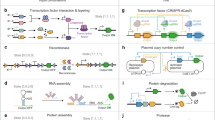
Abstract
Cells rely on complex molecular networks to perceive and process external and internal signals into tailored responses. These cellular abilities can be augmented and modified by integrating artificial gene circuitry for the engineering of cell-based therapeutics. In this Review, we outline the engineering principles that govern the design of synthetic gene networks, highlighting how the sensitivity, detection range and specificity of synthetic gene networks can be optimized for in vivo functionality. In particular, we examine synthetic molecular modules, including transcriptionally regulated, translationally regulated and post-translationally regulated circuits, that enable tailored adjustments in therapeutic cell functions based on dynamic disease-state cues, or that can be remotely controlled using clinically compatible external molecular or physical signals. Furthermore, we explore the potential of multi-input regulatable logic-gated programs to enhance the efficacy and safety of engineered cell immunotherapies for cancer treatment, and highlight the application of synthetic gene circuits for gene therapy and the design of therapeutic microbes. Finally, we examine how synthetic-biology-inspired therapies may benefit from evolving genome engineering technologies and synergy with artificial intelligence.
Key points
-
Synthetic macromolecular switches responding to external or endogenous signals at the DNA, RNA or protein level offer promising tools for developing safe and effective therapeutic cells.
-
Consideration of tradeoffs inherent to each regulatory mode, including dynamic ranges, response times, genetic footprint and component sources, is crucial for their optimal therapeutic application.
-
Synthetic transcriptional systems can be integrated in cancer gene and cell therapy products, and translational and post-translational switches are being explored in preclinical studies.
-
Machine learning models, trained on high-quality datasets, can aid in the design and optimization of synthetic macromolecular circuits to meet clinical requirements while reducing the time and cost of development.
-
To aid the clinical translation of large circuits integrating extracellularly controlled logical operations, gene transfer methods for ex vivo or in vivo cell engineering must be improved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Elowitz, M. B. & Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000).
Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000).
Kramer, B. P. et al. An engineered epigenetic transgene switch in mammalian cells. Nat. Biotechnol. 22, 867–870 (2004).
Tigges, M., Marquez-Lago, T. T., Stelling, J. & Fussenegger, M. A tunable synthetic mammalian oscillator. Nature 457, 309–312 (2009).
Madderson, O., Teixeira, A. P. & Fussenegger, M. Emerging mammalian gene switches for controlling implantable cell therapies. Curr. Opin. Chem. Biol. 64, 98–105 (2021).
Teixeira, A. P. & Fussenegger, M. Synthetic biology-inspired therapies for metabolic diseases. Curr. Opin. Biotechnol. 47, 59–66 (2017).
Bertschi, A. et al. Controlling therapeutic protein expression via inhalation of a butter flavor molecule. Nucleic Acids Res. 51, e28 (2023).
Galvan, S., Madderson, O., Xue, S., Teixeira, A. P. & Fussenegger, M. Regulation of transgene expression by the natural sweetener xylose. Adv. Sci. 9, e2203193 (2022).
Teixeira, A. P., Xue, S., Huang, J. & Fussenegger, M. Evolution of molecular switches for regulation of transgene expression by clinically licensed gluconate. Nucleic Acids Res. 51, e85 (2023).
Huang, J., Xue, S., Xie, Y.-Q., Teixeira, A. P. & Fussenegger, M. Ultrashort-peptide-responsive gene switches for regulation of therapeutic protein expression in mammalian cells. Adv. Sci. 11, e2309411 (2024).
Motta-Mena, L. B. et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nat. Chem. Biol. 10, 196–202 (2014).
Wang, X., Chen, X. & Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Methods 9, 266–269 (2012).
Stefanov, B. A. et al. Genetically encoded protein thermometer enables precise electrothermal control of transgene expression. Adv. Sci. 8, e2101813 (2021).
Nguyen, D. P. et al. Ligand-binding domains of nuclear receptors facilitate tight control of split CRISPR activity. Nat. Commun. 7, 12009 (2016).
Picard, D. Chaperoning steroid hormone action. Trends Endocrinol. Metab. 17, 229–235 (2006).
