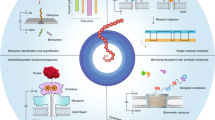
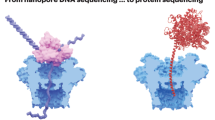
Abstract
Protein sequencing and the identification of post-translational modifications are key to understanding cellular signalling pathways and metabolic processes in health and disease. Nanopores, that is, nanometre-sized holes in a membrane, were previously put to use for DNA and RNA sequencing, offering single-molecule sensitivity and long read lengths. This prompted efforts to engineer nanopores for the high-throughput sequencing of peptides and proteins. In this Review, we discuss research towards single-molecule protein sequencing using biological nanopores, investigating how their sensitivity allows the discrimination of all 20 amino acids. We outline how fingerprinting of proteins is facilitated by using motor proteins and electro-osmotic flow to promote the slow translocation of proteins through nanopores. Moreover, we examine applications of nanopores to the identification of post-translational modifications, highlighting the potential of this technology for fundamental and clinical proteomic studies. Finally, we outline the advantages and limitations of nanopore systems for protein sequencing and the challenges that remain to be overcome for realizing de novo nanopore protein sequencing.
Key points
-
Twenty individual amino acids can be distinguished by biological nanopores.
-
Nanopores allow the discrimination of single-amino acid substitutions within proteins.
-
Electro-osmotic flow allows uncharged and heterogeneously charged proteins to translocate through nanopores.
-
Motor proteins reduce protein translocation speed, resulting in high-resolution signals.
-
Various post-translational modifications and their locations can be identified with nanopores.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kasianowicz, J. J., Brandin, E., Branton, D. & Deamer, D. W. Characterization of individual polynucleotide molecules using a membrane channel. Proc. Natl Acad. Sci. USA 93, 13770–13773 (1996).
Akeson, M., Branton, D., Kasianowicz, J. J., Brandin, E. & Deamer, D. W. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophys. J. 77, 3227–3233 (1999).
Meller, A., Nivon, L., Brandin, E., Golovchenko, J. & Branton, D. Rapid nanopore discrimination between single polynucleotide molecules. Proc. Natl Acad. Sci. USA 97, 1079–1084 (2000).
Deamer, D. W. & Branton, D. Characterization of nucleic acids by nanopore analysis. Acc. Chem. Res. 35, 817–825 (2002).
Deamer, D., Akeson, M. & Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 34, 518–524 (2016).
Wang, Y., Zhao, Y., Bollas, A., Wang, Y. & Au, K. F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 39, 1348–1365 (2021).
Chen, P. et al. Portable nanopore-sequencing technology: trends in development and applications. Front. Microbiol. 14, 1043967 (2023).
Dorey, A. & Howorka, S. Nanopore DNA sequencing technologies and their applications towards single-molecule proteomics. Nat. Chem. 16, 314–334 (2024).
Clarke, J. et al. Continuous base identification for single-molecule nanopore DNA sequencing. Nat. Nanotechnol. 4, 265–270 (2009).
Cockroft, S. L., Chu, J., Amorin, M. & Ghadiri, M. R. A single-molecule nanopore device detects DNA polymerase activity with single-nucleotide resolution. J. Am. Chem. Soc. 130, 818–820 (2008).
Kumar, S. et al. PEG-labeled nucleotides and nanopore detection for single molecule DNA sequencing by synthesis. Sci. Rep. 2, 684 (2012).
Manrao, E. A. et al. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase. Nat. Biotechnol. 30, 349–353 (2012).
Cherf, G. M. et al. Automated forward and reverse ratcheting of DNA in a nanopore at 5-Å precision. Nat. Biotechnol. 30, 344–348 (2012).
Branton, D. et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 26, 1146–1153 (2008).
Nivala, J., Marks, D. B. & Akeson, M. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nat. Biotechnol. 31, 247–250 (2013).
Nivala, J., Mulroney, L., Li, G., Schreiber, J. & Akeson, M. Discrimination among protein variants using an unfoldase-coupled nanopore. ACS Nano 8, 12365–12375 (2014).
Wei, X. et al. Engineering biological nanopore approaches toward protein sequencing. ACS Nano 17, 16369–16395 (2023).
