Abstract
Oxygen is essential for the viability of mammalian cells. Disruptions in circulation lead to a cessation of oxygen delivery, which causes decreased ATP production, intracellular acidosis and oedema. If blood flow is reintroduced, this initiates secondary cellular damage usually facilitating cell death. Nonetheless, such outcomes are not inevitable; cells from various organs have been recovered in vitro after extended periods without blood supply, with emerging technologies aimed at scaling up these findings. Perfusion systems, inspired by heart–lung machines, provide mechanical support by restoring circulation, regulating temperature, exchanging gases and modifying circulating perfusate with various pharmacological compounds. Together, perfusion systems and perfusates have mitigated cellular demise and recovered injured tissues, potentially revolutionizing resuscitation medicine and organ transplantation. This Review summarizes the biological mechanisms of cellular injury, perfusate modifications and mechanistic approaches for reinstating circulation, and offers perspectives on the future of organ and whole-body recovery.
Key points
-
Circulatory cessation causes detrimental metabolic changes in mammalian cells due to limited ATP and oxygen reserves, whereas restoring blood flow leads to even more pronounced secondary damage, termed ischaemia–reperfusion injury.
-
In vitro cultures first demonstrated that cell death is not inevitable as previously thought even hours after circulatory cessation, with viable cells successfully recovered from multiple organs.
-
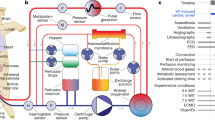
Perfusion systems, inspired by heart–lung machines, use perfusates with blood or synthetic agents to restore circulation, support metabolism and recover compromised tissues.
-
Multimodal perfusion approaches substantially improve organs or whole-body recovery following prolonged circulatory cessation.
-
Perfusion systems with new perfusates are slowly being translated into clinical settings with approaches towards whole-body or isolated-organ recovery in resuscitation and transplant medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hsia, C. C. W., Schmitz, A., Lambertz, M., Perry, S. F. & Maina, J. N. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr. Physiol. 3, 849–915 (2013).
Lee, P., Chandel, N. S. & Simon, M. C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283 (2020). This article provides a great overview of molecular oxygen and its importance in cellular bioenergetics.
Eltzschig, H. K. & Eckle, T. Ischemia and reperfusion—from mechanism to translation. Nat. Med. 17, 1391–1401 (2011).
Neumar, R. W. et al. Post-cardiac arrest syndrome. Circulation 118, 2452–2483 (2008).
Trump, B. F. & Harris, C. C. Human tissues in biomedical research. Hum. Pathol. 10, 245–248 (1979).
Giwa, S. et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 35, 530–542 (2017). This article is one of the first to provide an estimate of a true organ need in the world and the impact that different organ preservation strategies would have on global healthcare.
Orlando, G. & Keshavjee, S. Organ repair and regeneration: preserving organs in the regenerative medicine era (2021).
Daniele, S. G. et al. Brain vulnerability and viability after ischaemia. Nat. Rev. Neurosci. 22, 553–572 (2021). This article is one of the first to provide a comprehensive overview of brain ischaemia and multimodal strategies for targeting various cellular mechanisms following ischaemic injury.
Nasralla, D. et al. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018).
Markmann, J. F. et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: the OCS liver protect randomized clinical trial. JAMA Surg. 157, 189–198 (2022).
van Rijn, R. et al. Hypothermic machine perfusion in liver transplantation—a randomized trial. N. Engl. J. Med. 384, 1391–1401 (2021).
Eshmuminov, D. et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. 28, 189–198 (2020).
Clavien, P. A. et al. Transplantation of a human liver following 3 days of ex situ normothermic preservation. Nat. Biotechnol. 40, 1610–1616 (2022). This study is first of its kind describing the ability to perfuse ex vivo human liver for 3 days and then transplant it with great outcome after 1-year of follow-up.
Brasile, L. et al. Overcoming severe renal ischemia: the role of ex vivo warm perfusion. Transplantation 73, 897–901 (2002).
Weissenbacher, A. et al. Twenty-four-hour normothermic perfusion of discarded human kidneys with urine recirculation. Am. J. Transplant. 19, 178–192 (2019).
Hozain, A. E. et al. Xenogeneic cross-circulation for extracorporeal recovery of injured human lungs. Nat. Med. 26, 1102–1113 (2020).
Ali, A. et al. Successful 3-day lung preservation using a cyclic normothermic ex vivo lung perfusion strategy. EBioMedicine 83, 104210 (2022).
Mallea, J. M. et al. Remote ex vivo lung perfusion at a centralized evaluation facility. J. Heart Lung Transpl. 41, 1700–1711 (2022).
Vrselja, Z. et al. Restoration of brain circulation and cellular functions hours post-mortem. Nature 568, 336–343 (2019). This study demonstrates that circulation and cellular functions can be restored 4 h after death in the large mammalian brain.
Andrijevic, D. et al. Cellular recovery after prolonged warm ischaemia of the whole body. Nature 608, 405–412 (2022). This study demonstrates that circulation and cellular recovery across multiple vital organs can be achieved following 1 h of complete circulatory arrest in large mammals.
