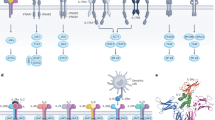
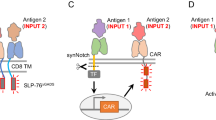
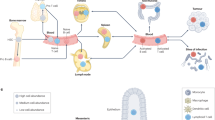
Abstract
Cytokines have pivotal roles in immunity, making them attractive as therapeutics for a variety of immune-related disorders. However, the widespread clinical use of cytokines has been limited by their short blood half-lives and severe side effects caused by low specificity and unfavourable biodistribution. Innovations in bioengineering have aided in advancing our knowledge of cytokine biology and yielded new technologies for cytokine engineering. In this Review, we discuss how the development of bioanalytical methods, such as sequencing and high-resolution imaging combined with genetic techniques, have facilitated a better understanding of cytokine biology. We then present an overview of therapeutics arising from cytokine re-engineering, targeting and delivery, mRNA therapeutics and cell therapy. We also highlight the application of these strategies to adjust the immunological imbalance in different immune-mediated disorders, including cancer, infection and autoimmune diseases. Finally, we look ahead to the hurdles that must be overcome before cytokine therapeutics can live up to their full potential.
Key points
-
Cytokines are crucial regulators of the immune system and have important roles in health and disease.
-
Recombinant cytokine therapy is hampered by the short blood half-life and severe side effects of cytokines.
-
Increased knowledge of cytokine biology converges with new bioengineering approaches to develop engineered cytokine therapeutics.
-
Cytokine therapeutics can be applied to modulate dysregulated immune responses in disorders such as cancer or autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
27 March 2023
A Correction to this paper has been published: https://doi.org/10.1038/s44222-023-00057-1
References
Chaplin, D. D. Overview of the immune response. J. Allergy Clin. Immunol. 125, S3–S23 (2010).
Altan-Bonnet, G. & Mukherjee, R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat. Rev. Immunol. 19, 205–217 (2019).
Stanley, A. C. & Lacy, P. Pathways for cytokine secretion. Physiology 25, 218–229 (2010).
Leonardi, C. L. et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 371, 1665–1674 (2008).
Saenz, S. A., Taylor, B. C. & Artis, D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226, 172–190 (2008).
Arango Duque, G. & Descoteaux, A. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5, 491 (2014).
Eyerich, S., Eyerich, K., Cavani, A. & Schmidt-Weber, C. IL-17 and IL-22: siblings, not twins. Trends Immunol. 31, 354–361 (2010).
Opal, S. M. & DePalo, V. A. Anti-inflammatory cytokines. Chest 117, 1162–1172 (2000).
Gharee-Kermani, M. & Pham, S. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr. Pharm. Des. 7, 1083–1103 (2001).
Hughes, C. E. & Nibbs, R. J. B. A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 (2018).
Borden, E. C. et al. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 6, 975–990 (2007).
Hamilton, J. A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 (2008).
Roan, F., Obata-Ninomiya, K. & Ziegler, S. F. Epithelial cell-derived cytokines: more than just signaling the alarm. J. Clin. Invest. 129, 1441–1451 (2019).
Smith, D. E. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin. Exp. Allergy 40, 200–208 (2010).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11, 85–97 (2011).
Ihle, J. N. Cytokine receptor signalling. Nature 377, 591–594 (1995).
O’Shea, J. J., Gadina, M. & Siegel, R. M. in Clinical Immunology 5th edn Ch. 9 (eds Rich, R. R. et al.) 127–155.e1 (2019).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23, 89–100.e5 (2018).
Guo, L., Junttila, I. S. & Paul, W. E. Cytokine-induced cytokine production by conventional and innate lymphoid cells. Trends Immunol. 33, 598 (2012).
Rousset, F., Garcia, E. & Banchereau, J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J. Exp. Med. 173, 705–710 (1991).
Tough, D. F., Borrow, P. & Sprent, J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272, 1947–1950 (1996).
Ross, M. E. & Caligiuri, M. A. Cytokine-induced apoptosis of human natural killer cells identifies a novel mechanism to regulate the innate immune response. Blood 89, 910–918 (1997).
Lin, J. X. et al. The role of shared receptor motifs and common stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 2, 331–339 (1995).