Pugh, G. C., Burns, J. R. & Howorka, S. Comparing proteins and nucleic acids for next-generation biomolecular engineering. Nat. Rev. Chem. 2, 113–130 (2018).
Huo, M.-Z., Li, M.-Y., Ying, Y.-L. & Long, Y.-T. Is the volume exclusion model practicable for nanopore protein sequencing? Anal. Chem. 93, 11364–11369 (2021).
Jayasinghe, L. & Wallace, J. E. Mutant pore. US patent 11186868 B2 (2021).
Aebersold, R. et al. How many human proteoforms are there? Nat. Chem. Biol. 14, 206–214 (2018).
Mallick, P. & Kuster, B. Proteomics: a pragmatic perspective. Nat. Biotechnol. 28, 695–709 (2010).
Polasky, D. A., Yu, F., Teo, G. C. & Nesvizhskii, A. I. Fast and comprehensive N- and O-glycoproteomics analysis with MSFragger-Glyco. Nat. Methods 17, 1125–1132 (2020).
Daly, L. A., Clarke, C. J., Po, A., Oswald, S. O. & Eyers, C. E. Considerations for defining +80 Da mass shifts in mass spectrometry-based proteomics: phosphorylation and beyond. Chem. Commun. 59, 11484–11499 (2023).
Xu, T., Wang, Q., Wang, Q. & Sun, L. Mass spectrometry-intensive top-down proteomics: an update on technology advancements and biomedical applications. Anal. Methods 16, 4664–4682 (2024).
Nicholson, J. A nanopore distance away from next-generation protein sequencing. Chem 8, 17–19 (2022).
Motone, K. & Nivala, J. Not if but when nanopore protein sequencing meets single-cell proteomics. Nat. Methods 20, 336–338 (2023).
Dekker, C. Solid-state nanopores. Nat. Nanotechnol. 2, 209–215 (2007).
Mayer, S. F., Cao, C. & Dal Peraro, M. Biological nanopores for single-molecule sensing. iScience 25, 104145 (2022).
Plesa, C. et al. Fast translocation of proteins through solid state nanopores. Nano Lett. 13, 658–663 (2013).
Restrepo-Pérez, L., Joo, C. & Dekker, C. Paving the way to single-molecule protein sequencing. Nat. Nanotechnol. 13, 786–796 (2018).
Xue, L. et al. Solid-state nanopore sensors. Nat. Rev. Mater. 5, 931–951 (2020).
Wang, H., Tang, H., Qiu, X. & Li, Y. Solid-state glass nanopipettes: functionalization and applications. Chem. Eur. J. 30, e202400281 (2024).
Chou, Y.-C., Masih Das, P., Monos, D. S. & Drndić, M. Lifetime and stability of silicon nitride nanopores and nanopore arrays for ionic measurements. ACS Nano 14, 6715–6728 (2020).
Graf, M. et al. Fabrication and practical applications of molybdenum disulfide nanopores. Nat. Protoc. 14, 1130–1168 (2019).
Wilson, J., Sloman, L., He, Z. & Aksimentiev, A. Graphene nanopores for protein sequencing. Adv. Funct. Mater. 26, 4830–4838 (2016).
Thomsen, R. P. et al. A large size-selective DNA nanopore with sensing applications. Nat. Commun. 10, 5655 (2019).
Tripathi, P. et al. Electrical unfolding of cytochrome c during translocation through a nanopore constriction. Proc. Natl Acad. Sci. USA 118, e2016262118 (2021).
Xing, Y., Dorey, A., Jayasinghe, L. & Howorka, S. Highly shape- and size-tunable membrane nanopores made with DNA. Nat. Nanotechnol. 17, 708–713 (2022).
Bošković, F. & Keyser, U. F. Nanopore microscope identifies RNA isoforms with structural colours. Nat. Chem. 14, 1258–1264 (2022).
Wang, F. et al. MoS2 nanopore identifies single amino acids with sub-1 Dalton resolution. Nat. Commun. 14, 2895 (2023).
Shen, Q. et al. Functionalized DNA-origami-protein nanopores generate large transmembrane channels with programmable size-selectivity. J. Am. Chem. Soc. 145, 1292–1300 (2023).