Kalogeris, T., Baines, C. P., Krenz, M. & Korthuis, R. J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 298, 229 (2012).
Wu, M. Y. et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell. Physiol. Biochem. 46, 1650–1667 (2018).
Zhang, M. et al. Ischemia–reperfusion injury: molecular mechanisms and therapeutic targets. Signal Transduct. Target Ther. 9, 12 (2024). This review greatly summarizes a more molecular aspect of IRI and potential therapeutic targets.
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Soares, R. O. S., Losada, D. M., Jordani, M. C., Évora, P. & Castro-E-Silva, O. Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int. J. Mol. Sci. 20, 5034 (2019).
Auten, R. L. & Davis, J. M. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatric Res. 66, 121–127 (2009).
Beg, A. A. Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23, 509–512 (2002).
Midwood, K. S. & Piccinini, A. M. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 672395 (2010).
Devarajan, P. Update on mechanisms of ischemic acute kidney injury. J. Am. Soc. Nephrol. 17, 1503–1520 (2006).
Li, L. et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia–reperfusion injury. Kidney Int. 74, 1526–1537 (2008).
Huen, S. C., Moecke, G. W. & Cantley, L. G. Macrophage-specific deletion of transforming growth factor-β1 does not prevent renal fibrosis after severe ischemia–reperfusion or obstructive injury. Am. J. Physiol. Ren. Physiol 305, 477–484 (2013).
Bonavia, A. & Singbartl, K. A review of the role of immune cells in acute kidney injury. Pediatric Nephrol. 33, 1629–1639 (2018).
Arumugam, T. V., Shiels, I. A., Woodruff, T. M., Neil Granger, D. & Taylor, S. M. The role of the complement system in ischemia–reperfusion injury. Shock 21, 401–409 (2004).
Micó-Carnero, M. et al. A potential route to reduce ischemia/reperfusion injury in organ preservation. Cells 11, 2763 (2022).
Vaseva, A. V. et al. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548 (2012).
Yang, H. et al. p53–cyclophilin D mediates renal tubular cell apoptosis in ischemia–reperfusion-induced acute kidney injury. Am. J. Physiol. Ren. Physiol 317, F1311–F1317 (2019).
Linkermann, A. et al. Two independent pathways of regulated necrosis mediate ischemia–reperfusion injury. Proc. Natl Acad. Sci. USA 110, 12024–12029 (2013).
Fuchs, Y. & Steller, H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 16, 329–344 (2015). This article provides a great comprehensive overview of various cell death pathways.
Liu, Y. et al. Research progress on the role of pyroptosis in myocardial ischemia–reperfusion injury. Cell 11, 3271 (2022).
Zheng, Y., Xu, X., Chi, F. & Cong, N. Pyroptosis: a newly discovered therapeutic target for ischemia–reperfusion injury. Biomolecules 12, 1625 (2022).
Pan, Y. et al. Targeting ferroptosis as a promising therapeutic strategy for ischemia–reperfusion injury. Antioxidants 11, 2196 (2022).
Nolan, J. P. et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 79, 350–379 (2008). This article is one of the first and most comprehensive reviews of PCAS.
Jozwiak, M., Bougouin, W., Geri, G., Grimaldi, D. & Cariou, A. Post-resuscitation shock: recent advances in pathophysiology and treatment. Ann. Intensive Care 10, 1–11 (2020).
Gando, S. & Wada, T. Disseminated intravascular coagulation in cardiac arrest and resuscitation. J. Thromb. Haemost. 17, 1205–1216 (2019).
Rothman, S. M. & Olney, J. W. Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann. Neurol. 19, 105–111 (1986).
Hayman, E. G., Patel, A. P., Kimberly, W. T., Sheth, K. N. & Simard, J. M. Cerebral edema after cardiopulmonary resuscitation: a therapeutic target following cardiac arrest? Neurocrit Care 28, 276–287 (2018).
Ames, A., Wright, R. L., Kowada, M., Thurston, J. M. & Majno, G. Cerebral ischemia. II. No-reflow phenomenon. Am. J. Pathol. 52, 437 (1968).
Del Zoppo, G. J. & Mabuchi, T. Cerebral microvessel responses to focal ischemia. J. Cereb. Blood Flow. Metab. 23, 879–894 (2003).
Kagstrom, E., Smith, M. L. & Siesjo, B. K. Local cerebral blood flow in the recovery period following complete cerebral ischemia in the rat. J. Cereb. Blood Flow. Metab. 3, 170–182 (1983).
Fischer, M. & Hossmann, K. A. No-reflow after cardiac arrest. Intensive Care Med. 21, 132–141 (1995). This article is one of the landmark studies on the no-reflow phenomenon after cardiac arrest.
Greif, D. M. & Eichmann, A. Vascular biology: brain vessels squeezed to death. Nature 508, 50–51 (2014).
Pulsinelli, W. A. Selective neuronal vulnerability: morphological and molecular characteristics. Prog. Brain Res. 63, 29–37 (1985).