Berraondo, P. et al. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 120, 6–15 (2019).
Ozaki, K. & Leonard, W. J. Cytokine and cytokine receptor pleiotropy and redundancy. J. Biol. Chem. 277, 29355–29358 (2002).
Ruddy, M. J. et al. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 279, 2559–2567 (2004).
Yoshimoto, T. et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-γ production. J. Immunol. 161, 3400–3407 (1998).
Waldmann, T. A. The shared and contrasting roles of interleukin-2 (IL-2) and IL-15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer Immunol. Res. 3, 219 (2015).
Royall, J. A. et al. Tumor necrosis factor and interleukin 1 alpha increase vascular endothelial permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 257, L399–L410 (1989).
Hafler, D. A. Cytokines and interventional immunology. Nat. Rev. Immunol. 7, 423–423 (2007).
Monaco, C., Nanchahal, J., Taylor, P. & Feldmann, M. Anti-TNF therapy: past, present and future. Int. Immunol. 27, 55–62 (2015).
Ly, K. et al. Anti IL-17 in psoriasis. Expert Rev. Clin. Immunol. 15, 1185–1194 (2019).
van de Veerdonk, F. L. et al. A guide to immunotherapy for COVID-19. Nat. Med. 28, 39–50 (2022).
Herman, A. C., Boone, T. C. & Lu, H. S. in Formulation, Characterization, and Stability of Protein Drugs (eds Pearlman, R. & Wang, Y. J.) 303–328 (Springer, 2002).
Rasenack, J. et al. Peginterferon alpha-2a (40kD) [Pegasys] improves HR-QOL outcomes compared with unmodified interferon alpha-2a [Roferon-A]. Pharmacoeconomics 21, 341–349 (2003).
Baldo, B. A. Side effects of cytokines approved for therapy. Drug Safety 37, 921–943 (2014).
Golomb, H. M. et al. Alpha-2 interferon therapy of hairy-cell leukemia: a multicenter study of 64 patients. J. Clin. Oncol. 4, 900–905 (1986).
Cohen, J. IL-12 deaths: explanation and a puzzle. Science 270, 908 (1995).
Atkins, M. B. et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 17, 2105–2116 (1999).
Saiki, R. K. et al. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239, 487–491 (1988).
Sanger, F., Nicklen, S. & Coulson, A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl Acad. Sci. USA 74, 5463–5467 (1977).
Karas, M. & Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 60, 2299–2301 (1988).
Tanaka, K. et al. Protein and polymer analyses up to mlz 100 000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2, 151–153 (1988).
Williamson, M. P., Havel, T. F. & Wüthrich, K. Solution conformation of proteinase inhibitor IIA from bull seminal plasma by 1H nuclear magnetic resonance and distance geometry. J. Mol. Biol. 182, 295–315 (1985).
Woolfson, M. M. The development of structural x-ray crystallography. Phys. Scr. 93, 032501 (2018).
Matadeen, R., Hon, W. C., Heath, J. K., Jones, E. Y. & Fuller, S. The dynamics of signal triggering in a gp130-receptor complex. Structure 15, 441–448 (2007).
Wang, X., Rickert, M., Garcia, K. C. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science 310, 1159–1163 (2005).
Gaffen, S. L. Signaling domains of the interleukin 2 receptor. Cytokine 14, 63–77 (2001).
Johnson, K., Granzow, R., Creasey, A. & Ciardelli, T. Ligand binding analyses and functional activity of interleukin-2 receptor ectodomains. Methods 6, 199–205 (1994).
Truneh, A. et al. Temperature-sensitive differential affinity of TRAIL for its receptors: DR5 is the highest affinity receptor. J. Biol. Chem. 275, 23319–23325 (2000).
Levin, A. M. et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 484, 529–533 (2012). The researchers employed directed evolution to engineer an IL-2 ‘superkine’ (MDNA11) with improved antitumour properties and reduced toxicity that is now undergoing a phase I/II clinical trial.
Lumsden, A. & Wilkinson, D. The promise of gene ablation. Nature 347, 335–336 (1990).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Kluger, M. J., Kozak, W., Leon, L. R. & Conn, C. A. The use of knockout mice to understand the role of cytokines in fever. Clin. Exp. Pharmacol. Physiol. 25, 141–144 (1998).
