Abstract
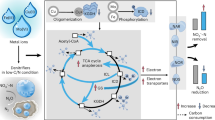
The artificial recycling of CO2 into value-added feedstocks and chemicals provides a sustainable approach to mitigate its greenhouse effect and realize a carbon-neutral economy, for which the direct and efficient utilization of CO2 reduction products without additional separation and purification remains challenging. Here an electrochemical–biological hybrid system has been developed to merge CO2 electrolysis with municipal wastewater treatment. In this set-up, the formate produced electrocatalytically (formate-e) in neutral electrolyte (1.0 M KHCO3) is directly applied as a carbon source and energy carrier for biological denitrification using activated sludge from municipal wastewater treatment plants, exhibiting an excellent nitrate nitrogen (NO3−-N) removal rate of ~3.06 mg l−1 h−1. Moreover, after long-term continuous operation of the tailored denitrification bioreactor, the formate-e displayed high denitrification rate of 1.08 mgNO3−-N per gram suspended solids per litre per hour, surpassing that of acetate, widely used as a commercial carbon source. Further environmental and techno-economic analyses suggest that integrating this electrochemical–biological hybrid system with an electrochemical recovery and separation system can significantly lower the cost of the electrolyte, thereby showing promise for the direct use of formate-e in industrial applications for wastewater treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the data that support the findings of this study are included in the Article and its Supplementary Information. Source data are provided with this paper. These data are also available via figshare at https://doi.org/10.6084/m9.figshare.29815832 (ref. 56).
References
Siegenthaler, U. & Sarmiento, J. L. Atmospheric carbon dioxide and the ocean. Nature 365, 119–125 (1993).
Liu, Z., Deng, Z., Davis, S. & Ciais, P. Monitoring global carbon emissions in 2022. Nat. Rev. Earth Environ. 4, 205–206 (2023).
MacDowell, N., Fennell, P. S., Shah, N. & Maitland, G. C. The role of CO2 capture and utilization in mitigating climate change. Nat. Clim. Change 7, 243–249 (2017).
Jordaan, S. M. & Wang, C. Electrocatalytic conversion of carbon dioxide for the Paris goals. Nat. Catal. 4, 915–920 (2021).
Overa, S., Feric, T. G., Park, A.-H. A. & Jiao, F. Tandem and hybrid processes for carbon dioxide utilization. Joule 5, 8–13 (2021).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Overa, S., Ko, B. H., Zhao, Y. & Jiao, F. Electrochemical approaches for CO2 conversion to chemicals: a journey toward practical applications. Acc. Chem. Res. 55, 638–648 (2022).
Liu, Z., Yang, H., Kutz, R. & Masel, R. I. CO2 electrolysis to CO and O2 at high selectivity, stability and efficiency using sustainion membranes. J. Electrochem. Soc. 165, J3371 (2018).
Han, N., Ding, P., He, L., Li, Y. & Li, Y. Promises of main group metal-based nanostructured materials for electrochemical CO2 reduction to formate. Adv. Energy Mater. 10, 1902338 (2020).
Li, C. W. & Kanan, M. W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 134, 7231–7234 (2012).
Zhang, G. et al. Selective CO2 electroreduction to methanol via enhanced oxygen bonding. Nat. Commun. 13, 7768 (2022).
Qiu, X.-F. et al. A stable and conductive covalent organic framework with isolated active sites for highly selective electroreduction of carbon dioxide to acetate. Angew. Chem. Int. Ed. 61, e202206470 (2022).
Zhang, J. et al. Accelerating electrochemical CO2 reduction to multi-carbon products via asymmetric intermediate binding at confined nanointerfaces. Nat. Commun. 14, 1298 (2023).
Xia, W. et al. Adjacent copper single atoms promote C–C coupling in electrochemical CO2 reduction for the efficient conversion of ethanol. J. Am. Chem. Soc. 145, 17253–17264 (2023).
Yang, P.-P. et al. Highly enhanced chloride adsorption mediates efficient neutral CO2 electroreduction over a dual-phase copper catalyst. J. Am. Chem. Soc. 145, 8714–8725 (2023).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Möller, T. et al. The product selectivity zones in gas diffusion electrodes during the electrocatalytic reduction of CO2. Energy Environ. Sci. 14, 5995–6006 (2021).
Ren, S. et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 365, 367–369 (2019).
