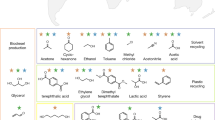
Abstract
Although research on computer-assisted planning of organic chemical syntheses dates back to the 1960s, it has only been in recent years that algorithms became powerful enough to autonomously design complete pathways, both in retrosynthetic and forward directions. Some of these modern programmes can plan chemically correct routes to complex targets and multiple such designs have now been validated by experiment. With chemical correctness secured, the next frontier for machines is to help design syntheses that are green (for example, in terms of conditions applied), sustainable (in terms of resources used) or circular (for example, in terms of waste feedstocks being revalorized). This Perspective argues that several parts of this challenge can benefit from close collaboration between synthetic chemists and bioengineers — for instance, to design better metrics of the environmental impact of syntheses or to develop algorithms with which to delineate the substrate scope of enzymatic transformations. Computers able to plan efficient and greener routes combining chemical and enzymatic steps will have a powerful and lasting impact on the production of fine chemicals.
Key points
-
Advances in reaction network theory and artificial intelligence have already enabled the development of several fully automated synthetic planners.
-
In terms of strictly chemical aspects, the best algorithms are currently on par with human experts. Moreover, they surpass humans in their ability to score syntheses against multiple criteria at once.
-
Multiparametric evaluation is important to prioritize syntheses that meet the demands of greenness and sustainability.
-
Bringing more enzymes into organic synthesis is a largely unmet need of the fine-chemical industry and yet another opportunity where chemistry, bioengineering and artificial intelligence can productively collaborate on algorithms to approximate the substrate scope of enzymes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Wang, J. Z., Lyon, W. L. & MacMillan, D. W. C. Alkene dialkylation by triple radical sorting. Nature 628, 104–109 (2024).
Murphy, M. A. Early industrial roots of green chemistry and the history of the BHC ibuprofen process invention and its quality connection. Found. Chem. 20, 121–165 (2018).
Hoyos, P., Pace, V. & Alcántara, A. R. Biocatalyzed synthesis of statins: a sustainable strategy for the preparation of valuable drugs. Catalysts 9, 260 (2019).
European Chemical Agency. Chemicals in a circular economy https://chemicalsinourlife.echa.europa.eu/chemicals-in-a-circular-economy (2020).
European Commission. A new circular economy plan for a cleaner and more competitive europe https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1583933814386&uri=COM:2020:98:FIN (2020).
Neslen, A. EU abandons promise to ban toxic chemicals in consumer products. The Guardian https://www.theguardian.com/environment/2023/oct/16/eu-abandons-promise-ban-toxic-chemicals-consumer-products (16 October 2023).
Stahel, W. R. Circular economy. Nature 531, 435–438 (2016).
Winans, K., Kendall, A. & Deng, H. The history and current applications of the circular economy concept. Renew. Sust. Ener. Rev. 68, 825–833 (2017).
Keijer, T., Bakker, V. & Slootweg, J. C. Circular chemistry to enable a circular economy. Nat. Chem. 11, 190–195 (2019).
Kümmerer, K., Clark, J. H. & Zuin, V. G. Rethinking chemistry for a circular economy. Science 367, 369–370 (2020).
Kümmerer, K. Sustainable chemistry: a future guiding principle. Angew. Chem. Int. Ed. Engl. 56, 16420–16421 (2017).
Mutlu, H. & Barner, L. Getting the terms right: green, sustainable, or circular chemistry? Macromol. Chem. Phys. 223, 2200111 (2022).
Poliakoff, M., Fitzpatrick, J. M., Farren, T. R. & Anastas, P. T. Green chemistry: science and politics of change. Science 297, 807–810 (2002).
Anastas, P. T. & Warner, J. Green Chemistry: Theory and Practice (Oxford University Press, 1998).
Anastas, P. T. in: Benign by Design. ACS Symposium Series Ch 1 (eds Anastas, P. T. & Farris, C. A.) (American Chemical Society, 1994).
Hutzinger, O. The greening of chemistry — is it sustainable? Environ. Sci. Pollut. Res. 6, 123 (1999).
Fantke, P. et al. Transition to sustainable chemistry through digitalization. Chem 7, 2866–2882 (2021).