Beerli, R. R., Schopfer, U., Dreier, B. & Barbas, C. F. III Chemically regulated zinc finger transcription factors. J. Biol. Chem. 275, 32617–32627 (2000).
Mercer, A. C. et al. Regulation of endogenous human gene expression by ligand-inducible TALE transcription factors. ACS Synth. Biol. 3, 723–730 (2014).
Webster, N. J., Green, S., Jin, J. R. & Chambon, P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell 54, 199–207 (1988).
Gersbach, C. A., Gaj, T. & Barbas, C. F. III Synthetic zinc finger proteins: the advent of targeted gene regulation and genome modification technologies. Acc. Chem. Res. 47, 2309–2318 (2014).
Moscou, M. J. & Bogdanove, A. J. A simple cipher governs DNA recognition by TAL effectors. Science 326, 1501 (2009).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
McCarty, N. S., Graham, A. E., Studena, L. & Ledesma-Amaro, R. Multiplexed CRISPR technologies for gene editing and transcriptional regulation. Nat. Commun. 11, 1281 (2020).
Li, H. S. et al. Multidimensional control of therapeutic human cell function with synthetic gene circuits. Science 378, 1227–1234 (2022).
Franko, N. et al. Integrated compact regulators of protein activity enable control of signaling pathways and genome-editing in vivo. Cell Discov. 10, 9 (2024).
Wei, C. T. et al. A chemically controlled Cas9 switch enables temporal modulation of diverse effectors. Nat. Chem. Biol. 19, 981–991 (2023).
Kleinjan, D. A., Wardrope, C., Nga Sou, S. & Rosser, S. J. Drug-tunable multidimensional synthetic gene control using inducible degron-tagged dCas9 effectors. Nat. Commun. 8, 1191 (2017).
Banaszynski, L. A., Chen, L. C., Maynard-Smith, L. A., Ooi, A. G. & Wandless, T. J. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 (2006).
Nakahara, E., Mullapudi, V., Collier, G. E., Joachimiak, L. A. & Hulleman, J. D. Development of a new DHFR-based destabilizing domain with enhanced basal turnover and applicability in mammalian systems. ACS Chem. Biol. 17, 2877–2889 (2022).
Miyazaki, Y., Imoto, H., Chen, L. C. & Wandless, T. J. Destabilizing domains derived from the human estrogen receptor. J. Am. Chem. Soc. 134, 3942–3945 (2012).
Franko, N., Teixeira, A. P., Xue, S., Charpin-El Hamri, G. & Fussenegger, M. Design of modular autoproteolytic gene switches responsive to anti-coronavirus drug candidates. Nat. Commun. 12, 6786 (2021).
Inoue, T., Heo, W. D., Grimley, J. S., Wandless, T. J. & Meyer, T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods 2, 415–418 (2005).
Gao, Y. et al. Complex transcriptional modulation with orthogonal and inducible dCas9 regulators. Nat. Methods 13, 1043–1049 (2016).
Mahameed, M., Wang, P., Xue, S. & Fussenegger, M. Engineering receptors in the secretory pathway for orthogonal signalling control. Nat. Commun. 13, 7350 (2022).
Bertschi, A., Wang, P., Galvan, S., Teixeira, A. P. & Fussenegger, M. Combinatorial protein dimerization enables precise multi-input synthetic computations. Nat. Chem. Biol. 19, 767–777 (2023).
Palli, S. R., Kapitskaya, M. Z., Kumar, M. B. & Cress, D. E. Improved ecdysone receptor-based inducible gene regulation system. Eur. J. Biochem. 270, 1308–1315 (2003).
Beltran, J. et al. Rapid biosensor development using plant hormone receptors as reprogrammable scaffolds. Nat. Biotechnol. 40, 1855–1861 (2022).
Rihtar, E. et al. Chemically inducible split protein regulators for mammalian cells. Nat. Chem. Biol. 19, 64–71 (2023).
Chin, S. E. et al. A simeprevir-inducible molecular switch for the control of cell and gene therapies. Nat. Commun. 14, 7753 (2023).