Fragasso, A., Schmid, S. & Dekker, C. Comparing current noise in biological and solid-state nanopores. ACS Nano 14, 1338–1349 (2020).
Mohammadi, M. M. & Bavi, O. DNA sequencing: an overview of solid-state and biological nanopore-based methods. Biophys. Rev. 14, 99–110 (2021).
Alfaro, J. A. et al. The emerging landscape of single-molecule protein sequencing technologies. Nat. Methods 18, 604–617 (2021).
Yu, L. et al. Unidirectional single-file transport of full-length proteins through a nanopore. Nat. Biotechnol. 41, 1130–1139 (2023).
Martin-Baniandres, P. et al. Enzyme-less nanopore detection of post-translational modifications within long polypeptides. Nat. Nanotechnol. 18, 1335–1340 (2023).
Sauciuc, A., Morozzo della Rocca, B., Tadema, M. J., Chinappi, M. & Maglia, G. Translocation of linearized full-length proteins through an engineered nanopore under opposing electrophoretic force. Nat. Biotechnol. 42, 1275–1281 (2024).
Brinkerhoff, H., Kang, A. S. W., Liu, J., Aksimentiev, A. & Dekker, C. Multiple rereads of single proteins at single-amino acid resolution using nanopores. Science 374, 1509–1513 (2021).
Chen, Z. et al. Controlled movement of ssDNA conjugated peptide through Mycobacterium smegmatis porin A (MspA) nanopore by a helicase motor for peptide sequencing application. Chem. Sci. 12, 15750–15756 (2021).
Yan, S. et al. Single molecule ratcheting motion of peptides in a Mycobacterium smegmatis porin A (MspA) nanopore. Nano Lett. 21, 6703–6710 (2021).
Motone, K. et al. Multi-pass, single-molecule nanopore reading of long protein strands. Nature 633, 662–669 (2024).
Meller, A., Nivon, L. & Branton, D. Voltage-driven DNA translocations through a nanopore. Phys. Rev. Lett. 86, 3435–3438 (2001).
Butler, T. Z., Pavlenok, M., Derrington, I. M., Niederweis, M. & Gundlach, J. H. Single-molecule DNA detection with an engineered MspA protein nanopore. Proc. Natl Acad. Sci. USA 105, 20647–20652 (2008).
Derrington, I. M. et al. Subangstrom single-molecule measurements of motor proteins using a nanopore. Nat. Biotechnol. 33, 1073–1075 (2015).
Ensslen, T., Sarthak, K., Aksimentiev, A. & Behrends, J. C. Resolving isomeric posttranslational modifications using a biological nanopore as a sensor of molecular shape. J. Am. Chem. Soc. 144, 16060–16068 (2022).
Song, L. et al. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 274, 1859–1865 (1996).
Iacovache, I. et al. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat. Commun. 7, 12062 (2016).
Faller, M., Niederweis, M. & Schulz, G. E. The structure of a mycobacterial outer-membrane channel. Science 303, 1189–1192 (2004).
Goyal, P. et al. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature 516, 250–253 (2014).
Zhang, M. et al. Real-time detection of 20 amino acids and discrimination of pathologically relevant peptides with functionalized nanopore. Nat. Methods 21, 609–618 (2024).
Wang, K. et al. Unambiguous discrimination of all 20 proteinogenic amino acids and their modifications by nanopore. Nat. Methods 21, 92–101 (2024).
Zhou, W., Qiu, H., Guo, Y. & Guo, W. Molecular insights into distinct detection properties of α-hemolysin, MspA, CsgG, and aerolysin nanopore sensors. J. Phys. Chem. B 124, 1611–1618 (2020).
Crnković, A., Srnko, M. & Anderluh, G. Biological nanopores: engineering on demand. Life 11, 27 (2021).
Huang, G., Voet, A. & Maglia, G. FraC nanopores with adjustable diameter identify the mass of opposite-charge peptides with 44 dalton resolution. Nat. Commun. 10, 835 (2019).
Pavlenok, M., Yu, L., Herrmann, D., Wanunu, M. & Niederweis, M. Control of subunit stoichiometry in single-chain MspA nanopores. Biophys. J. 121, 742–754 (2022).
Ouldali, H. et al. Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nat. Biotechnol. 38, 176–181 (2020).