Jentzer, J. C., Chonde, M. D. & Dezfulian, C. Myocardial dysfunction and shock after cardiac arrest. Biomed. Res. Int. 2015, 314796 (2015).
Chalkias, A. & Xanthos, T. Pathophysiology and pathogenesis of post-resuscitation myocardial stunning. Heart Fail. Rev. 17, 117–128 (2012).
Hajjar, R. J. et al. Myofilament calcium regulation in human myocardium. Circulation 101, 1679–1685 (2000).
Van Eyk, J. E. & Murphy, A. M. The role of troponin abnormalities as a cause for stunned myocardium. Coron. Artery Dis. http://journals.lww.com/coronary-artery (2001).
Kloner, R. A., King, K. S. & Harrington, M. G. No-reflow phenomenon in the heart and brain. Am. J. Physiol. Heart Circ. Physiol 315, H550–H562 (2018).
Ferenz, K. B. & Steinbicker, A. U. Artificial oxygen carriers—past, present and the future—a review of the most innovative and clinically relevant concepts. J. Pharmacol. Exp. Ther. 369, 300–310 (2019).
Jahr, J. S., Guinn, N. R., Lowery, D. R., Shore-Lesserson, L. & Shander, A. Blood substitutes and oxygen therapeutics: a review. Anesth. Analg. 132, 119–129 (2021).
Cao, M. et al. Hemoglobin-based oxygen carriers: potential applications in solid organ preservation. Front. Pharmacol. 12, 760215 (2021).
Teh, E. S., Zal, F., Polard, V., Menasché, P. & Chambers, D. J. HEMO2life as a protective additive to Celsior solution for static storage of donor hearts prior to transplantation. Artif. Cells Nanomed. Biotechnol. 45, 717–722 (2017).
Christoforidis, G. A. et al. Effect of early Sanguinate (PEGylated carboxyhemoglobin bovine) infusion on cerebral blood flow to the ischemic core in experimental middle cerebral artery occlusion. J. Neurointerv. Surg. 14, 1253–1257 (2021).
Abuchowski, A. SANGUINATE (PEGylated carboxyhemoglobin bovine): mechanism of action and clinical update. Artif. Organs 41, 346–350 (2017).
Mito, T. et al. Decreased damage from transient focal cerebral ischemia by transfusion of zero-link hemoglobin polymers in mouse. Stroke 40, 278–284 (2009).
Reynolds, P. S., Barbee, R. W., Skaflen, M. D. & Ward, K. R. Low-volume resuscitation cocktail extends survival after severe hemorrhagic shock. Shock 28, 45–52 (2007).
Paradis, N. A. Dose–response relationship between aortic infusions of polymerized bovine hemoglobin and return of circulation in a canine model of ventricular fibrillation and advanced cardiac life support. Crit. Care Med. 25, 476–483 (1997).
Kaneda, T., Ku, K., Inoue, T., Onoe, M. & Oku, H. Postischemic reperfusion injury can be attenuated by oxygen tension control. Jpn. Circ. J. 65, 213–218 (2001).
Taverne, Y. J. et al. Normalization of hemoglobin-based oxygen carrier-201 induced vasoconstriction: targeting nitric oxide and endothelin. J. Appl. Physiol. 122, 1227–1237 (2017).
Jahr, J. S., MacKenzie, C., Pearce, L. B., Pitman, A. & Greenburg, A. G. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J. Trauma. Injury Infect. Crit. Care 64, 1484–1497 (2008).
Natanson, C., Kern, S. J., Lurie, P., Banks, S. M. & Wolfe, S. M. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 299, 2304–2312 (2008).
Follette, D. M. et al. Reducing postischemic damage by temporary modification of reperfusate calcium, potassium, pH, and osmolarity. J. Thorac. Cardiovasc. Surg. 82, 221–238 (1981).
Leaf, A. Cell swelling. Circulation 48, 455–458 (1973).
Inan, N. et al. Effect of hydroxyethyl starch 130/0.4 on ischaemia/reperfusion in rabbit skeletal muscle. Eur. J. Anaesthesiol. 26, 160–165 (2009).
Hahn, C. et al. Hypertonic saline infusion during resuscitation from out-of-hospital cardiac arrest: a matched-pair study from the German Resuscitation Registry. Resuscitation 85, 628–636 (2014).
Mosbah, I. B. et al. Effects of polyethylene glycol and hydroxyethyl starch in University of Wisconsin preservation solution on human red blood cell aggregation and viscosity. Transpl. Proc. 38, 1229–1235 (2006).
Hauet, T. et al. Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys. Kidney Int. 62, 654–667 (2002).
Juarez-Moreno, K., Ayala, M. & Vazquez-Duhalt, R. Antioxidant capacity of poly(ethylene glycol) (PEG) as protection mechanism against hydrogen peroxide inactivation of peroxidases. Appl. Biochem. Biotechnol. 177, 1364–1373 (2015).
Ge, W. et al. Effects of polyethylene glycol-20k on coronary perfusion pressure and postresuscitation myocardial and cerebral function in a rat model of cardiac arrest. J. Am. Heart Assoc. 9, e014232 (2020).