Konermann, S. et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 (2013).
Muller, K. et al. A red/far-red light-responsive bi-stable toggle switch to control gene expression in mammalian cells. Nucleic Acids Res. 41, e77 (2013).
Chen, R. Deep brain optogenetics without intracranial surgery. Nat. Biotechnol. 39, 161–164 (2021).
Li, C. & Samulski, R. J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 21, 255–272 (2020).
Cui, M. et al. A single-component, light-assisted uncaging switch for endoproteolytic release. Nat. Chem. Biol. 20, 353–364 (2024).
Dolberg, T. B. et al. Computation-guided optimization of split protein systems. Nat. Chem. Biol. 17, 531–539 (2021).
Kroeze, W. K. et al. PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol. 22, 362–369 (2015).
Mansouri, M. et al. Smart-watch-programmed green-light-operated percutaneous control of therapeutic transgenes. Nat. Commun. 12, 3388 (2021).
Morsut, L. et al. Engineering customized cell sensing and response behaviors using synthetic Notch receptors. Cell 164, 780–791 (2016).
Zhu, I. et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell 185, 1431–1443 e1416 (2022).
Barnea, G. et al. The genetic design of signaling cascades to record receptor activation. Proc. Natl Acad. Sci. USA 105, 64–69 (2008).
Kipniss, N. H. et al. Engineering cell sensing and responses using a GPCR-coupled CRISPR-Cas system. Nat. Commun. 8, 2212 (2017).
Haellman, V., Saxena, P., Jiang, Y. & Fussenegger, M. Rational design and optimization of synthetic gene switches for controlling cell-fate decisions in pluripotent stem cells. Metab. Eng. 65, 99–110 (2021).
Bai, P. et al. A fully human transgene switch to regulate therapeutic protein production by cooling sensation. Nat. Med. 25, 1266–1273 (2019).
Labanieh, L. et al. Enhanced safety and efficacy of protease-regulated CAR-T cell receptors. Cell 185, 1745–1763 e1722 (2022).
Scheller, L., Strittmatter, T., Fuchs, D., Bojar, D. & Fussenegger, M. Generalized extracellular molecule sensor platform for programming cellular behavior. Nat. Chem. Biol. 14, 723–729 (2018).
Strittmatter, T. et al. Programmable DARPin-based receptors for the detection of thrombotic markers. Nat. Chem. Biol. 18, 1125–1134 (2022).
Grusch, M. et al. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J. 33, 1713–1726 (2014).
Krawczyk, K., Scheller, L., Kim, H. & Fussenegger, M. Rewiring of endogenous signaling pathways to genomic targets for therapeutic cell reprogramming. Nat. Commun. 11, 608 (2020).
Huang, J., Xue, S., Buchmann, P., Teixeira, A. P. & Fussenegger, M. An electrogenetic interface to program mammalian gene expression by direct current. Nat. Metab. 5, 1395–1407 (2023).
Beilstein, K., Wittmann, A., Grez, M. & Suess, B. Conditional control of mammalian gene expression by tetracycline-dependent hammerhead ribozymes. ACS Synth. Biol. 4, 526–534 (2015).
Goldfless, S. J., Belmont, B. J., de Paz, A. M., Liu, J. F. & Niles, J. C. Direct and specific chemical control of eukaryotic translation with a synthetic RNA–protein interaction. Nucleic Acids Res. 40, e64 (2012).
Strobel, B. et al. A small-molecule-responsive riboswitch enables conditional induction of viral vector-mediated gene expression in mice. ACS Synth. Biol. 9, 1292–1305 (2020).
Endo, K., Stapleton, J. A., Hayashi, K., Saito, H. & Inoue, T. Quantitative and simultaneous translational control of distinct mammalian mRNAs. Nucleic Acids Res. 41, e135 (2013).
Saito, H. et al. Synthetic translational regulation by an L7Ae-kink-turn RNP switch. Nat. Chem. Biol. 6, 71–78 (2010).
Wagner, T. E. et al. Small-molecule-based regulation of RNA-delivered circuits in mammalian cells. Nat. Chem. Biol. 14, 1043–1050 (2018).
