies for selective formation of high added value chemicals. Catal. Today 340, 128–144 (2020).
Rolf, J. et al. Inorganic scaling in membrane desalination: models, mechanisms, and characterization methods. Environ. Sci. Technol. 56, 7484–7511 (2022).
Lee, J., von Gunten, U. & Kim, J.-H. Persulfate-based advanced oxidation: critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 54, 3064–3081 (2020).
Xing, S., Li, W., Liu, B., Wu, Y. & Gao, Y. Removal of ciprofloxacin by persulfate activation with CuO: a pH-dependent mechanism. Chem. Eng. J. 382, 122837 (2020).
Chen, C. et al. Membrane-catalysis integrated system for contaminants degradation and membrane fouling mitigation: a review. Sci. Total Environ. 904, 166220 (2023).
Liu, Y. et al. Progress and challenges in structural, in situ and operando characterization of single-atom catalysts by X-ray based synchrotron radiation techniques. Chem. Soc. Rev. 53, 11850–11887 (2024).
Yao, Y. et al. High performance polyester reverse osmosis desalination membrane with chlorine resistance. Nat. Sustain. 4, 138–146 (2021).
Yao, Y. et al. More resilient polyester membranes for high-performance reverse osmosis desalination. Science 384, 333–338 (2024).
Wang, R. & Lin, S. Membrane design principles for ion-selective electrodialysis: an analysis for Li/Mg separation. Environ. Sci. Technol. 58, 3552–3563 (2024).
Wang, R. & Lin, S. Pore model for nanofiltration: history, theoretical framework, key predictions, limitations, and prospects. J. Membr. Sci. 620, 118809 (2021).
Wang, L. et al. Salt and water transport in reverse osmosis membranes: beyond the solution-diffusion model. Environ. Sci. Technol. 55, 16665–16675 (2021).
Richards, L. A., Schäfer, A. I., Richards, B. S. & Corry, B. The importance of dehydration in determining ion transport in narrow pores. Small 8, 1701–1709 (2012).
Lu, C. et al. In situ characterization of dehydration during ion transport in polymeric nanochannels. J. Am. Chem. Soc. 143, 14242–14252 (2021).
Richard Bowen, W. & Wahab Mohammad, A. Diafiltration by nanofiltration: prediction and optimization. AIChE J. 44, 1799–1812 (1998).
Childress, A. E. & Elimelech, M. Relating nanofiltration membrane performance to membrane charge (electrokinetic) characteristics. Environ. Sci. Technol. 34, 3710–3716 (2000).
Coronell, O., Mariñas, B. J., Zhang, X. & Cahill, D. G. Quantification of functional groups and modeling of their ionization behavior in the active layer of FT30 reverse osmosis membrane. Environ. Sci. Technol. 42, 5260–5266 (2008).
Bruni, L. & Bandini, S. The role of the electrolyte on the mechanism of charge formation in polyamide nanofiltration membranes. J. Membr. Sci. 308, 136–151 (2008).
Chaudhury, S. G. The Donnan membrane equilibrium. Nature 152, 76–77 (1943).
Dechadilok, P. & Deen, W. M. Hindrance factors for diffusion and convection in pores. Ind. Eng. Chem. Res. 45, 6953–6959 (2006).
Wang, J. et al. A critical review of transport through osmotic membranes. J. Membr. Sci. 454, 516–537 (2014).
Duan, Y. Design principles of catalytic reactive membranes for water treatment. Figshare https://doi.org/10.6084/m9.figshare.29333090 (2025).
Acknowledgements
This research was partially funded by the Yale University Superfund Research Program (SRP), which is supported by a grant from the National Institute of Environmental Health Sciences, National Institutes of Health (award no. P42ES033815) and the Rice Center for Membrane Excellence (RiCeME). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Conceptualization: Y.D. and M.E. Methodology: Y.D., R.W., A.N.S. and M.E. Formal analysis: Y.D., R.W., A.N.S. and M.E. Writing—original draft preparation: Y.D. Writing—review and editing: Y.D., R.W., A.N.S. and M.E. Supervision: M.E. Funding acquisition: M.E. All authors have read and agreed to the published version of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Qi Sun, Gaofeng Zeng and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, Tables 1 and 2 and Texts 1–7.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, Y., Wang, R., Shocron, A.N. et al. Design principles of catalytic reactive membranes for water treatment. Nat Water 3, 949–962 (2025). https://doi.org/10.1038/s44221-025-00467-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00467-y