Abstract
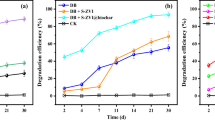
The valorization of chlorinated organic pollutants in water, such as 1,2-dichloroethane (1,2-DCA), into value-added products, such as ethylene, offers a sustainable remediation strategy but is limited by low efficiency and selectivity. Here we present a bioinspired system, consisting of cobalamin (vitamin B12) cofactor and microscale zero-valent iron (mZVI), that dechlorinates 1,2-DCA to ethylene with a rate constant of 0.066 h−1 and near-100% selectivity. mZVI creates a moderately reducing environment that reduces cob(III)alamin (the original B12 species) to cob(II)alamin, which forms an organocobalt–1,2-DCA complex and drives proton-independent dihaloelimination, avoiding unwanted hydrogenation and ethylene over-reduction. The strategy is effective for various chlorinated alkanes, alkenes and aromatics, high concentrations of 1,2-DCA in wastewater and mixed pollutants in groundwater. Mechanochemically anchoring B12 onto mZVI enables assembly in a column reactor for continuous 1,2-DCA removal, achieving a more than tenfold reduction in costs compared with conventional redox processes. This work demonstrates a cost-effective approach to pollutant remediation and resource recovery through the rational modulation of B12 redox chemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information.
Change history
18 September 2025
In the version of the article initially published, the peer reviewer Jordi Palau’s name appeared incorrectly and has now been amended in the HTML and PDF versions of the article.
References
Leow, D., Li, G., Mei, T.-S. & Yu, J.-Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Liang, Y., Lin, F., Adeli, Y., Jin, R. & Jiao, N. Efficient electrocatalysis for the preparation of (hetero)aryl chlorides and vinyl chloride with 1,2‐dichloroethane. Angew. Chem. Int. Ed. 58, 4566–4570 (2019).
Sherwood, J. European restrictions on 1,2‐dichloroethane: C–H activation research and development should be liberated and not limited. Angew. Chem. Int. Ed. 57, 14286–14290 (2018).
Wang, X.-C. et al. Ligand-enabled meta-C–H activation using a transient mediator. Nature 519, 334–338 (2015).
Phipps, R. J. & Gaunt, M. J. A meta-selective copper-catalyzed C–H bond arylation. Science 323, 1593–1597 (2009).
Wang, Z., Yin, J., Zhou, F., Liu, Y. & You, J. Multicomponent reactions of pyridines to give ring‐fused pyridiniums: in situ activation strategy using 1,2‐dichloroethane as a vinyl equivalent. Angew. Chem. Int. Ed. 58, 254–258 (2018).
Choi, C. et al. CO2-promoted electrocatalytic reduction of chlorinated hydrocarbons. J. Am. Chem. Soc. 146, 8486–8491 (2024).
Choi, C. et al. Efficient electrocatalytic valorization of chlorinated organic water pollutant to ethylene. Nat. Nanotechnol. 18, 160–167 (2022).
Ma, J. et al. Vapor intrusion risk of lead scavengers 1,2-dibromoethane (EDB) and 1,2-dichloroethane (DCA). Environ. Pollut. 213, 825–832 (2016).
Seok, J., Phan, N. T. Y., Kim, J.-C., Shin, H. & Choi, M. Catalytic synergy between Lewis acidic alumina and Pt in hydrodechlorination for plastic chemical recycling. J. Am. Chem. Soc. 146, 23881–23890 (2024).
Jiang, L. et al. Geobacter sp. strain IAE dihaloeliminates 1,1,2-trichloroethane and 1,2-dichloroethane. Environ. Sci. Technol. 56, 3430–3440 (2022).
Palau, J. et al. Hydrogen isotope fractionation during the biodegradation of 1,2-dichloroethane: potential for pathway identification using a multi-element (C, Cl, and H) isotope approach. Environ. Sci. Technol. 51, 10526–10535 (2017).
Chen, X. et al. Microdroplet-mediated multiphase cycling in a cloud of water drives chemoselective electrolysis. J. Am. Chem. Soc. 146, 29742–29750 (2024).