Liu, R. et al. Optogenetic control of RNA function and metabolism using engineered light-switchable RNA-binding proteins. Nat. Biotechnol. 40, 779–786 (2022).
Nakanishi, H. et al. Light-controllable RNA-protein devices for translational regulation of synthetic mRNAs in mammalian cells. Cell Chem. Biol. 28, 662–674 (2021).
Weber, A. M. et al. A blue light receptor that mediates RNA binding and translational regulation. Nat. Chem. Biol. 15, 1085–1092 (2019).
Shao, J. et al. Engineered poly(A)-surrogates for translational regulation and therapeutic biocomputation in mammalian cells. Cell Res. 34, 31–46 (2024).
Zhong, G., Wang, H., Bailey, C. C., Gao, G. & Farzan, M. Rational design of aptazyme riboswitches for efficient control of gene expression in mammalian cells. eLife 5, e18858 (2016).
Hou, Q. & Jaffrey, S. R. Synthetic biology tools to promote the folding and function of RNA aptamers in mammalian cells. RNA Biol. 20, 198–206 (2023).
Chen, Y. Y., Jensen, M. C. & Smolke, C. D. Genetic control of mammalian T-cell proliferation with synthetic RNA regulatory systems. Proc. Natl Acad. Sci. USA 107, 8531–8536 (2010).
Xiang, J. S. et al. Massively parallel RNA device engineering in mammalian cells with RNA-Seq. Nat. Commun. 10, 4327 (2019).
Zhong, G. et al. A reversible RNA on-switch that controls gene expression of AAV-delivered therapeutics in vivo. Nat. Biotechnol. 38, 169–175 (2020).
Wang, E. T. et al. Alternative isoform regulation in human tissue transcriptomes. Nature 456, 470–476 (2008).
Fukunaga, K. et al. Small-molecule aptamer for regulating RNA functions in mammalian cells and animals. J. Am. Chem. Soc. 145, 7820–7828 (2023).
Luo, L. M., Jea, J. D. Y., Wang, Y., Chao, P. W. & Yen, L. S. Control of mammalian gene expression by modulation of polyA signal cleavage at 5′ UTR. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01989-0 (2024).
Monteys, A. M. et al. Regulated control of gene therapies by drug-induced splicing. Nature 596, 291–295 (2021).
Vogel, M., Weigand, J. E., Kluge, B., Grez, M. & Suess, B. A small, portable RNA device for the control of exon skipping in mammalian cells. Nucleic Acids Res. 46, e48 (2018).
Georgakopoulos-Soares, I. et al. Alternative splicing modulation by G-quadruplexes. Nat. Commun. 13, 2404 (2022).
Schmok, J. C. et al. Large-scale evaluation of the ability of RNA-binding proteins to activate exon inclusion. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-02014-0 (2024).
Kormann, M. S. et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 29, 154–157 (2011).
Geall, A. J. et al. Nonviral delivery of self-amplifying RNA vaccines. Proc. Natl Acad. Sci. USA 109, 14604–14609 (2012).
Mc Cafferty, S. et al. In vivo validation of a reversible small molecule-based switch for synthetic self-amplifying mRNA regulation. Mol. Ther. 29, 1164–1173 (2021).
Yesbolatova, A. et al. The auxin-inducible degron 2 technology provides sharp degradation control in yeast, mammalian cells, and mice. Nat. Commun. 11, 5701 (2020).
Noviello, G., Gjaltema, R. A. F. & Schulz, E. G. CasTuner is a degron and CRISPR/Cas-based toolkit for analog tuning of endogenous gene expression. Nat. Commun. 14, 3225 (2023).
Nabet, B. et al. Rapid and direct control of target protein levels with VHL-recruiting dTAG molecules. Nat. Commun. 11, 4687 (2020).
Nabet, B. et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 14, 431–441 (2018).
Agbowuro, A. A., Huston, W. M., Gamble, A. B. & T, J. D. Proteases and protease inhibitors in infectious diseases. Medicinal Res. Rev. 38, 1295–1331 (2018).