Piguet, F. et al. Identification of single amino acid differences in uniformly charged homopolymeric peptides with aerolysin nanopore. Nat. Commun. 9, 966 (2018).
Baaken, G. et al. High-resolution size-discrimination of single nonionic synthetic polymers with a highly charged biological nanopore. ACS Nano 9, 6443–6449 (2015).
Chavis, A. E. et al. Single molecule nanopore spectrometry for peptide detection. ACS Sens. 2, 1319–1328 (2017).
Delahaye, C. & Nicolas, J. Sequencing DNA with nanopores: troubles and biases. PLoS ONE 16, e0257521 (2021).
Zhang, Y. et al. Peptide sequencing based on host–guest interaction-assisted nanopore sensing. Nat. Methods 21, 102–109 (2024).
Peeters, J. M., Hazendonk, T. G., Beuvery, E. C. & Tesser, G. I. Comparison of four bifunctional reagents for coupling peptides to proteins and the effect of the three moieties on the immunogenicity of the conjugates. J. Immunol. Methods 120, 133–143 (1989).
Zhang, L. et al. Photoredox-catalyzed decarboxylative C-terminal differentiation for bulk- and single-molecule proteomics. ACS Chem. Biol. 16, 2595–2603 (2021).
De Rosa, L., Di Stasi, R., Romanelli, A. & D’Andrea, L. D. Exploiting protein N-terminus for site-specific bioconjugation. Molecules 26, 3521 (2021).
Afshar Bakshloo, M. et al. Polypeptide analysis for nanopore-based protein identification. Nano Res. 15, 9831–9842 (2022).
Boukhet, M. et al. Probing driving forces in aerolysin and α-hemolysin biological nanopores: electrophoresis versus electroosmosis. Nanoscale 8, 18352–18359 (2016).
Li, M. & Muthukumar, M. Electro-osmotic flow in nanoconfinement: solid-state and protein nanopores. J. Chem. Phys. 160, 084905 (2024).
Wen, C., Schmid, S. & Dekker, C. Understanding electrophoresis and electroosmosis in nanopore sensing with the help of the nanopore electro-osmotic trap. ACS Nano 18, 20449–20458 (2024).
Liu, Y. et al. Machine learning assisted simultaneous structural profiling of differently charged proteins in a Mycobacterium smegmatis porin A (MspA) electroosmotic trap. J. Am. Chem. Soc. 144, 757–768 (2022).
Mehrafrooz, B. et al. Electro-osmotic flow generation via a sticky ion action. ACS Nano 18, 17521–17533 (2024).
Sauciuc, A. et al. Blobs form during the single-file transport of proteins across nanopores. Proc. Natl Acad. Sci. USA 121, e2405018121 (2024).
Baldelli, M. et al. Controlling electroosmosis in nanopores without altering the nanopore sensing region. Adv. Mater. 36, 2401761 (2024).
Gu, L.-Q., Cheley, S. & Bayley, H. Electroosmotic enhancement of the binding of a neutral molecule to a transmembrane pore. Proc. Natl Acad. Sci. USA 100, 15498–15503 (2003).
Nova, I. C. et al. Investigating asymmetric salt profiles for nanopore DNA sequencing with biological porin MspA. PLoS ONE 12, e0181599 (2017).
Nova, I. C. et al. Detection of phosphorylation post-translational modifications along single peptides with nanopores. Nat. Biotechnol. 42, 710–714 (2023).
Craig, J. M. et al. Determining the effects of DNA sequence on Hel308 helicase translocation along single-stranded DNA using nanopore tweezers. Nucleic Acids Res. 47, 2506–2513 (2019).
Bhattacharya, S., Yoo, J. & Aksimentiev, A. Water mediates recognition of DNA sequence via ionic current blockade in a biological nanopore. ACS Nano 10, 4644–4651 (2016).
Katsikis, P. D., Ishii, K. J. & Schliehe, C. Challenges in developing personalized neoantigen cancer vaccines. Nat. Rev. Immunol. 24, 213–227 (2024).
Yewdell, J. W. MHC class I immunopeptidome: past, present, and future. Mol. Cell. Proteom. 21, 100230 (2022).