Deng, Y.-D. et al. The complete degradation of 1,2-dichloroethane in Escherichia coli by metabolic engineering. J. Hazard. Mater. 472, 134476 (2024).
Huo, K. et al. Creating an efficient 1,2-dichloroethane-mineralizing bacterium by a combination of pathway engineering and promoter engineering. Sci. Total Environ. 878, 163140 (2023).
Totten, L. A. & Roberts, A. L. Calculated one- and two-electron reduction potentials and related molecular descriptors for reduction of alkyl and vinyl halides in water. Crit. Rev. Environ. Sci. Technol. 31, 175–221 (2001).
Liu, T. et al. Modular assembly of arenes, ethylene and heteroarenes for the synthesis of 1,2-arylheteroaryl ethanes. Nat. Chem. 16, 1705–1714 (2024).
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Palau, J. et al. Distinct dual C–Cl isotope fractionation patterns during anaerobic biodegradation of 1,2-dichloroethane: potential to characterize microbial degradation in the field. Environ. Sci. Technol. 51, 2685–2694 (2017).
Gan, G. et al. Identification of catalytic active sites in nitrogen-doped carbon for electrocatalytic dechlorination of 1,2-dichloroethane. ACS Catal. 9, 10931–10939 (2019).
Gan, G. et al. Nature of intrinsic defects in carbon materials for electrochemical dechlorination of 1,2-dichloroethane to ethylene. ACS Catal. 11, 14284–14292 (2021).
Cheon, S. et al. Neighboring catalytic sites are essential for electrochemical dechlorination of 2-chlorophenol. J. Am. Chem. Soc. 146, 25151–25157 (2024).
Gan, G. et al. Active sites in single-atom Fe–Nx–C nanosheets for selective electrochemical dechlorination of 1,2-dichloroethane to ethylene. ACS Nano 14, 9929–9937 (2020).
Nunez Garcia, A., Boparai, H. K. & O’Carroll, D. M. Enhanced dechlorination of 1,2-dichloroethane by coupled nano iron-dithionite treatment. Environ. Sci. Technol. 50, 5243–5251 (2016).
Gan, G. et al. Metal–nitrogen–carbon single‐atom aerogels as self‐supporting electrodes for dechlorination of 1,2‐dichloroethane. Adv. Funct. Mater. 32, 2206263 (2022).
Min, Y. et al. Mimicking reductive dehalogenases for efficient electrocatalytic water dechlorination. Nat. Commun. 14, 5134 (2023).
Wang, S. et al. Genomic characterization of three unique Dehalococcoides that respire on persistent polychlorinated biphenyls. Proc. Natl Acad. Sci. USA 111, 12103–12108 (2014).
Bommer, M. et al. Structural basis for organohalide respiration. Science 346, 455–458 (2014).
Ren, C. et al. A bioinspired molybdenum catalyst for aqueous perchlorate reduction. J. Am. Chem. Soc. 143, 7891–7896 (2021).
Qin, J. et al. An enzyme-mimic single Fe-N3 atom catalyst for the oxidative synthesis of nitriles via C–C bond cleavage strategy. Sci. Adv. 8, eadd1267 (2022).
Xu, N., Zhang, X., Guo, P.-C., Xie, D.-H. & Sheng, G.-P. Biological self-protection inspired engineering of nanomaterials to construct a robust bio-nano system for environmental applications. Sci. Adv. 10, eadp2179 (2024).
Zhang, S. et al. Insight into the mechanism underlying Dehalococcoides mccartyi strain CBDB1-mediated B12-dependent aromatic reductive dehalogenation. Environ. Sci. Technol. 57, 10773–10781 (2023).
Kunze, C. et al. Cobamide-mediated enzymatic reductive dehalogenation via long-range electron transfer. Nat. Commun. 8, 15858 (2017).
Wang, S. et al. Electron transport chains in organohalide-respiring bacteria and bioremediation implications. Biotechnol. Adv. 36, 1194–1206 (2018).