Chung, H. K. et al. Tunable and reversible drug control of protein production via a self-excising degron. Nat. Chem. Biol. 11, 713–720 (2015).
Jacobs, C. L., Badiee, R. K. & Lin, M. Z. StaPLs: versatile genetically encoded modules for engineering drug-inducible proteins. Nat. Methods 15, 523–526 (2018).
Tague, E. P., Dotson, H. L., Tunney, S. N., Sloas, D. C. & Ngo, J. T. Chemogenetic control of gene expression and cell signaling with antiviral drugs. Nat. Methods 15, 519–522 (2018).
Mahameed, M., Xue, S., Stefanov, B. A., Hamri, G. C. & Fussenegger, M. Engineering a rapid insulin release system controlled by oral drug administration. Adv. Sci. 9, e2105619 (2022).
Mansouri, M., Ray, P. G., Franko, N., Xue, S. & Fussenegger, M. Design of programmable post-translational switch control platform for on-demand protein secretion in mammalian cells. Nucleic Acids Res. 51, e1 (2023).
Praznik, A. et al. Regulation of protein secretion through chemical regulation of endoplasmic reticulum retention signal cleavage. Nat. Commun. 13, 1323 (2022).
Wang, X. et al. A programmable protease-based protein secretion platform for therapeutic applications. Nat. Chem. Biol. 20, 432–442 (2024).
Vlahos, A. E. et al. Protease-controlled secretion and display of intercellular signals. Nat. Commun. 13, 912 (2022).
Wang, J. H., Gessler, D. J., Zhan, W., Gallagher, T. L. & Gao, G. P. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Target Ther. 9, 78 (2024).
Barrett, J. A. et al. Regulated intratumoral expression of IL-12 using a RheoSwitch Therapeutic System® (RTS®) gene switch as gene therapy for the treatment of glioma. Cancer Gene Ther. 25, 106–116 (2018).
Chiocca, E. A. et al. Regulatable interleukin-12 gene therapy in patients with recurrent high-grade glioma: results of a phase 1 trial. Sci. Transl. Med. 11, eaaw5680 (2019).
Cirella, A. et al. Novel strategies exploiting interleukin-12 in cancer immunotherapy. Pharmacol. Ther. 239, 108189 (2022).
Cripe, T. P. et al. Leveraging gene therapy to achieve long-term continuous or controllable expression of biotherapeutics. Sci. Adv. 8, eabm1890 (2022).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Weber, E. W. et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science 372, 49 (2021).
Marofi, F. et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res. Ther. 12, 81 (2021).
Majzner, R. G. & Mackall, C. L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 8, 1219–1226 (2018).
Choe, J. H. et al. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci. Transl. Med. 13, eabe7378 (2021).
Hyrenius-Wittsten, A. et al. SynNotch CAR circuits enhance solid tumor recognition and promote persistent antitumor activity in mouse models. Sci. Transl. Med. 13, eabd8836 (2021).
Hernandez-Lopez, R. A. et al. T cell circuits that sense antigen density with an ultrasensitive threshold. Science 371, 1166–1171 (2021).
Tousley, A. M. et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 615, 507–516 (2023).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Minutolo NG, S. P. et al. Quantitative control of gene-engineered T-cell activity through the covalent attachment of targeting ligands to a universal immune receptor. J. Am. Chem. Soc. 142, 6554–6568 (2020).
Stepanov, A. V. et al. Control of the antitumour activity and specificity of CAR T cells via organic adapters covalently tethering the CAR to tumour cells. Nat. Biomed. Eng. 8, 529–543 (2024).
Li, H. S. et al. High-performance multiplex drug-gated CAR circuits. Cancer Cell 40, 1294–1305 e1294 (2022).
Allen, G. M. et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science 378, 1186 (2022).
Maldini, C. R., Ellis, G. I. & Riley, J. L. CAR T cells for infection, autoimmunity and allotransplantation. Nat. Rev. Immunol. 18, 605–616 (2018).