Beyersdorf, F., Trummer, G., Benk, C. & Pooth, J.-S. Application of cardiac surgery techniques to improve the results of cardiopulmonary resuscitation after cardiac arrest: controlled automated reperfusion of the whole body. JTCVS Open 8, 47–52 (2021).
Orrenius, S., Zhivotovsky, B. & Nicotera, P. Regulation of cell death: the calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 4, 552–565 (2003).
Klein, H. H. et al. Treatment of reperfusion injury with intracoronary calcium channel antagonists and reduced coronary free calcium concentration in regionally ischemic, reperfused porcine hearts. J. Am. Coll. Cardiol. 13, 1395–1401 (1989).
Pooth, J. S. et al. Effects of prolonged serum calcium suppression during extracorporeal cardiopulmonary resuscitation in pigs. Biomedicines 11, 2612 (2023).
Kang, S. W. et al. Neuroprotective effects of magnesium-sulfate on ischemic injury mediated by modulating the release of glutamate and reduced of hyperreperfusion. Brain Res. 1371, 121–128 (2011).
Gorelick, P. B. & Ruland, S. IMAGES and FAST-MAG: magnesium for acute ischaemic stroke. Lancet Neurol. 3, 330 (2004).
Liu, B. et al. The role of magnesium in cardiac arrest. Front. Nutr. 11, 1387268 (2024).
Ishaq, G. M. et al. Effects of α-tocopherol and ascorbic acid in the severity and management of traumatic brain injury in albino rats. J. Neurosci. Rural. Pract. 4, 292–297 (2013).
Azari, O. et al. Protective effects of hydrocortisone, vitamin C and E alone or in combination against renal ischemia–reperfusion injury in rat. Iran. J. Pathol. 10, 272 (2015).
Hsu, C. C. & Wang, J. J. l-Ascorbic acid and ɑ-tocopherol attenuates liver ischemia–reperfusion induced of cardiac function impairment. Transpl. Proc. 44, 933–936 (2012).
Anderson, M. F., Nilsson, M., Eriksson, P. S. & Sims, N. R. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neurosci. Lett. 354, 163–165 (2004).
Kobayashi, H. et al. The effects of γ-glutamylcysteine ethyl ester, a prodrug of glutathione, on ischemia–reperfusion-induced liver injury in rats. Transplantation 54, 414–418 (1992).
Tsai, M. S. et al. Combination of intravenous ascorbic acid administration and hypothermia after resuscitation improves myocardial function and survival in a ventricular fibrillation cardiac arrest model in the rat. Acad. Emerg. Med. 21, 257–265 (2014).
Adlam, V. J. et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia–reperfusion injury. FASEB J. 19, 1088–1095 (2005).
Zinovkin, R. A. & Zamyatnin, A. A. Mitochondria-targeted drugs. Curr. Mol. Pharmacol. 12, 202–214 (2019).
Murphy, M. P. & Hartley, R. C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug. Discov. 17, 865–886 (2018).
Matthews, R. T., Yang, L., Browne, S., Baik, M. & Beal, M. F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl Acad. Sci. USA 95, 8892–8897 (1998).
Damian, M. S. et al. Coenzyme Q10 combined with mild hypothermia after cardiac arrest: a preliminary study. Circulation 110, 3011–3016 (2004).
Dambrova, M., Zuurbier, C. J., Borutaite, V., Liepinsh, E. & Makrecka-Kuka, M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free. Radic. Biol. Med. 165, 24–37 (2021).
Satomi, S. et al. Branched-chain amino acids-induced cardiac protection against ischemia/reperfusion injury. Life Sci. 245, 117368 (2020).
Vreugdenhil, P. K., Marsh, D. C. & Southard, J. H. Comparison of isolated hepatocytes and tissue slices for study of liver hypothermic preservation/reperfusion injury. Cryobiology 33, 430–435 (1996).
Schuster, H. et al. Protective effects of regulatory amino acids on ischaemia–reperfusion injury in the isolated perfused rat liver. Scand. J. Gastroenterol. 41, 1342–1349 (2006).
Yu, Y. et al. Treatment with d-β-hydroxybutyrate protects heart from ischemia/reperfusion injury in mice. Eur. J. Pharmacol. 829, 121–128 (2018).
Tajima, T. et al. β-Hydroxybutyrate attenuates renal ischemia–reperfusion injury through its anti-pyroptotic effects. Kidney Int. 95, 1120–1137 (2019).
Ysebaert, D. K. et al. Effect of immunosuppression on damage, leukocyte infiltration, and regeneration after severe warm ischemia/reperfusion renal injury. Kidney Int. 64, 864–873 (2003).
Pratschke, S. et al. Tacrolimus preconditioning of rat liver allografts impacts glutathione homeostasis and early reperfusion injury. J. Surg. Res. 176, 309–316 (2012).
Cour, M. et al. Inhibition of mitochondrial permeability transition to prevent the post-cardiac arrest syndrome: a pre-clinical study. Eur. Heart J. 32, 226–235 (2011).