Zhou, Y., Ganganahalli, R., Verma, S., Tan, H. R. & Yeo, B. S. Production of C3–C6 acetate esters via CO electroreduction in a membrane electrode assembly cell. Angew. Chem. Int. Ed. 61, e202202859 (2022).
Gu, Z. et al. Efficient electrocatalytic CO2 reduction to C2+ alcohols at defect-site-rich Cu surface. Joule 5, 429–440 (2021).
Seger, B., Robert, M. & Jiao, F. Best practices for electrochemical reduction of carbon dioxide. Nat. Sustain. 6, 236–238 (2023).
Zheng, T. et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nat. Catal. 5, 388–396 (2022).
Bi, H. et al. Biofuel synthesis from carbon dioxide via a bio-electrocatalysis system. Chem Catal. 3, 100557 (2023).
Hann, E. C. et al. A hybrid inorganic–biological artificial photosynthesis system for energy-efficient food production. Nat. Food 3, 461–471 (2022).
Wang, X.-H., Wang, X., Huppes, G., Heijungs, R. & Ren, N.-Q. Environmental implications of increasingly stringent sewage discharge standards in municipal wastewater treatment plants: case study of a cool area of China. J. Clean. Prod. 94, 278–283 (2015).
Ghafari, S., Hasan, M. & Aroua, M. K. Bio-electrochemical removal of nitrate from water and wastewater—a review. Bioresour. Technol. 99, 3965–3974 (2008).
Xu, Z., Dai, X. & Chai, X. Effect of different carbon sources on denitrification performance, microbial community structure and denitrification genes. Sci. Total Environ. 634, 195–204 (2018).
Tian, Y. et al. Glycine adversely affects enhanced biological phosphorus removal. Water Res. 209, 117894 (2022).
Gu, X. et al. Influence mechanism of C/N ratio on heterotrophic nitrification- aerobic denitrification process. Bioresour. Technol. 343, 126116 (2022).
Qiu, G. et al. Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources. Water Res. 149, 496–510 (2019).
Xue, Z. et al. An alternative carbon source withdrawn from anaerobic fermentation of soybean wastewater to improve the deep denitrification of tail water. Biochem. Eng. J. 132, 217–224 (2018).
Kargi, F. & Uygur, A. Effect of carbon source on biological nutrient removal in a sequencing batch reactor. Bioresour. Technol. 89, 89–93 (2003).
Wang, T. et al. Halogen-incorporated Sn catalysts for selective electrochemical CO2 reduction to formate. Angew. Chem. Int. Ed. 62, e202211174 (2023).
Zhu, J. et al. Surface passivation for highly active, selective, stable, and scalable CO2 electroreduction. Nat. Commun. 14, 4670 (2023).
Liu, L., Jiang, J., Jin, S., Xia, Z. & Tang, M. Hydrothermal synthesis of β-bismuth oxide nanowires from particles. CrystEngComm 13, 2529–2532 (2011).
Wang, Z. et al. Carbon-confined indium oxides for efficient carbon dioxide reduction in a solid-state electrolyte flow cell. Angew. Chem. Int. Ed. 61, e202200552 (2022).
Zheng, Y. et al. Toward more efficient carbon-based electrocatalysts for hydrogen peroxide synthesis: roles of cobalt and carbon defects in two-electron ORR catalysis. Nano Lett. 23, 1100–1108 (2023).
Bi, J. et al. High-rate CO2 electrolysis to formic acid over a wide potential window: an electrocatalyst comprised of indium nanoparticles on chitosan-derived graphene. Angew. Chem. Int. Ed. 62, e202307612 (2023).
Wagner, A., Sahm, C. D. & Reisner, E. Towards molecular understanding of local chemical environment effects in electro- and photocatalytic CO2 reduction. Nat. Catal. 3, 775–786 (2020).
Wu, Q. et al. Nanograin-boundary-abundant Cu2O-Cu nanocubes with high C2+ selectivity and good stability during electrochemical CO2 reduction at a current density of 500 mA/cm2. ACS Nano 17, 12884–12894 (2023).
Xing, Z., Hu, L., Ripatti, D. S., Hu, X. & Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 12, 136 (2021).
Arán-Ais, R. M., Scholten, F., Kunze, S., Rizo, R. & Roldan Cuenya, B. The role of in situ generated morphological motifs and Cu(I) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat. Energy 5, 317–325 (2020).
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
Hung, S.-F. et al. Operando X-ray absorption spectroscopic studies of the carbon dioxide reduction reaction in a modified flow cell. Catal. Sci. Technol. 12, 2739–2743 (2022).