Ingman, W. V. & Jones, R. L. Cytokine knockouts in reproduction: the use of gene ablation to dissect roles of cytokines in reproductive biology. Hum. Reprod. Update 14, 179–192 (2008).
McCaughtry, T. M. et al. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J. Exp. Med. 209, 2263–2276 (2012).
Archambault, L. S., Trzilova, D., Gonia, S., Gale, C. & Wheeler, R. T. Intravital imaging reveals divergent cytokine and cellular immune responses to Candida albicans and Candida parapsilosis. mBio 10, e00266-19 (2019).
van de Donk, P. P. et al. Interleukin-2 PET imaging in patients with metastatic melanoma before and during immune checkpoint inhibitor therapy. Eur. J. Nucl. Med. Mol. Imaging 48, 4369–4376 (2021).
Park, L. M., Lannigan, J. & Jaimes, M. C. OMIP‐069: forty‐color full spectrum flow cytometry panel for deep immunophenotyping of major cell subsets in human peripheral blood. Cytometry 97, 1044 (2020).
Zhang, T., Warden, A. R., Li, Y. & Ding, X. Progress and applications of mass cytometry in sketching immune landscapes. Clin. Transl Med. 10, e206 (2020).
Iyer, A., Hamers, A. A. J. & Pillai, A. B. CyTOF® for the masses. Front. Immunol. 13, 815828 (2022).
Cano-Gamez, E. et al. Single-cell transcriptomics identifies an effectorness gradient shaping the response of CD4+ T cells to cytokines. Nat. Commun. 11, 1801 (2020).
Jiang, P. et al. Systematic investigation of cytokine signaling activity at the tissue and single-cell levels. Nat. Methods 18, 1181–1191 (2021).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 20, 375–388 (2020).
Hahn, N. et al. The orphan cytokine receptor CRLF3 emerged with the origin of the nervous system and is a neuroprotective erythropoietin receptor in locusts. Front. Mol. Neurosci. 12, 251 (2019).
Carter, P. Site-directed mutagenesis. Biochemistry 237, 1–7 (1986).
Packer, M. S. & Liu, D. R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 16, 379–394 (2015).
Krieg, C., Létourneau, S., Pantaleo, G. & Boyman, O. Improved IL-2 immunotherapy by selective stimulation of IL-2 receptors on lymphocytes and endothelial cells. Proc. Natl Acad. Sci. USA 107, 11906–11911 (2010).
Junttila, I. S. et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat. Chem. Biol. 8, 990–998 (2012).
Mueller, T. D., Zhang, J.-L., Sebald, W. & Duschl, A. Structure, binding, and antagonists in the IL-4/IL-13 receptor system. Biochim. Biophys. Acta Mol. Cell Res. 1592, 237–250 (2002).
Gorby, C. et al. Engineered IL-10 variants elicit potent immunomodulatory effects at low ligand doses. Sci. Signal. 13, eabc0653 (2020).
Zhou, T. et al. IL-18BP is a secreted immune checkpoint and barrier to IL-18 immunotherapy. Nature 583, 609–614 (2020).
Spangler, J. B., Moraga, I., Mendoza, J. L. & Garcia, K. C. Insights into cytokine–receptor interactions from cytokine engineering. Annu. Rev. Immunol. 33, 139–167 (2015).
Saxton, R. A. et al. The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity 54, 660–672.e9 (2021).
Saxton, R. A. et al. Structure-based decoupling of the pro- and anti-inflammatory functions of interleukin-10. Science 371, eabc8433 (2021).
Mendoza, J. L. et al. Structure of the IFNγ receptor complex guides design of biased agonists. Nature 567, 56–60 (2019).
Glassman, C. R. et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell 184, 983–999.e24 (2021).
Carmenate, T. et al. Human IL-2 mutein with higher antitumor efficacy than wild type IL-2. J. Immunol. 190, 6230–6238 (2013).
Chen, X. et al. A novel human IL-2 mutein with minimal systemic toxicity exerts greater antitumor efficacy than wild-type IL-2. Cell Death Dis. 9, 989 (2018).
Shanafelt, A. B. et al. A T-cell-selective interleukin 2 mutein exhibits potent antitumor activity and is well tolerated in vivo. Nat. Biotechnol. 18, 1197–1202 (2000).