Argaud, L. et al. Effect of cyclosporine in nonshockable out-of-hospital cardiac arrest: the CYRUS randomized clinical trial. JAMA Cardiol. 1, 557–565 (2016).
Cunningham, C. A., Coppler, P. J. & Skolnik, A. B. The immunology of the post-cardiac arrest syndrome. Resuscitation 179, 116–123 (2022).
Kazmi, S., Salehi-Pourmehr, H., Sadigh-Eteghad, S. & Farhoudi, M. The efficacy and safety of interleukin-1 receptor antagonist in stroke patients: a systematic review. J. Clin. Neurosci. 120, 120–128 (2024).
Meyer, M. A. S. et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (The IMICA Trial): a double-blinded, placebo-controlled, single-center, randomized, clinical trial. Circulation 143, 1841–1851 (2021).
Penn, J. et al. Efficacy and safety of corticosteroids in cardiac arrest: a systematic review, meta-analysis and trial sequential analysis of randomized control trials. Crit. Care 27, 12 (2023).
Yaoita, H., Ogawa, K., Maehara, K. & Maruyama, Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation 97, 276–281 (1998).
Koudstaal, S. et al. Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur. J. Clin. Invest. 45, 150–159 (2015).
Van Hout, G. P. J. et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 38, 828–836 (2017).
Zheng, G. et al. The selective NLRP3-inflammasome inhibitor MCC950 mitigates post-resuscitation myocardial dysfunction and improves survival in a rat model of cardiac arrest and resuscitation. Cardiovasc. Drugs Ther. 37, 423–433 (2023).
Ni, J. et al. Hydrogen sulfide reduces pyroptosis and alleviates ischemia–reperfusion-induced acute kidney injury by inhibiting NLRP3 inflammasome. Life Sci. 284, 119466 (2021).
Dhani, S., Zhao, Y. & Zhivotovsky, B. A long way to go: caspase inhibitors in clinical use. Cell Death Dis. 2021, 1–13 (2021).
Vereczki, V. et al. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J. Cereb. Blood Flow. Metab. 26, 821–835 (2006).
Shou, B. L. et al. Arterial oxygen and carbon dioxide tension and acute brain injury in extracorporeal cardiopulmonary resuscitation patients: analysis of the extracorporeal life support organization registry. J. Heart Lung Transplant. 42, 503–511 (2023).
Dulla, C. G. et al. Adenosine and ATP link pCO2 to cortical excitability via pH. Neuron 48, 1011–1023 (2005).
Bagul, A., Hosgood, S. A., Kaushik, M. & Nicholson, M. L. Carbon monoxide protects against ischemia–reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation 85, 576–581 (2008).
McFarlane, L., Nelson, P., Dugbartey, G. J. & Sener, A. Pre-treatment of transplant donors with hydrogen sulfide to protect against warm and cold ischemia–reperfusion injury in kidney and other transplantable solid organs. Int. J. Mol. Sci. 24, 3518 (2023).
Minamishima, S. et al. Hydrogen sulfide improves survival after cardiac arrest and cardiopulmonary resuscitation via a nitric oxide synthase 3-dependent mechanism in mice. Circulation 120, 888–896 (2009).
Wang, P. et al. Carbon monoxide improves neurologic outcomes by mitochondrial biogenesis after global cerebral ischemia induced by cardiac arrest in rats. Int. J. Biol. Sci. 12, 1000 (2016).
Derwall, M. et al. Hydrogen sulfide does not increase resuscitability in a porcine model of prolonged cardiac arrest. Shock 34, 190–195 (2010).
Wollborn, J. et al. Carbon monoxide exerts functional neuroprotection after cardiac arrest using extracorporeal resuscitation in pigs. Crit. Care Med. 48, E299–E307 (2020).
De Deken, J., Rex, S., Monbaliu, D., Pirenne, J. & Jochmans, I. The efficacy of noble gases in the attenuation of ischemia reperfusion injury: a systematic review and meta-analyses. Crit. Care Med. 44, e886–e896 (2016).
Martens, A. et al. A porcine ex vivo lung perfusion model with maximal argon exposure to attenuate ischemia–reperfusion injury. Med. Gas. Res. 7, 28 (2017).
Smith, S. F., Adams, T., Hosgood, S. A. & Nicholson, M. L. The administration of argon during ex vivo normothermic perfusion in an experimental model of kidney ischemia–reperfusion injury. J. Surg. Res. 218, 202–208 (2017).
Laitio, R. et al. Effect of inhaled xenon on cerebral white matter damage in comatose survivors of out-of-hospital cardiac arrest: a randomized clinical trial. JAMA 315, 1120–1128 (2016).
Hayashida, K. et al. Inhaled gases as therapies for post-cardiac arrest syndrome: a narrative review of recent developments. Front. Med. 7, 586229 (2021).
Tamura, T. et al. Efficacy of inhaled hydrogen on neurological outcome following brain ischaemia during post-cardiac arrest care (HYBRID II): a multi-centre, randomised, double-blind, placebo-controlled trial. eClinicalMedicine 58, 101907 (2023).