Boparai, J. K. & Sharma, P. K. Mini review on antimicrobial peptides, sources, mechanism and recent applications. Protein Pept. Lett. 27, 4–16 (2020).
Koch, C. et al. Nanopore sequencing of DNA-barcoded probes for highly multiplexed detection of microRNA, proteins and small biomarkers. Nat. Nanotechnol. 18, 1483–1491 (2023).
Jain, M., Olsen, H. E., Paten, B. & Akeson, M. The Oxford Nanopore MinION: delivery of nanopore sequencing to the genomics community. Genome Biol. 17, 239 (2016).
Zhang, S. et al. Bottom-up fabrication of a proteasome–nanopore that unravels and processes single proteins. Nat. Chem. 13, 1192–1199 (2021).
Barkow, S. R., Levchenko, I., Baker, T. A. & Sauer, R. T. Polypeptide translocation by the AAA+ ClpXP protease machine. Chem. Biol. 16, 605–612 (2009).
Cordova, J. C. et al. Stochastic but highly coordinated protein unfolding and translocation by the ClpXP proteolytic machine. Cell 158, 647–658 (2014).
Flynn, J. M. et al. Overlapping recognition determinants within the ssrA degradation tag allow modulation of proteolysis. Proc. Natl Acad. Sci. USA 98, 10584–10589 (2001).
Deribe, Y. L., Pawson, T. & Dikic, I. Post-translational modifications in signal integration. Nat. Struct. Mol. Biol. 17, 666–672 (2010).
El Kennani, S., Crespo, M., Govin, J. & Pflieger, D. Proteomic analysis of histone variants and their PTMs: strategies and pitfalls. Proteomes 6, 29 (2018).
Park, J. S. et al. Brain somatic mutations observed in Alzheimer’s disease associated with aging and dysregulation of tau phosphorylation. Nat. Commun. 10, 3090 (2019).
Costa, A. F., Campos, D., Reis, C. A. & Gomes, C. Targeting glycosylation: a new road for cancer drug discovery. Trends Cancer 6, 757–766 (2020).
Restrepo-Pérez, L., Wong, C. H., Maglia, G., Dekker, C. & Joo, C. Label-free detection of post-translational modifications with a nanopore. Nano Lett. 19, 7957–7964 (2019).
Lucas, F. L. R. et al. The manipulation of the internal hydrophobicity of FraC nanopores augments peptide capture and recognition. ACS Nano 15, 9600–9613 (2021).
Rajkovic, A. et al. Cyclic rhamnosylated elongation factor P establishes antibiotic resistance in Pseudomonas aeruginosa. mBio 6, e00823-15 (2015).
Versloot, R. C. A. et al. Quantification of protein glycosylation using nanopores. Nano Lett. 22, 5357–5364 (2022).
Li, S. et al. T232K/K238Q aerolysin nanopore for mapping adjacent phosphorylation sites of a single tau peptide. Small Methods 4, 2000014 (2020).
Afshar Bakshloo, M. et al. Discrimination between alpha-synuclein protein variants with a single nanometer-scale pore. ACS Chem. Neurosci. 14, 2517–2526 (2023).
Cao, C. et al. Deep learning-assisted single-molecule detection of protein post-translational modifications with a biological nanopore. ACS Nano 18, 1504–1515 (2024).
Cao, C. et al. Aerolysin nanopores decode digital information stored in tailored macromolecular analytes. Sci. Adv. 6, eabc2661 (2020).
Liu, J. & Aksimentiev, A. Molecular determinants of current blockade produced by peptide transport through a nanopore. ACS Nanosci. Au 4, 21–29 (2024).
Chen, X. et al. Resolving sulfation posttranslational modifications on a peptide hormone using nanopores. ACS Nano 18, 28999–29007 (2024).
Niu, H. et al. Direct mapping of tyrosine sulfation states in native peptides by nanopore. Nat. Chem. Biol. https://doi.org/10.1038/s41589-024-01734-x (2024).
Franks, A., Airoldi, E. & Slavov, N. Post-transcriptional regulation across human tissues. PLoS Comput. Biol. 13, e1005535 (2017).
Sinitcyn, P. et al. MaxDIA enables library-based and library-free data-independent acquisition proteomics. Nat. Biotechnol. 39, 1563–1573 (2021).