Peterson, L. B. et al. A long-lived IL-2 mutein that selectively activates and expands regulatory T cells as a therapy for autoimmune disease. J. Autoimmun. 95, 1–14 (2018).
Ghelani, A. et al. Defining the threshold IL-2 signal required for induction of selective Treg cell responses using engineered IL-2 muteins. Front. Immunol. 11, 1106 (2020).
Visweswaraiah, J. et al. Generation of PT101, a highly selective IL-2 mutein for treatment of autoimmune diseases. Ann. Rheum. Dis. 80, 13–13 (2021).
Khoryati, L. et al. An IL-2 mutein engineered to promote expansion of regulatory T cells arrests ongoing autoimmunity in mice. Sci. Immunol. 5, eaba5264 (2020).
Milton Harris, J. & Chess, R. B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
Molineux, G. Pegylation: engineering improved biopharmaceuticals for oncology. Pharmacotherapy 23, 3S–8S (2003).
Meyers, F. J., Paradise, C., Scudder, S. A., Goodman, G. & Konrad, M. A phase I study including pharmacokinetics of polyethylene glycol conjugated interleukin-2. Clin. Pharmacol. Ther. 49, 307–313 (1991).
Mattijssen, V. et al. Intratumoral PEG-interleukin-2 therapy in patients with locoregionally recurrent head and neck squamous-cell carcinoma. Ann. Oncol. 5, 957–960 (1994).
Emmerich, J. et al. IL-10 directly activates and expands tumor-resident CD8+ T cells without de novo infiltration from secondary lymphoid organs. Cancer Res. 72, 3570–3581 (2012).
Naing, A. et al. PEGylated IL-10 (pegilodecakin) induces systemic immune activation, CD8+ T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell 34, 775–791.e3 (2018).
Ptacin, J. L. et al. An engineered IL-2 reprogrammed for anti-tumor therapy using a semi-synthetic organism. Nat. Commun. 12, 4785 (2021). The researchers developed a rationally PEGylated form of IL-2 (THOR-707) that selectively expands and activates effector T cells and NK cells and is undergoing a phase I/II clinical trial.
Charych, D. H. et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res. 22, 680–690 (2016).
Dixit, N. et al. NKTR-358: a novel regulatory T-cell stimulator that selectively stimulates expansion and suppressive function of regulatory T cells for the treatment of autoimmune and inflammatory diseases. J. Transl Autoimmun. 4, 100103 (2021).
Bristol Myers Squibb. Nektar and Bristol Myers Squibb announce update on clinical development program for bempegaldesleukin (BEMPEG) in pdivo (nivolumab). BMS https://news.bms.com/news/details/2022/Nektar-and-Bristol-Myers-Squibb-Announce-Update-on-Clinical-Development-Program-for-Bempegaldesleukin-BEMPEG-in-Combination-with-Opdivo-nivolumab/default.aspx (2022).
Miyazaki, T. et al. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J. Immunother. Cancer 9, e002024 (2021).
Noren, C. J., Anthony-Cahill, S. J., Griffith, M. C. & Schultz, P. G. A general method for site-specific incorporation of unnatural amino acids into proteins. Science 244, 182–188 (1989).
Yang, Q. & Lai, S. K. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdisc. Rev. Nanomed. Nanobiotechnol. 7, 655–677 (2015).
Sellaturay, P., Nasser, S., Islam, S., Gurugama, P. & Ewan, P. W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 51, 861–863 (2021).
Anishchenko, I. et al. De novo protein design by deep network hallucination. Nature 600, 547–552 (2021).
Biswas, S., Khimulya, G., Alley, E. C., Esvelt, K. M. & Church, G. M. Low-N protein engineering with data-efficient deep learning. Nat. Methods 18, 389–396 (2021).
Pan, X. & Kortemme, T. Recent advances in de novo protein design: principles, methods, and applications. J. Biol. Chem. 296, 100558 (2021).
Huang, P.-S., Boyken, S. E. & Baker, D. The coming of age of de novo protein design. Nature 537, 320–327 (2016).
Goldenzweig, A. et al. Automated structure- and sequence-based design of proteins for high bacterial expression and stability. Mol. Cell 63, 337–346 (2016).