Lee, H. M., Choi, J. W. & Choi, M. S. Role of nitric oxide and protein S-nitrosylation in ischemia–reperfusion injury. Antioxidants 11, 57 (2021).
Moncada, S. & Erusalimsky, J. D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 3, 214–220 (2002).
Bonfoco, E., Krainc, D., Ankarcrona, M., Nicotera, P. & Lipton, S. A. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-d-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc. Natl Acad. Sci. USA 92, 7162–7166 (1995).
Miyazaki, Y. & Ichinose, F. Nitric oxide in post-cardiac arrest syndrome. J. Cardiovasc. Pharmacol. 75, 508–515 (2020).
Brücken, A. et al. Brief inhalation of nitric oxide increases resuscitation success and improves 7-day-survival after cardiac arrest in rats: a randomized controlled animal study. Crit. Care 19, 1–13 (2015).
Derwall, M. et al. Inhaled nitric oxide improves transpulmonary blood flow and clinical outcomes after prolonged cardiac arrest: a large animal study. Crit. Care 19, 1–10 (2015).
Patel, J. K. et al. Inhaled nitric oxide in adults with in-hospital cardiac arrest: a feasibility study. Nitric Oxide 115, 30–33 (2021).
Rayego-Mateos, S. et al. The optimization of renal graft preservation temperature to mitigate cold ischemia–reperfusion injury in kidney transplantation. Int. J. Mol. Sci. 24, 567 (2022).
Choi, H. A., Badjatia, N. & Mayer, S. A. Hypothermia for acute brain injury—mechanisms and practical aspects. Nat. Rev. Neurol. 2012, 214–222 (2012).
Hägerdal, M., Harp, J. & Siesjö, B. K. Effect of hypothermia upon organ phosphates, glycolytic metabolites, citric acid cycle intermediates and associated amino acids in rat cerebral cortex. J. Neurochem. 24, 743–748 (1975).
Kimura, A., Sakurada, S., Ohkuni, H., Todome, Y. & Kurata, K. Moderate hypothermia delays proinflammatory cytokine production of human peripheral blood mononuclear cells. Crit. Care Med. 30, 1499–1502 (2002).
Van Breukelen, F., Krumschnabel, G. & Podrabsky, J. E. Vertebrate cell death in energy-limited conditions and how to avoid it: what we might learn from mammalian hibernators and other stress-tolerant vertebrates. Apoptosis 15, 386–399 (2010).
Dankiewicz, J. et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N. Engl. J. Med. 384, 2283–2294 (2021).
Lascarrou, J.-B. et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N. Engl. J. Med. 381, 2327–2337 (2019).
Moers, C. et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 360, 1460–1461 (2009).
Minor, T. & von Horn, C. Rewarming injury after cold preservation. Int. J. Mol. Sci. 20, 2059 (2019).
Hosgood, S. A. et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: a randomized controlled trial. Nat. Med. 29, 1511–1519 (2023).
Zlatev, H., von Horn, C., Kaths, M., Paul, A. & Minor, T. Clinical use of controlled oxygenated rewarming of kidney grafts prior to transplantation by ex vivo machine perfusion. A pilot study. Eur. J. Clin. Invest. 52, e13691 (2022).
Minor, T. et al. Controlled oxygenated rewarming as novel end‐ischemic therapy for cold stored liver grafts. A randomized controlled trial. Clin. Transl. Sci. 15, 2918 (2022).
Tamari, Y., Lee-Sensiba, K., Leonard, E. F., Parnell, V. & Tortolani, A. J. The effects of pressure and flow on hemolysis caused by Bio-Medicus centrifugal pumps and roller pumps: guidelines for choosing a blood pump. J. Thorac. Cardiovasc. Surg. 106, 997–1007 (1993).
Saczkowski, R., Maklin, M., Mesana, T., Boodhwani, M. & Ruel, M. Centrifugal pump and roller pump in adult cardiac surgery: a meta-analysis of randomized controlled trials. Artif. Organs 36, 668–676 (2012).
Kreibich, M. et al. Improved outcome in an animal model of prolonged cardiac arrest through pulsatile high pressure controlled automated reperfusion of the whole body. Artif. Organs 42, 992–1000 (2018).
Allen, B. S., Ko, Y., Buckberg, G. D. & Tan, Z. Studies of isolated global brain ischaemia: II. Controlled reperfusion provides complete neurologic recovery following 30 min of warm ischaemia—the importance of perfusion pressure. Eur. J. Cardiothorac. Surg. 41, 1147 (2012).
Vincent, D. E. et al. Pulsatile ECMO: the future of mechanical circulatory support for severe cardiogenic shock. JACC Basic Transl. Sci. 9, 456–458 (2024).
von Horn, C. & Minor, T. Isolated kidney perfusion: the influence of pulsatile flow. Scand. J. Clin. Lab. Invest. 78, 131–135 (2018).
Tawa, P. et al. Continuous versus pulsatile flow in 24-hour vascularized composite allograft machine perfusion in swine: a pilot study. J. Surg. Res. 283, 1145–1153 (2023).