Huang, J., Xue, S., Teixeira, A. P. & Fussenegger, M. A gene-switch platform interfacing with reactive oxygen species enables transcription fine-tuning by soluble and volatile pharmacologics and food additives. Adv. Sci. (Weinh.) 11, e2306333 (2024).
Kemmer, C. et al. Self-sufficient control of urate homeostasis in mice by a synthetic circuit. Nat. Biotechnol. 28, 355–360 (2010).
Schukur, L., Geering, B., Charpin-El Hamri, G. & Fussenegger, M. Implantable synthetic cytokine converter cells with AND-gate logic treat experimental psoriasis. Sci. Transl. Med. 7, 318ra201 (2015).
Zhao, H. et al. Tuning of cellular insulin release by music for real-time diabetes control. Lancet Diabetes Endocrinol. 11, 637–640 (2023).
Zhou, Y. et al. A small and highly sensitive red/far-red optogenetic switch for applications in mammals. Nat. Biotechnol. 40, 262–272 (2022).
Naldini, L. Gene therapy returns to centre stage. Nature 526, 351–360 (2015).
Birocchi, F. et al. Targeted inducible delivery of immunoactivating cytokines reprograms glioblastoma microenvironment and inhibits growth in mouse models. Sci. Transl. Med. 14, eabl4106 (2022).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019).
Wang, B. et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 5, 429–440 (2021).
Fong, C. Y., Gauthaman, K. & Bongso, A. Teratomas from pluripotent stem cells: a clinical hurdle. J. Cell Biochem. 111, 769–781 (2010).
Mehta, A. et al. Interim phase I clinical data of FT819-101, a study of the first-ever, off-the-shelf, iPSC-derived TCR-less CD19 CAR T-cell therapy for patients with relapsed/refractory B-cell malignancies. Blood 140, 4577–4578 (2022).
Charbonneau, M. R., Isabella, V. M., Li, N. & Kurtz, C. B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 11, 1738 (2020).
Hwang, I. Y. et al. Engineered probiotic can eliminate and prevent gut infection in animal models. Nat. Commun. 8, 15028 (2017).
Koh, E. et al. Engineering probiotics to inhibit Clostridioides difficile infection by dynamic regulation of intestinal metabolism. Nat. Commun. 13, 3834 (2022).
Pawelek, J. M., Low, K. B. & Bermudes, D. Bacteria as tumour-targeting vectors. Lancet Oncol. 4, 548–556 (2003).
Din, M. O. et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 536, 81–85 (2016).
Chowdhury, S. et al. Programmable bacteria induce durable tumor regression and systemic antitumor immunity. Nat. Med. 25, 1057–1063 (2019).
Gurbatri, C. R. et al. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 12, eaax0876 (2020).
Bell, H. N. et al. Microenvironmental ammonia enhances T cell exhaustion in colorectal cancer. Cell Metab. 35, 134–149 e136 (2023).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021).
Adolfsen, K. J. et al. Improvement of a synthetic live bacterial therapeutic for phenylketonuria with biosensor-enabled enzyme engineering. Nat. Commun. 12, 6215 (2021).
Vockley, J. et al. Efficacy and safety of a synthetic biotic for treatment of phenylketonuria: a phase 2 clinical trial. Nat. Metab. 5, 1685–1690 (2023).
Kurtz, C. B. et al. An engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. Sci. Transl. Med. 11, eaau7975 (2019).
Rottinghaus, A. G., Ferreiro, A., Fishbein, S. R. S., Dantas, G. & Moon, T. S. Genetically stable CRISPR-based kill switches for engineered microbes. Nat. Commun. 13, 672 (2022).
Baek, M. et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Yeh, A. H. et al. De novo design of luciferases using deep learning. Nature 614, 774–780 (2023).
Carinhas, N., Oliveira, R., Alves, P. M., Carrondo, M. J. & Teixeira, A. P. Systems biotechnology of animal cells: the road to prediction. Trends Biotechnol. 30, 377–385 (2012).
Teixeira, A. P., Alves, C., Alves, P. M., Carrondo, M. J. & Oliveira, R. Hybrid elementary flux analysis/nonparametric modeling: application for bioprocess control. BMC Bioinforma. 8, 30 (2007).