Khersonsky, O. et al. Automated design of efficient and functionally diverse enzyme repertoires. Mol. Cell 72, 178–186.e5 (2018).
Correia, B. E. et al. Proof of principle for epitope-focused vaccine design. Nature 507, 201–206 (2014).
Silva, D. A. et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature 565, 186–191 (2019). This research is the first demonstration of how de novo protein design can be used to engineer an improved cytokine-receptor agonist (Neo-2/15) which is now in phase I/II clinical trials (NL-201).
Wang, J., Cao, H., Zhang, J. Z. H. & Qi, Y. Computational protein design with deep learning neural networks. Sci. Rep. 8, 6349 (2018).
Lin, E., Lin, C. H. & Lane, H. Y. De novo peptide and protein design using generative adversarial networks: an update. J. Chem. Inf. Model. 62, 761–774 (2022).
Leman, J. K. et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat. Methods 17, 665–680 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Yen, M. et al. Facile discovery of surrogate cytokine agonists. Cell 185, 1414–1430.e19 (2022).
Moraga, I. et al. Synthekines are surrogate cytokine and growth factor agonists that compel signaling through non-natural receptor dimers. eLife 6, 22882 (2017).
Emmerich, J. et al. Abstract 1744: STK-012, an α/β selective IL-2 mutein for the activation of the antigen-activated T cells in solid tumor. Cancer Res. 81, 1744–1744 (2021).
Ren, J. et al. Interleukin-2 superkines by computational design. Proc. Natl Acad. Sci. USA 119, e2117401119 (2022).
Harvill, E. T. & Morrison, S. L. An IgG3–IL2 fusion protein activates complement, binds FcγRI, generates LAK activity and shows enhanced binding to the high affinity IL-2R. Immunotechnology 1, 95–105 (1995).
Peng, L. S., Penichet, M. L. & Morrison, S. L. A single-chain IL-12 IgG3 antibody fusion protein retains antibody specificity and IL-12 bioactivity and demonstrates antitumor activity. J. Immunol. 163, 250–258 (1999).
Halin, C. et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat. Biotechnol. 20, 264–269 (2002).
Castellani, P. et al. The fibronectin isoform containing the ED-B oncofetal domain: a marker of angiogenesis. Int. J. Cancer 59, 612–618 (1994).
Fallon, J. et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget 5, 1869–1884 (2014). The researchers fused IL-12 to a histone-binding antibody to engineer an immunocytokine target’s exposed DNA found at the site of the tumour.
Zhang, L. et al. Imaging the alternatively spliced D domain of tenascin C in preclinical models of inflammatory bowel disease. Mol. Imaging Biol. https://doi.org/10.1007/s11307-022-01758-6 (2022).
Tzeng, A., Kwan, B. H., Opel, C. F., Navaratna, T. & Wittrup, K. D. Antigen specificity can be irrelevant to immunocytokine efficacy and biodistribution. Proc. Natl Acad. Sci. USA 112, 3320–3325 (2015).
Spangler, J. B. et al. Engineering a single-agent cytokine/antibody fusion that selectively expands regulatory T cells for autoimmune disease therapy. J. Immunol. 201, 2094–2106 (2018).
Huyghe, L. et al. Safe eradication of large established tumors using neovasculature-targeted tumor necrosis factor-based therapies. EMBO Mol. Med. 12, e11223 (2020).
Cauwels, A. & Tavernier, J. Tolerizing strategies for the treatment of autoimmune diseases: from ex vivo to in vivo strategies. Front. Immunol. 11, 674 (2020).
Hank, J. A. et al. Immunogenicity of the Hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin. Cancer Res. 15, 5923–5930 (2009).
Xu, S. et al. The role of collagen in cancer: from bench to bedside. J. Transl Med. 17, 309 (2019).
Momin, N. et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl Med. 11, 2614 (2019). The researchers fused pro-inflammatory cytokines to a collagen-binding protein to engineer cytokine fusion proteins with increased tumour retention.
Agarwal, Y. et al. Intratumourally injected alum-tethered cytokines elicit potent and safer local and systemic anticancer immunity. Nat. Biomed. Eng. 6, 129–143 (2022).
Mansurov, A. et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. 4, 531–543 (2020).