Brüggenwirth, I. M. et al. A comparative study of single and dual perfusion during end-ischemic subnormothermic liver machine preservation. Transplant. Direct. 4, e400 (2018).
Itoh, H. et al. Effect of the pulsatile extracorporeal membrane oxygenation on hemodynamic energy and systemic microcirculation in a piglet model of acute cardiac failure. Artif. Organs 40, 19–26 (2016).
Betit, P. Technical advances in the field of ECMO. Respir. Care 63, 1162–1173 (2018).
Murphy, G. S., Hessel, E. A. & Groom, R. C. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth. Analg. 108, 1394–1417 (2009).
Boodram, S. & Evans, E. Use of leukocyte-depleting filters during cardiac surgery with cardiopulmonary bypass: a review. J. Extra Corpor. Technol. 40, 27 (2008).
Naruka, V. et al. Use of cytokine filters during cardiopulmonary bypass: systematic review and meta-analysis. Heart Lung Circ. 31, 1493–1503 (2022).
Iskender, I. et al. Cytokine filtration modulates pulmonary metabolism and edema formation during ex vivo lung perfusion. J. Heart Lung Transplant. 37, 283–291 (2018).
Noda, K. et al. Targeting circulating leukocytes and pyroptosis during ex vivo lung perfusion improves lung preservation. Transplantation 101, 2841–2849 (2017).
Huber, W., Zanner, R., Schneider, G., Schmid, R. & Lahmer, T. Assessment of regional perfusion and organ function: less and non-invasive techniques. Front. Med. 6, 50 (2019).
Eshmuminov, D. & Clavien, P. A. Long-term dynamic ex vivo organ preservation. Nat. Rev. Gastroenterol. Hepatol. 20, 267–268 (2022).
Eltzschig, H. K. Prolonging cellular life after hypoxic death. N. Engl. J. Med. 387, 2089–2091 (2022).
Li, X. et al. Alleviation of ischemia–reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbecks Arch. Surg. 392, 345–351 (2007).
Wu, Y., Liu, Y., Li, M., Liu, Z. & Gong, J. IRAK-4-shRNA prevents ischemia/reperfusion injury via different perfusion periods through the portal vein after liver transplantation in rat. Transpl. Proc. 48, 2803–2808 (2016).
Gao, Q. et al. Gene therapy: will the promise of optimizing lung allografts become reality? Front. Immunol. https://doi.org/10.3389/fimmu.2022.931524 (2022).
Cypel, M. et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci. Transl. Med. 1, 4ra9 (2009).
Daddi, N. et al. Recipient intramuscular cotransfection of naked plasmid transforming growth factor β1 and interleukin 10 ameliorates lung graft ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 124, 259–269 (2002).
Brasile, L., Henry, N., Orlando, G. & Stubenitsky, B. Potentiating renal regeneration using mesenchymal stem cells. Transplantation 103, 307–313 (2019).
Podestà, M. A., Remuzzi, G. & Casiraghi, F. Mesenchymal stromal cell therapy in solid organ transplantation. Front. Immunol. 11, 618243 (2021).
Thompson, E. R. et al. Novel delivery of cellular therapy to reduce ischemia reperfusion injury in kidney transplantation. Am. J. Transplant. 21, 1402–1414 (2021).
Lohmann, S. et al. Mesenchymal stromal cell treatment of donor kidneys during ex vivo normothermic machine perfusion: a porcine renal autotransplantation study. Am. J. Transplant. 21, 2348–2359 (2021).
Hayashida, K. et al. Mitochondrial transplantation therapy for ischemia reperfusion injury: a systematic review of animal and human studies. J. Transl. Med. 19, 1–15 (2021).
Guariento, A. et al. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 162, 992–1001 (2021).
Hozain, A. E. et al. Multiday maintenance of extracorporeal lungs using cross-circulation with conscious swine. J. Thorac. Cardiovasc. Surg. 159, 1640 (2020).
Wu, W. K. et al. Xenogeneic cross-circulation for physiological support and recovery of ex vivo human livers. Hepatology 78, 820–834 (2023).
Montgomery, R. A., Mehta, S. A., Parent, B. & Griesemer, A. Next steps for the xenotransplantation of pig organs into humans. Nat. Med. 28, 1533–1536 (2022).
Kiyohara, Y., Kampaktsis, P. N., Briasoulis, A. & Kuno, T. Extracorporeal membrane oxygenation-facilitated resuscitation in out-of-hospital cardiac arrest: a meta-analysis of randomized controlled trials. J. Cardiovasc. Med. 24, 414–419 (2023).
Yannopoulos, D. et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet 396, 1807–1816 (2020).
Trummer, G. et al. Treatment of refractory cardiac arrest by controlled reperfusion of the whole body: a multicenter, prospective observational study. J. Clin. Med. 13, 56 (2024).
Belohlavek, J. et al. Effect of intra-arrest transport, extracorporeal cardiopulmonary resuscitation, and immediate invasive assessment and treatment on functional neurologic outcome in refractory out-of-hospital cardiac arrest: a randomized clinical trial. JAMA 327, 737 (2022).