Notin, P., Rollins, N., Gal, Y., Sander, C. & Marks, D. Machine learning for functional protein design. Nat. Biotechnol. 42, 216–228 (2024).
Bryant, D. H. et al. Deep diversification of an AAV capsid protein by machine learning. Nat. Biotechnol. 39, 691–696 (2021).
Zhu, D. et al. Optimal trade-off control in machine learning-based library design, with application to adeno-associated virus (AAV) for gene therapy. Sci. Adv. 10, eadj3786 (2024).
Kelsic, E. D. & GM, C. Challenges and opportunities of machine-guided capsid engineering for gene therapy. Cell Gene Ther. Insights. 5, 523–536 (2019).
Ichikawa, D. M. et al. A universal deep-learning model for zinc finger design enables transcription factor reprogramming. Nat. Biotechnol. 41, 1117–1129 (2023).
Thean, D. G. L. et al. Machine learning-coupled combinatorial mutagenesis enables resource-efficient engineering of CRISPR-Cas9 genome editor activities. Nat. Commun. 13, 2219 (2022).
Zhang, P. et al. Deep flanking sequence engineering for efficient promoter design using DeepSEED. Nat. Commun. 14, 6309 (2023).
Sumi, S., Hamada, M. & Saito, H. Deep generative design of RNA family sequences. Nat. Methods 21, 435–443 (2024).
Daniels, K. G. et al. Decoding CAR T cell phenotype using combinatorial signaling motif libraries and machine learning. Science 378, 1194–1200 (2022).
Castellanos-Rueda, R. et al. speedingCARs: accelerating the engineering of CAR T cells by signaling domain shuffling and single-cell sequencing. Nat. Commun. 13, 6555 (2022).
Di Stasi, A. et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 365, 1673–1683 (2011).
Weinberg, B. H. et al. Large-scale design of robust genetic circuits with multiple inputs and outputs for mammalian cells. Nat. Biotechnol. 35, 453–462 (2017).
Zhu, R., Del Rio-Salgado, J. M., Garcia-Ojalvo, J. & Elowitz, M. B. Synthetic multistability in mammalian cells. Science 375, eabg9765 (2022).
Irving, M., Lanitis, E., Migliorini, D., Ivics, Z. & Guedan, S. Choosing the right tool for genetic engineering: clinical lessons from chimeric antigen receptor-T cells. Hum. Gene Ther. 32, 1044–1058 (2021).
Fraietta, J. A. et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 558, 307–312 (2018).
Nobles, C. L. et al. CD19-targeting CAR T cell immunotherapy outcomes correlate with genomic modification by vector integration. J. Clin. Invest. 130, 673–685 (2020).
Rodrigues, A. F., Alves, P. M. & Coroadinha, A. in Viral Gene Therapy (ed. Xu, K.) 15–40 (IntechOpen, 2011).
Ye, L. et al. AAV-mediated delivery of a Sleeping Beauty transposon and an mRNA-encoded transposase for the engineering of therapeutic immune cells. Nat. Biomed. Eng. 8, 132–148 (2024).
Fiorenza, S., Ritchie, D. S., Ramsey, S. D., Turtle, C. J. & Roth, J. A. Value and affordability of CAR T-cell therapy in the United States. Bone Marrow Transpl. 55, 1706–1715 (2020).
Marin, D. et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat. Med. 30, 772–784 (2024).
Bachanova, V. et al. Safety and efficacy of FT596, a first-in-class, multi-antigen targeted, off-the-shelf, iPSC-derived CD19 CAR NK cell therapy in relapsed/refractory B-cell lymphoma. Blood 138, 823 (2021).
Cichocki, F., van der Stegen, S. J. C. & Miller, J. S. Engineered and banked iPSCs for advanced NK- and T-cell immunotherapies. Blood 141, 846–855 (2023).
Iriguchi, S. et al. A clinically applicable and scalable method to regenerate T-cells from iPSCs for off-the-shelf T-cell immunotherapy. Nat. Commun. 12, 430 (2021).