Deng, P. et al. Bismuth oxides with enhanced bismuth–oxygen structure for efficient electrochemical reduction of carbon dioxide to formate. ACS Catal. 10, 743–750 (2020).
Li, Z. et al. In situ investigations on Bi-based electrocatalyst construction and reaction dynamic monitoring toward efficient CO2 reduction. Chem Catal. 3, 100767 (2023).
Liang, X.-D. et al. In-situ constructing Bi@Bi2O2CO3 nanosheet catalyst for ampere-level CO2 electroreduction to formate. Nano Energy 114, 108638 (2023).
Lu, H., Chandran, K. & Stensel, D. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 64, 237–254 (2014).
Kelly, J. J. et al. DNA microarray detection of nitrifying bacterial 16s rRNA in wastewater treatment plant samples. Water Res. 39, 3229–3238 (2005).
Srinandan, C. S., D’souza, G., Srivastava, N., Nayak, B. B. & Nerurkar, A. S. Carbon sources influence the nitrate removal activity, community structure and biofilm architecture. Bioresour. Technol. 117, 292–299 (2012).
McIlroy, S. J. et al. Identification of active denitrifiers in full-scale nutrient removal wastewater treatment systems. Environ. Microbiol. 18, 50–64 (2016).
McIlroy, S. J. et al. ‘Candidatus Competibacter’-lineage genomes retrieved from metagenomes reveal functional metabolic diversity. ISME J. 8, 613–624 (2014).
Dong, N., Zeng, Z., Russenberger, M., Zhou, L. & Zhuang, W.-Q. Investigating cake layer development and functional genes in formate- and acetate-driven heterotrophic denitrifying AnMBRs. Chem. Eng. J. 485, 149623 (2024).
Wang, P. et al. Integrated system for electrolyte recovery, product separation, and CO2 capture in CO2 reduction. Nat. Commun. 16, 731 (2025).
Chen, J. et al. Relating the carbon sources to denitrifying community in full-scale wastewater treatment plants. Chemosphere 361, 142329 (2024).
Wu, Q. et al. Realizing the practical application of CO2 electroreduction for urban wastewater denitrification. figshare https://doi.org/10.6084/m9.figshare.29815832 (2025).
Acknowledgements
G.C., G.Q. and W.-W.Z. thank the support of the National Natural Science Foundation of China (22471077, 21971070, 52270035 and 22374066), the Guangdong Basic and Applied Basic Research Foundation (2022A1515012047), the Guangdong Innovative and Entrepreneurial Research Team Program (2019ZT08L075), the Guangdong Pearl River Talent Program (2019QN01L159, 2019QN01L125), the Science and Technology Program of Guangzhou (202103040002, 2025A04J7050) and the National Key R&D Program of China (2023YFC3708505). K.-S.P., Y.-Y.C. and S.-F.H. acknowledge the support from the National Science and Technology Council, Taiwan (Contract Number NSTC 114-2628-M-A49-005). We thank the support from the Yushan Young Scholar Program (MOE-114-YSFMS-0010-003-P2) and the Center for Emergent Functional Matter Science, Ministry of Education, Taiwan. X.W. acknowledges support through an ECS grant from the Research Grants Council of the Hong Kong Special Administrative Region (project number 21300323) and the CityU start-up fund (project number 9610600).
Author information
Authors and Affiliations
Contributions
G.C. and G.Q. conceived the idea. Q.W., R.D., H.W. and P.W. prepared the catalysts and conducted the CO2 electroreduction tests. S.J., J.C. and Q.W. conducted a denitrification batch experiment. Y.Q., K.Y., Y.Z. and L.Z. contributed to thorough discussions on this work. K.-S.P., Y.-Y.C. and S.-F.H. carried out the operando XAS measurements. X.-Q.T. and W.-J.O. conducted the LCA analysis. G.Q., W.-W.Z. and G.C. proposed the idea, designed the experiments, analysed the data and wrote the paper with editing from G.Q. and X.W. G.C. supervised and coordinated all investigators in this project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Ke Xie, Cesar Torres and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–39, Tables 1–12 and references.
Supplementary Data
Source data for Supplementary Figs. 1–39.
Source data
Source Data Figs. 2–5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Q., Ji, S., Chen, J. et al. Realizing the practical application of CO2 electroreduction for urban wastewater denitrification. Nat Water (2025). https://doi.org/10.1038/s44221-025-00516-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44221-025-00516-6