Szymkuć, S. et al. Computer‐assisted synthetic planning: the end of the beginning. Angew. Chem. Int. Ed. Engl. 55, 5904–5937 (2016).
Mikulak-Klucznik, B. et al. Computational planning of the synthesis of complex natural products. Nature 588, 83–88 (2020).
Klucznik, T. et al. Efficient syntheses of diverse, medicinally relevant targets planned by computer and executed in the laboratory. Chem 4, 522–532 (2018).
Lin, Y., Zhang, R., Wang, D. & Cernak, T. Computer-aided key step generation in alkaloid total synthesis. Science 379, 453–457 (2023).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Levin, I. et al. Merging enzymatic and synthetic chemistry with computational synthesis planning. Nat. Commun. 13, 7747 (2022).
Sankaranarayanan, K., Klavs, F. & Jensen, K. F. Computer-assisted multistep chemoenzymatic retrosynthesis using a chemical synthesis planner. Chem. Sci. 14, 6467–6475 (2023).
Gao, W., Mercado, R. & Coley, C. W. Amortized tree generation for bottom-up synthesis planning and synthesizable molecular design. Preprint at https://doi.org/10.48550/arXiv.2110.06389 (2021).
Joung, J. F. et al. Reproducing reaction mechanisms with machine learning models trained on a large‐scale mechanistic dataset. Angew. Chem. Int. Ed. Engl. 63, e202411296 (2024).
Genheden, S. et al. AiZynthFinder: a fast, robust and flexible open-source software for retrosynthetic planning. J. Cheminformatics 12, 70 (2020).
Shields, J. D. et al. AiZynth impact on medicinal chemistry practice at AstraZeneca. RSC Med. Chem. 15, 1085–1095 (2024).
Wołos, A. et al. Synthetic connectivity, emergence, and self-regeneration in the network of prebiotic chemistry. Science 369, eaaw1955 (2020).
Wołos, A. et al. Computer-designed repurposing of chemical wastes into drugs. Nature 604, 668–676 (2022).
Żądło-Dobrowolska, A. et al. Computational synthesis design for controlled degradation and revalorization. Nat. Synth. 3, 643–654 (2024).
Klucznik, T. et al. Computational prediction of complex cationic rearrangement outcomes. Nature 625, 508–515 (2024).
Roszak, R., Gadina, L., Wołos, A. et al. Systematic, computational discovery of multicomponent and one-pot reactions. Nat. Commun. 15, 10285 (2024).
Strieth-Kalthoff, F. et al. Artificial intelligence for retrosynthetic planning needs both data and expert knowledge. J. Am. Chem. Soc. 146, 11005–11017 (2024).
Mikulak-Klucznik, B., Klucznik, T., Beker, W., Moskal, M. & Grzybowski, B. A. Catalyst: curtailing the scalable supply of fentanyl by using chemical AI. Chem 10, 1319–1326 (2024).
CAS. CAS SciFinder Discovery Platform™ https://www.cas.org/solutions/cas-scifinder-discovery-platform/cas-scifinder/synthesis-planning (accessed 2024).
Elsevier. Reaxys predictive retrosynthesis accelerates retrosynthetic analysis https://www.elsevier.com/products/reaxys/predictive-retrosynthesis (accessed 2024).
Finnigan, W. et al. RetroBioCat as a computer-aided synthesis planning tool for biocatalytic reactions and cascades. Nat. Catal. 4, 98–104 (2021).
Liu, X., Li, H. & Zhao, H. Chemoenzymatic synthesis planning by evaluating the synthetic potential in biocatalysis and chemocatalysis. Preprint at https://doi.org/10.26434/chemrxiv-2024-hnl71 (2024).
Zeng, T., Jin, Z., Zheng, S., Yu, T. & Wu, R. Developing BioNavi for hybrid retrosynthesis planning. JACS Au 4, 2492–2502 (2024).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018).
Liu, B. et al. Retrosynthetic reaction prediction using neural sequence-to-sequence models. ACS Cent. Sci. 3, 1103–1113 (2017).
Schwaller, P. et al. Predicting retrosynthetic pathways using transformer-based models and a hyper-graph exploration strategy. Chem. Sci. 11, 3316–3325 (2020).
Corey, E. J. & Wipke, W. T. Computer-assisted design of complex organic syntheses. Science 166, 178–192 (1969).