Ishihara, J. et al. Targeted antibody and cytokine cancer immunotherapies through collagen affinity. Sci. Transl Med. 11, 3259 (2019).
Rautio, J., Meanwell, N. A., Di, L. & Hageman, M. J. The expanding role of prodrugs in contemporary drug design and development. Nat. Rev. Drug Discov. 17, 559–587 (2018).
López-Otín, C. & Matrisian, L. M. Emerging roles of proteases in tumour suppression. Nat. Rev. Cancer 7, 800–808 (2007).
Hsu, E. J. et al. A cytokine receptor-masked IL2 prodrug selectively activates tumor-infiltrating lymphocytes for potent antitumor therapy. Nat. Commun. 12, 2768 (2021).
O’Neil, J. et al. Tumor-selective activity of XTX202, a protein-engineered IL-2, in mice without peripheral toxicities in nonhuman primates. J. Clin. Oncol. 39, 2563–2563 (2021). The researchers developed an IL-2 prodrug that is inactive until the inactivation unit is cleaved by tumour-associated proteases.
Mansurov, A. et al. Masking the immunotoxicity of interleukin-12 by fusing it with a domain of its receptor via a tumour-protease-cleavable linker. Nat. Biomed. Eng. 6, 819–829 (2022).
Nirschl, C. et al. WTX-124 is a novel IL-2 pro-drug that is conditionally activated in tumors and drives antitumor immunity in murine syngeneic cancer models. J. Immunother. Cancer 9, A747–A747 (2021).
Steiner, P. et al. Conditionally activated IL-12 or IFNα indukineTM molecules inhibit syngeneic lymphoma tumor growth in mice, induce anti-tumor immune responses and are tolerated in non-human primates. Blood 138, 2258–2258 (2021).
Rosen, D. B. et al. TransConTM IL-2 β/γ: a novel long-acting prodrug of receptor-biased IL-2 designed for improved pharmocokinetics and optimal activation of T cells for the treatment of cancer [abstract 4507]. Immunology 80, 4507–4507 (2020).
Zhang, Y., Li, N., Suh, H. & Irvine, D. J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 9, 6 (2018). The researchers anchored IL-2 and anti-CD137 on the surface of liposomes to improve tumour accumulation and decrease systemic toxicity.
Kamaly, N. et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano 10, 5280–5292 (2016).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Kawasaki, T. & Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 5, 461 (2014).
Nance, K. D. & Meier, J. L. Modifications in an emergency: the role of N1-methylpseudouridine in COVID-19 vaccines. ACS Cent. Sci. 7, 748–756 (2021).
Andries, O. et al. N1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release 217, 337–344 (2015).
Karikó, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Alameh, M.-G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e7 (2021).
Li, C. et al. Mechanisms of innate and adaptive immunity to the Pfizer–BioNTech BNT162b2 vaccine. Nat. Immunol. 23, 543–555 (2022).
Li, Y. et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1, 882–893 (2020).
Liu, J. Q. et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release 345, 306–313 (2022).
Hewitt, S. L. et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36g, and OX40L mRNAs. Sci. Transl Med. 11, 9143 (2019). In this study, a cocktail of mRNA-encoding cytokines (IL-23, IL-36γ and OX40L) was intratumorally administered for cancer treatment.
Hotz, C. et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl Med. 13, eabc7804 (2021).
Jain, R. et al. MicroRNAs enable mRNA therapeutics to selectively program cancer cells to self-destruct. Nucleic Acid. Ther. 28, 285–296 (2018).
Melero, I., Castanon, E., Alvarez, M., Champiat, S. & Marabelle, A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 18, 558–576 (2021).
Beck, J. D. et al. mRNA therapeutics in cancer immunotherapy. Mol. Cancer 20, 69 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04455620 (2023).
Lai, I. et al. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J. Immunother. Cancer 6, 125 (2018). In this study, a lipid nanoparticle encapsulating mRNA encoding IL-12 was intravenously administered to treat hepatocellular carcinoma.
Lei, S. et al. Efficient colorectal cancer gene therapy with IL-15 mRNA nanoformulation. Mol. Pharm. 17, 3378–3391 (2020).
Tahtinen, S. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23, 532–542 (2022).