Dai, X. et al. Massively parallel knock-in engineering of human T cells. Nat. Biotechnol. 41, 1239–1255 (2023).
Shy, B. R. et al. High-yield genome engineering in primary cells using a hybrid ssDNA repair template and small-molecule cocktails. Nat. Biotechnol. 41, 521–531 (2023).
Zhang, X. et al. Harnessing eukaryotic retroelement proteins for transgene insertion into human safe-harbor loci. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02137-y (2024).
Mahata, B. et al. Compact engineered human mechanosensitive transactivation modules enable potent and versatile synthetic transcriptional control. Nat. Methods 20, 1716–1728 (2023).
Agarwal, S. et al. In vivo generation of CAR T cells selectively in human CD4+ lymphocytes. Mol. Ther. 28, 1783–1794 (2020).
Hamilton, J. R. et al. In vivo human T cell engineering with enveloped delivery vehicles. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-02085-z (2024).
Greco, F. V., Pandi, A., Erb, T. J., Grierson, C. S. & Gorochowski, T. E. Harnessing the central dogma for stringent multi-level control of gene expression. Nat. Commun. 12, 1738 (2021).
Zhang, C. et al. Synthetic gene circuit-based assay with multilevel switch enables background-free and absolute quantification of circulating tumor DNA. Research 6, 0217 (2023).
Macarthur, B. D., Ma’ayan, A. & Lemischka, I. R. Systems biology of stem cell fate and cellular reprogramming. Nat. Rev. Mol. Cell Biol. 10, 672–681 (2009).
O’Shaughnessy, E. C., Palani, S., Collins, J. J. & Sarkar, C. A. Tunable signal processing in synthetic MAP kinase cascades. Cell 144, 119–131 (2011).
Bashor, C. J. et al. Complex signal processing in synthetic gene circuits using cooperative regulatory assemblies. Science 364, 593–597 (2019).
Pedone, E. et al. A tunable dual-input system for on-demand dynamic gene expression regulation. Nat. Commun. 10, 4481 (2019).
Qian, Y., Huang, H. H., Jimenez, J. I. & Del Vecchio, D. Resource competition shapes the response of genetic circuits. ACS Synth. Biol. 6, 1263–1272 (2017).
Tycko, J. et al. Development of compact transcriptional effectors using high-throughput measurements in diverse contexts. Preprint at bioRxiv https://doi.org/10.1101/2023.05.12.540558 (2023).
Wang, H., Ye, H., Xie, M., Daoud El-Baba, M. & Fussenegger, M. Cosmetics-triggered percutaneous remote control of transgene expression in mice. Nucleic Acids Res. 43, e91 (2015).
Emami, K. H. & Carey, M. A synergistic increase in potency of a multimerized VP16 transcriptional activation domain. EMBO J. 11, 5005–5012 (1992).
Christie, M. et al. Structural biology and regulation of protein import into the nucleus. J. Mol. Biol. 428, 2060–2090 (2016).
Cautain, B., Hill, R., de Pedro, N. & Link, W. Components and regulation of nuclear transport processes. FEBS J. 282, 445–462 (2015).
Piraner, D. I., Abedi, M. H., Moser, B. A., Lee-Gosselin, A. & Shapiro, M. G. Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat. Chem. Biol. 13, 75–80 (2017).
Acknowledgements
The authors disclose support for this work from the European Research Council (grant number 785800) and from the Swiss National Science Foundation (NCCR Molecular Systems Engineering).
Author information
Authors and Affiliations
Contributions
A.P.T. and M.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Hirohide Saito; Nisheth Reddy, who co-reviewed with Wendell Lim; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teixeira, A.P., Fussenegger, M. Synthetic macromolecular switches for precision control of therapeutic cell functions. Nat Rev Bioeng 2, 1005–1022 (2024). https://doi.org/10.1038/s44222-024-00235-9
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44222-024-00235-9
This article is cited by
-
Mammalian synthetic gene circuits for biopharmaceutical development & manufacture
npj Systems Biology and Applications (2025)
-
Designing supramolecular catalytic systems for mammalian synthetic metabolism
Nature Reviews Materials (2025)