Gelernter, H. L. et al. Empirical explorations of SYNCHEM. Science 197, 1041–1049 (1977).
Hanessian, S., Franco, J. & Larouche, B. The psychobiological basis of heuristic synthesis planning — man, machine and the Chiron approach. Pure Appl. Chem. 62, 1887–1910 (1990).
Hendrickson, J. B. Systematic synthesis design. 6. Yield analysis and convergency. J. Am. Chem. Soc. 99, 5439–5450 (1977).
Corey, E. J. Robert Robinson Lecture. Retrosynthetic thinking — essentials and examples. Chem. Soc. Rev. 17, 111–133 (1988).
Corey, E. J. & Cheng, X.-M. The Logic of Chemical Synthesis (Wiley, 1989).
Grzybowski, B. A., Badowski, T., Molga, T., Molga, K. & Szymkuć, S. Network search algorithms and scoring functions for advanced-level computerized synthesis planning. Wiley Interdiscip. Rev. Comput. Mol. Sci. 13, e1630 (2023).
Badowski, T., Molga, K. & Grzybowski, B. A. Selection of cost-effective yet chemically diverse pathways from the networks of computer-generated retrosynthetic plans. Chem. Sci. 10, 4640–4651 (2019).
Kowalik, M. et al. Parallel optimization of synthetic pathways within the network of organic chemistry. Angew. Chem. Int. Ed. Engl. 51, 7928–7932 (2012).
Trost, B. M. Atom economy. A challenge for organic synthesis. Angew. Chem. Int. Ed. Engl. 34, 259–281 (1995).
Borovika, A. et al. The PMI Predictor app to enable green-by-design chemical synthesis. Nat. Sustain. 2, 1034–1040 (2019).
Andraos, J. Relationships between step and cumulative PMI and E-factors: implications on estimating material efficiency with respect to charting synthesis optimization strategies. Green Process. Synth. 8, 324–336 (2019).
Adams, J. P. et al. Development of GSK’s reagent guides — embedding sustainability into reagent selection. Green Chem. 15, 1542 (2013).
Henderson, R. K., Hill, A. P., Redman, A. M. & Sneddon, H. F. Development of GSK’s acid and base selection guides. Green Chem. 17, 945–949 (2015).
Henderson, R. K. et al. Expanding GSK’s solvent selection guide — embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 13, 854 (2011).
Gu, X. et al. Application of transition-metal catalysis, biocatalysis, and flow chemistry as state-of-the-art technologies in the synthesis of LCZ696. J. Org. Chem. 85, 6844–6853 (2020).
Cybulski, O., Quintana, C., Siek, M. & Grzybowski, B. A. Stirring‐controlled synthesis of ultrastable, fluorescent silver nanoclusters. Small 20, 2400306 (2024).
Novick, S. J. et al. Engineering an amine transaminase for the efficient production of a chiral sacubitril precursor. ACS Catal. 6, 3762–3770 (2021).
Richard, A. M. et al. The Tox21 10K compound library: collaborative chemistry advancing toxicology. Chem. Res. Toxicol. 34, 189–216 (2021).
Schmidt, M. & Pei, L. Synthetic toxicology: where engineering meets biology and toxicology. Toxicol. Sci. 120, S2024–S2224 (2011).
Huang, R. et al. Tox21 challenge to build predictive models of nuclear receptor and stress response pathways as mediated by exposure to environmental chemicals and drugs. Front. Environ. Sci. 3, 85 (2016).
Hemmerich, J. & Ecker, G. F. In Silico toxicology: from structure–activity relationships towards deep learning and adverse outcome pathways. Wiley Interdiscip. Rev. Comput. Mol. Sci. 10, e1475 (2020).
Sheldon, R. A. The E factor 25 years on: the rise of green chemistry and sustainability. Green Chem. 19, 18–43 (2017).
Guinée, J. Handbook on Life Cycle Assessment (Springer, 2002).
Aggarwal, N. et al. Microbial engineering strategies to utilize waste feedstock for sustainable bioproduction. Nat. Rev. Bioeng. 2, 155–174 (2024).
Thatcher, G. R. J. & Weldon, H. NO problem for nitroglycerin: organic nitrate chemistry and therapy. Chem. Soc. Rev. 27, 331–337 (1998).