Heckel, B. & Elsner, M. Exploring mechanisms of biotic chlorinated alkane reduction: evidence of nucleophilic substitution (SN2) with vitamin B12. Environ. Sci. Technol. 56, 6325–6336 (2022).
Payne, K. A. P. et al. Reductive dehalogenase structure suggests a mechanism for B12-dependent dehalogenation. Nature 517, 513–516 (2014).
McCauley, K. M. et al. Properties and reactivity of chlorovinylcobalamin and vinylcobalamin and their implications for vitamin B12-catalyzed reductive dechlorination of chlorinated alkenes. J. Am. Chem. Soc. 127, 1126–1136 (2005).
Guo, M. & Chen, Y. Coenzyme cobalamin: biosynthesis, overproduction and its application in dehalogenation—a review. Rev. Environ. Sci. Biotechnol. 17, 259–284 (2018).
Dewey, C., Juillot, F., Fendorf, S. & Bargar, J. R. Seasonal oxygenation of contaminated floodplain soil releases Zn to porewater. Environ. Sci. Technol. 57, 4841–4851 (2023).
Gong, L., Qiu, X., Tratnyek, P. G., Liu, C. & He, F. FeNX(C)-coated microscale zero-valent iron for fast and stable trichloroethylene dechlorination in both acidic and basic pH conditions. Environ. Sci. Technol. 55, 5393–5402 (2021).
Gu, Y. et al. Sulfidation mitigates the passivation of zero valent iron at alkaline pHs: experimental evidences and mechanism. Water Res. 159, 233–241 (2019).
Low, A. et al. Isolation, characterization and bioaugmentation of an acidotolerant 1,2-dichloroethane respiring Desulfitobacterium species from a low pH aquifer. FEMS Microbiol. Ecol. 95, fiz055 (2019).
Schmidt, M., Lege, S. & Nijenhuis, I. Comparison of 1,2-dichloroethane, dichloroethene and vinyl chloride carbon stable isotope fractionation during dechlorination by two Dehalococcoides strains. Water Res. 52, 146–154 (2014).
Jeong, W.-G., Kim, J.-G. & Baek, K. Removal of 1,2-dichloroethane in groundwater using Fenton oxidation. J. Hazard. Mater. 428, 128253 (2022).
Zhou, Z. et al. Insights into enhanced removal of 1,2-dichloroethane by amorphous boron-enhanced Fenton system: performances and mechanisms. J. Hazard. Mater. 420, 126589 (2021).
Chen, W. et al. In-situ activation of persulfate by emplaced magnetite nanoparticles for degradation of 1,2-dichloroethane in porous media. Water Res. 268, 122574 (2025).
Sun, Z. et al. Vitamin B12 (CoII) initiates the reductive defluorination of branched perfluorooctane sulfonate (br-PFOS) in the presence of sulfide. Chem. Eng. J. 423, 130149 (2021).
Assaf-Anid, N., Hayes, K. F. & Vogel, T. M. Reduction dechlorination of carbon tetrachloride by cobalamin(II) in the presence of dithiothreitol: mechanistic study, effect of redox potential and pH. Environ. Sci. Technol. 28, 246–252 (1994).
Huang, C.-C., Lo, S.-L. & Lien, H.-L. Vitamin B12-mediated hydrodechlorination of dichloromethane by bimetallic Cu/Al particles. Chem. Eng. J. 273, 413–420 (2015).
Kim, Y. H. & Carraway, E. R. Reductive dechlorination of PCE and TCE by vitamin B12 and ZVMs. Environ. Technol. 23, 1135–1145 (2002).
Kliegman, S. & McNeill, K. Reconciling disparate models of the involvement of vinyl radicals in cobalamin-mediated dechlorination reactions. Environ. Sci. Technol. 43, 8961–8967 (2009).
Huang, D. et al. Elucidating the role of single-atom Pd for electrocatalytic hydrodechlorination. Environ. Sci. Technol. 55, 13306–13316 (2021).
He, F. et al. Dechlorination of excess trichloroethene by bimetallic and sulfidated nanoscale zero-valent iron. Environ. Sci. Technol. 52, 8627–8637 (2018).