Veiga, N. et al. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 9, 4493 (2018).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
van Leent, M. M. T. et al. Regulating trained immunity with nanomedicine. Nat. Rev. Mater. 7, 465–481 (2022).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Seimetz, D., Heller, K. & Richter, J. Approval of first CAR-Ts: have we solved all hurdles for ATMPs? Cell Med. 11, 215517901882278 (2019).
Morotti, M. et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br. J. Cancer 124, 1759–1776 (2021).
Gajewski, T. F. et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 25, 268–276 (2013).
Koneru, M., Purdon, T. J., Spriggs, D., Koneru, S. & Brentjens, R. J. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 4, e994446 (2015).
Yeku, O. O., Purdon, T. J., Koneru, M., Spriggs, D. & Brentjens, R. J. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 7, 10541 (2017).
Zhang, L. et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin. Cancer Res. 21, 2278–2288 (2015).
Shah, N. N. & Fry, T. J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 16, 372–385 (2019).
Yee, C. et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA 99, 16168–16173 (2002).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Andersen, R. et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin. Cancer Res. 22, 3734–3745 (2016).
Lee, J. M. et al. Direct and indirect antitumor effects by human peripheral blood lymphocytes expressing both chimeric immune receptor and interleukin-2 in ovarian cancer xenograft model. Cancer Gene Ther. 17, 742–750 (2010).
Sockolosky, J. T. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018). The researchers developed an orthogonal IL-2 cytokine–receptor complex that selectively potentiates engineered T cells for engineered cell therapies.
Zhang, Q. et al. A human orthogonal IL-2 and IL-2Rβ system enhances CAR T cell expansion and antitumor activity in a murine model of leukemia. Sci. Transl Med. 13, 6986 (2021).
Hirai, T. et al. Selective expansion of regulatory T cells using an orthogonal IL-2/IL-2 receptor system facilitates transplantation tolerance. J. Clin. Invest. 131, e139991 (2021).
Taylor, P. A. et al. L-selectinhi but not the L-selectinlo CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood 104, 3804–3812 (2004).
Mo, F. et al. An engineered IL-2 partial agonist promotes CD8+ T cell stemness. Nature 597, 544–548 (2021).
Pohl-Guimarães, F. et al. RNA-modified T cells mediate effective delivery of immunomodulatory cytokines to brain tumors. Mol. Ther. 27, 837–849 (2019).
Roybal, K. T. et al. Engineering T cells with customized therapeutic response programs using synthetic Notch receptors. Cell 167, 419–432.e16 (2016).
Yao, W. et al. Intratumoral injection of dendritic cells overexpressing interleukin-12 inhibits melanoma growth. Oncol. Rep. 42, 370–376 (2019).
Minkis, K. et al. Type 2 bias of T cells expanded from the blood of melanoma patients switched to type 1 by IL-12p70 mRNA-transfected dendritic cells. Cancer Res. 68, 9441–9450 (2008).
Tatsumi, T. et al. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity 1. Cancer Res. 63, 6378–6386 (2003).
Bontkes, H. J. et al. Dendritic cells transfected with interleukin-12 and tumor-associated antigen messenger RNA induce high avidity cytotoxic T cells. Gene Ther. 14, 366–375 (2007).
Naka, T. et al. Tumor vaccine therapy against recrudescent tumor using dendritic cells simultaneously transfected with tumor RNA and granulocyte macrophage colony-stimulating factor RNA. Cancer Sci. 99, 407–413 (2008).
Ljunggren, H. G. & Kärre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 11, 237–244 (1990).
van den Bergh, J. et al. Transpresentation of interleukin-15 by IL-15/IL-15Rα mRNA-engineered human dendritic cells boosts antitumoral natural killer cell activity. Oncotarget 6, 44123–44133 (2015).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020).
Kang, M. et al. Nanocomplex-mediated in vivo programming to chimeric antigen receptor-M1 macrophages for cancer therapy. Adv. Mater. 33, 2103258 (2021).
Wang, S. et al. CAR-macrophage: an extensive immune enhancer to fight cancer. eBioMedicine 76, 103873 (2022).
Kaczanowska, S. et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell 184, 2033–2052.e21 (2021).
Nash, A. M. et al. Clinically translatable cytokine delivery platform for eradication of intraperitoneal tumors. Sci. Adv. 8, 1032 (2022).