Suverein, M. M. et al. Early extracorporeal CPR for refractory out-of-hospital cardiac arrest. N. Engl. J. Med. 388, 299–309 (2023).
Raphalen, J. H. et al. Kidneys recovered from brain dead cardiac arrest patients resuscitated with ECPR show similar one-year graft survival compared to other donors. Resuscitation 190, 109883 (2023).
James, L. et al. Donation after circulatory death heart transplantation using normothermic regional perfusion: the NYU protocol. JTCVS Tech. 17, 111 (2023).
Wall, A. E. et al. The American Society of Transplant Surgeons consensus statement on normothermic regional perfusion. Transplantation 108, 312–318 (2024).
Johnston, C. J. C., Sherif, A. E. & Oniscu, G. C. Transplantation of discarded livers: the complementary role of normothermic regional perfusion. Nat. Commun. 12, 4471 (2021).
De Beule, J. et al. A systematic review and meta-analyses of regional perfusion in donation after circulatory death solid organ transplantation. Transpl. Int. 34, 2046–2060 (2021).
Smith, D. E. et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J. Thorac. Cardiovasc. Surg. 164, 557–568.e1 (2022).
Schemmer, P. et al. Transplanting marginal organs in the era of modern machine perfusion and advanced organ monitoring. Front. Immunol. 1, 631 (2020).
Kaths, J. M. et al. Continuous normothermic ex vivo kidney perfusion improves graft function in donation after circulatory death pig kidney transplantation. Transplantation 101, 754 (2017).
Czigany, Z. et al. Hypothermic oxygenated machine perfusion reduces early allograft injury and improves post-transplant outcomes in extended criteria donation liver transplantation from donation after brain death: results from a multicenter randomized controlled trial (HOPE ECD-DBD). Ann. Surg. 274, 705–712 (2021).
Risbey, C. W. G. & Pulitano, C. Normothermic ex vivo machine perfusion for liver transplantation: a systematic review of progress in humans. J. Clin. Med. 12, 3718 (2023).
Ardehali, A. et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 385, 2577–2584 (2015).
Cypel, M. et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N. Engl. J. Med. 364, 1431–1440 (2011).
Warnecke, G. et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir. Med. 6, 357–367 (2018).
Langmuur, S. J. J. et al. Normothermic ex situ heart perfusion with the organ care system for cardiac transplantation: a meta-analysis. Transplantation 106, 1745–1753 (2022).
Trummer, G., Benk, C. & Beyersdorf, F. Controlled automated reperfusion of the whole body after cardiac arrest. J. Thorac. Dis. 11, S1464 (2019).
Taunyane, I. C. et al. Preserved brain morphology after controlled automated reperfusion of the whole body following normothermic circulatory arrest time of up to 20 minutes. Eur. J. Cardiothorac. Surg. 50, 1025–1034 (2016).
Croome, K. P. Introducing machine perfusion into routine clinical practice for liver transplantation in the United States: the moment has finally come. J. Clin. Med. 12, 909 (2023).
Parente, A. et al. Trends and obstacles to implement dynamic perfusion concepts for clinical liver transplantation: results from a global web-based survey. J. Clin. Med. 12, 3765 (2023).
Suverein, M. M. et al. Ethics of ECPR research. Resuscitation 169, 136–142 (2021).
American College of Physicians. Ethics, determination of death, and organ transplantation in normothermic regional perfusion (NRP) with controlled donation after circulatory determination of death (cDCD): American College of Physicians Statement of Concern; https://www.acponline.org/acp_policy/policies/ethics_determination_of_death_and_organ_transplantation_in_nrp_2021.pdf (2021).
Parent, B., Caplan, A., Moazami, N. & Montgomery, R. A. Response to American College of Physician’s statement on the ethics of transplant after normothermic regional perfusion. Am. J. Transplant. 22, 1307–1310 (2022).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization of the article, data search and discussion of content. The initial draft of the manuscript was authored by D.A., with contributions from all co-authors. D.A. and A.S. were responsible for the generation of all figures. Subsequently, all authors reviewed and edited the manuscript prior to its submission.
Corresponding author
Ethics declarations
Competing interests
N.S. is a co-founder of Bexorg, in which he holds equity and serves on the board. R.A.M. has received research funds from Lung Biotechnology, a wholly owned subsidiary of United Therapeutics Corporation, PBC; serves on the advisory board of eGenesis; and has been a strategic advisor for Recombinetics. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Korkut Uygun, who co-reviewed with Raphaela Bento; Peter Friend, who co-reviewed with Richard Dumbill; Constantin Coussios, who co-reviewed with Tamsyn Clark; and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andrijevic, D., Spajic, A., Hameed, I. et al. Mechanisms and strategies for organ recovery. Nat Rev Bioeng 3, 596–611 (2025). https://doi.org/10.1038/s44222-025-00293-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44222-025-00293-7
This article is cited by
-
Normothermic human kidney preservation drives iron accumulation and ferroptosis
Nature Communications (2025)