Chiu, P.-C. & Reinhard, M. Transformation of carbon tetrachloride by reduced vitamin B12 in aqueous cysteine solution. Environ. Sci. Technol. 30, 1882–1889 (1996).
Bae, S., Collins, R. N., Waite, T. D. & Hanna, K. Advances in surface passivation of nanoscale zerovalent iron: a critical review. Environ. Sci. Technol. 52, 12010–12025 (2018).
Li, M. et al. Kirkendall effect boosts phosphorylated nZVI for efficient heavy metal wastewater treatment. Angew. Chem. Int. Ed. 60, 17115–17122 (2021).
Wei, K. et al. Strained zero‐valent iron for highly efficient heavy metal removal. Adv. Funct. Mater. 32, 2200498 (2022).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2011).
Cai, S. et al. Cations facilitate sulfidation of zero-valent iron by elemental sulfur: mechanism and dechlorination application. Water Res. 242, 120262 (2023).
Kang, Q. et al. A synthetic cell-free 36-enzyme reaction system for vitamin B12 production. Nat. Commun. 14, 5177 (2023).
Gong, L. et al. Coincorporation of N and S into zero-valent iron to enhance TCE dechlorination: kinetics, electron efficiency, and dechlorination capacity. Environ. Sci. Technol. 55, 16088–16098 (2021).
Frisch, M. et al. Gaussian 09, Revision D.01 (Gaussian, Inc., 2013).
Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 161, 082503 (2024).
Zhang, N. et al. Crystal engineering of TiO2 for enhanced catalytic oxidation of 1,2-dichloroethane on a Pt/TiO2 catalyst. Environ. Sci. Technol. 57, 7086–7096 (2023).
Yu, X. et al. High selectivity to HCl for the catalytic removal of 1,2-dichloroethane over RuP/3DOM WOx: insights into the effects of P-doping and H2O introduction. Environ. Sci. Technol. 55, 14906–14916 (2021).
El-Sharnouby, O., Boparai, H. K., Herrera, J. & O’Carroll, D. M. Aqueous-phase catalytic hydrodechlorination of 1,2-dichloroethane over palladium nanoparticles (nPd) with residual borohydride from nPd synthesis. Chem. Eng. J. 342, 281–292 (2018).
VanStone, N., Elsner, M., Lacrampe-Couloume, G., Mabury, S. & Lollar, B. S. Potential for identifying abiotic chloroalkane degradation mechanisms using carbon isotopic fractionation. Environ. Sci. Technol. 42, 126–132 (2007).
Liu, X., Vellanki, B. P., Batchelor, B. & Abdel-Wahab, A. Degradation of 1,2-dichloroethane with advanced reduction processes (ARPs): effects of process variables and mechanisms. Chem. Eng. J. 237, 300–307 (2014).
Koenig, J. C. et al. Particles and enzymes: combining nanoscale zero valent iron and organochlorine respiring bacteria for the detoxification of chloroethane mixtures. J. Hazard. Mater. 308, 106–112 (2016).
Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (grant nos. 42225704 to F.H., 42421005 to Z.W. and W2411032 to F.H.) and the Fundamental Research Funds for the Central Universities (grant no. JUSRP202407001 to F.H.).
Author information
Authors and Affiliations
Contributions
F.H. and B.X. conceived and designed the project. H.W., C.C., Z.C. and S.C. conducted the experiments and HRMS characterizations. B.Z., Y.Z. and B.L. carried out the electrochemical characterizations and DFT calculations. H.W., M.Y., B.L., Y.Z. and C.C. wrote the paper. F.H., B.G., Z.W. and B.X. helped with data analysis and paper polishing. F.H. and Z.W. supervised the project. All authors discussed the results and made rational suggestions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Jordi Palau and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–42, Tables 1–5 and References.
Source data
Source Data Figs. 1
Source data for Figs. 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Cheng, C., Zhao, B. et al. Efficient and selective dechlorination of chlorinated organic pollutants by cob(II)alamin and zero-valent iron. Nat Water 3, 1208–1218 (2025). https://doi.org/10.1038/s44221-025-00499-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00499-4