Mosallaei, M. et al. Genetically engineered mesenchymal stem cells: targeted delivery of immunomodulatory agents for tumor eradication. Cancer Gene Ther. 27, 854–868 (2020).
Razeghian, E. et al. Mesenchymal stem/stromal cells as a vehicle for cytokine delivery: an emerging approach for tumor immunotherapy. Front. Med. 8, 1405 (2021).
Adams, G. P. & Weiner, L. M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 23, 1147–1157 (2005).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Perrillo, R. Benefits and risks of interferon therapy for hepatitis B. Hepatology 49, S103–S111 (2009).
Panitch, H. S. Interferons in multiple sclerosis. Drugs 44, 946–962 (1992).
Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 13, 688–696 (1995).
Bennett, C. L., Djulbegovic, B., Norris, L. B. & Armitage, J. O. Colony-stimulating factors for febrile neutropenia during cancer therapy. N. Engl. J. Med. 368, 1131–1139 (2013).
Zhang, P., Sun, F., Liu, S. & Jiang, S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J. Control. Release 244, 184–193 (2016).
Mitra, S. et al. Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity 42, 826–838 (2015).
Mould, D. R. & Green, B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs 24, 23–39 (2010).
Ghoreschi, K. et al. Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat. Med. 9, 40–46 (2002).
Tulpule, A. et al. Interleukin-4 in the treatment of AIDS-related Kaposi’s sarcoma. Ann. Oncol. 8, 79–83 (1997).
Sportès, C. et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205, 1701–1714 (2008).
Rosenberg, S. A. et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J. Immunother. 29, 313–319 (2006).
Buruiana, F. E., Solà, I. & Alonso-Coello, P. Recombinant human interleukin 10 for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2012, CD005109 (2010).
Colombel, J. F. et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut 49, 42–46 (2001).
Chernoff, A. E. et al. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J. Immunol. 154, 5492–5499 (1995).
Conlon, K. C. et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol. 33, 74–82 (2015).
Petrella, T. M. et al. Final efficacy results of NCIC CTG IND.202: a randomized phase II study of recombinant interleukin-21 (rIL21) in patients with recurrent or metastatic melanoma (MM). J. Clin. Oncol. 31, 9032–9032 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00873756 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00508625 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00400764 (2011).
Pina, A. S., Lowe, C. R. & Roque, A. C. A. Challenges and opportunities in the purification of recombinant tagged proteins. Biotechnol. Adv. 32, 366–381 (2014).
Malyala, P. & Singh, M. Endotoxin limits in formulations for preclinical research. J. Pharm. Sci. 97, 2041–2044 (2008).
Freitag, R. in Animal Cell Biotechnology Vol. 1104 (ed. Pörtner, R.) 419–458 (Humana, 2014).
Author information
Authors and Affiliations
Contributions
J.D., T.A., A.M.H., M.G.N. and W.J.M.M. wrote the manuscript. All authors researched data for the article, provided substantial contributions to discussion of content and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
W.J.M.M., L.A.B.J. and M.G.N. are scientific co-founders of and have equity in Trained Therapeutix Discovery. W.J.M.M. and M.G.N. have consulting agreements with Trained Therapeutix Discovery. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Yizhou Dong, Frances Balkwill, Beatrice Malacrida and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deckers, J., Anbergen, T., Hokke, A.M. et al. Engineering cytokine therapeutics. Nat Rev Bioeng 1, 286–303 (2023). https://doi.org/10.1038/s44222-023-00030-y
Published:
Issue date:
DOI: https://doi.org/10.1038/s44222-023-00030-y
This article is cited by
-
Enhancing immunotherapy with tumour-responsive nanomaterials
Nature Reviews Clinical Oncology (2025)
-
A peptide conjugate enables systemic injection of the morpholino inducer and more durable induction of T3H38 ribozyme-controlled AAV transgene in mice
Gene Therapy (2025)
-
Long-acting IL-2 release from pressure-fused biomineral tablets promotes antitumor immune response
Nature Cancer (2025)
-
Combined delivery of IL12 and an IL18 mutant without IL18BP-binding activity by an adenoviral vector enhances tumor specific immunity
Scientific Reports (2025)
-
The genomic architecture of circulating cytokine levels points to drug targets for immune-related diseases
Communications Biology (2025)