Cox, J. Prediction of peptide mass spectral libraries with machine learning. Nat. Biotechnol. 41, 33–43 (2023).
Deslignière, E. et al. Ultralong transients enhance sensitivity and resolution in Orbitrap-based single-ion mass spectrometry. Nat. Methods 21, 619–622 (2024).
Ye, Z. et al. One-Tip enables comprehensive proteome coverage in minimal cells and single zygotes. Nat. Commun. 15, 2474 (2024).
Daly, L. A. et al. Custom workflow for the confident identification of sulfotyrosine-containing peptides and their discrimination from phosphopeptides. J. Proteome Res. 22, 3754–3772 (2023).
Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 21, 729–749 (2020).
Bagdonaite, I. et al. Glycoproteomics. Nat. Rev. Methods Primers 2, 48 (2022).
Reed, B. D. et al. Real-time dynamic single-molecule protein sequencing on an integrated semiconductor device. Science 378, 186–192 (2022).
Filius, M. et al. Full-length single-molecule protein fingerprinting. Nat. Nanotechnol. 19, 652–659 (2024).
Castro-Wallace, S. L. et al. Nanopore DNA sequencing and genome assembly on the international space station. Sci. Rep. 7, 18022 (2017).
Quick, J. et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 530, 228–232 (2016).
Yu, L. et al. Stable polymer bilayers for protein channel recordings at high guanidinium chloride concentrations. Biophys. J. 120, 1537–1541 (2021).
Vreeker, E. et al. Hybrid lipid-block copolymer membranes enable stable reconstitution of a wide range of nanopores and robust sampling of serum. Preprint at bioRxiv https://doi.org/10.1101/2024.05.16.594548 (2024).
Lutz, I. D. et al. Top-down design of protein architectures with reinforcement learning. Science 380, 266–273 (2023).
Xu, C. et al. Computational design of transmembrane pores. Nature 585, 129–134 (2020).
Notin, P., Rollins, N., Gal, Y., Sander, C. & Marks, D. Machine learning for functional protein design. Nat. Biotechnol. 42, 216–228 (2024).
Eisenstein, M. Seven technologies to watch in 2024. Nature 625, 844–848 (2024).
de Lannoy, C., Lucas, F. L. R., Maglia, G. & de Ridder, D. In silico assessment of a novel single-molecule protein fingerprinting method employing fragmentation and nanopore detection. iScience 24, 103202 (2021).
Afshar Bakshloo, M. et al. Nanopore-based protein identification. J. Am. Chem. Soc. 144, 2716–2725 (2022).
Cao, C. et al. Mapping the sensing spots of aerolysin for single oligonucleotides analysis. Nat. Commun. 9, 2823 (2018).
Wu, J., Yamashita, T., Hamilton, A. D., Thompson, S. & Luo, J. Single-molecule nanopore dielectrophoretic trapping of α-synuclein with lipid membranes. Cell Rep. Phys. Sci. 4, 101243 (2023).
Bošković, F. et al. Simultaneous identification of viruses and viral variants with programmable DNA nanobait. Nat. Nanotechnol. 18, 290–298 (2023).
Acknowledgements
We thank B. Albada, E. Bertosin, E. van der Sluis and L. Yu for a critical reading of the manuscript. This work was supported by funding from the Dutch Research Council (NWO) project NWO-I680 (SMPS), European Research Council Advanced Grant 883684 and US National Institutes of Health National Human Genome Research Institute project HG012544-01.
Author information
Authors and Affiliations
Contributions
J.R. and X.C. surveyed the literature for this article. All authors contributed to writing the manuscript. C.D. supervised the work.
Corresponding author
Ethics declarations
Competing interests
C.D. is named inventor on a patent on protein sequencing with nanopores, which is licensed to Oxford Nanopore Technologies. Beyond that, the authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Abdelghani Oukhaled and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ritmejeris, J., Chen, X. & Dekker, C. Single-molecule protein sequencing with nanopores. Nat Rev Bioeng 3, 303–316 (2025). https://doi.org/10.1038/s44222-024-00260-8
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44222-024-00260-8
This article is cited by
-
Fabrication of cytotoxic mirror image nanopores
Nature Communications (2025)