Shimizu, S. et al. Pyridine and pyridine derivatives. Ullmanns Encycl. Indust. Chem. https://doi.org/10.1002/14356007.a22_399 (2000).
Greenlee, M. L. et al. Antifungal agents. WO Patent 2010019203A1 (2010).
Pramanik, C. et al. Commercial manufacturing of propofol: simplifying the isolation process and control on related substances. Org. Proc. Res. Dev. 18, 152–156 (2014).
Markham, A. Mobocertinib: first approval. Drugs 81, 2069–2074 (2021).
Andrews, S. W. et al. 2-Aryl- and 2-heteroaryl-substituted 2-pyridazin-3(2H)-one compounds as inhibitors of FGFR tyrosine kinases. US Patent 10766881B2 (2020).
Metcalf, B. et al. Discovery of GBT440, an orally bioavailable R-State stabilizer of sickle cell hemoglobin. ACS Med. Chem. Lett. 8, 321–326 (2017).
Gil, J. J. F. et al. Process and intermediates for the preparation of voxelotor. US Patent 20220073493A1 (2022).
Zhang, Y., Qi, M. Y., Tang, Z. R. & Xu, Y. J. Photoredox-catalyzed plastic waste conversion: nonselective degradation versus selective synthesis. ACS Catal. 13, 3575–3590 (2023).
Sobol, Ł., Dyjakon, A. & Soukup, K. Dioxins and furans in biochars, hydrochars and torreficates produced by thermochemical conversion of biomass: a review. Environ. Chem. Lett. 21, 2225–2249 (2023).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022).
Coates, G. W. & Getzler, Y. D. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Zhang, F. et al. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 370, 437–441 (2020).
Trang, B. et al. Low-temperature mineralization of perfluorocarboxylic acids. Science 377, 839–845 (2022).
He, J., Ritalahti, K. M., Yang, K. L., Koenigsberg, S. S. & Löffler, F. E. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424, 62–65 (2003).
Liang, X. et al. Highly efficient NaNO2‐catalyzed destruction of trichlorophenol using molecular oxygen. Angew. Chem. Int. Ed. Engl. 44, 5520–5523 (2005).
Kumamaru, T. et al. Enhanced degradation of polychlorinated biphenyls by directed evolution of biphenyl dioxygenase. Nat. Biotechnol. 16, 663–666 (1998).
Meunier, B. Catalytic degradation of chlorinated phenols. Science 296, 270–271 (2002).
Smith, B. M. Catalytic methods for the destruction of chemical warfare agents under ambient conditions. Chem. Soc. Rev. 37, 470–478 (2008).
Rathi, B. S. & Kumar, P. S. Sustainable approach on the biodegradation of azo dyes: a short review. Curr. Opin. Green Sustain. Chem. 33, 100578 (2022).
Antonetti, C., Licursi, D., Fulignati, S., Valentini, G. & Raspolli Galletti, A. M. New frontiers in the catalytic synthesis of levulinic acid: from sugars to raw and waste biomass as starting feedstock. Catalysts 6, 196 (2016).
Liu, F. et al. Continuously processing waste lignin into high-value carbon nanotube fibers. Nat. Commun. 13, 5755 (2022).
Sun, Z., Balint, F., de Santi, A., Saravanakumar, E. & Barta, K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 118, 614–678 (2018).
Lee, K., Jing, Y., Wang, Y. & Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem. 6, 635–652 (2022).
Zhou, X. et al. Discovery of novel inhibitors of human phosphoglycerate dehydrogenase by activity-directed combinatorial chemical synthesis strategy. Bioorg. Chem. 115, 105159 (2021).
Surivet, J. P. et al. Design, synthesis, and characterization of novel tetrahydropyran-based bacterial topoisomerase inhibitors with potent anti-gram-positive activity. J. Med. Chem. 56, 7396–7415 (2013).
Agouridas, C. et al. Synthesis and antibacterial activity of ketolides (6-O-methyl-3-oxoerythromycin derivatives): a new class of antibacterials highly potent against macrolide-resistant and -susceptible respiratory pathogens. J. Med. Chem. 41, 4080–4100 (1998).
GlaxoSmithKline Beecham P. L. C. Nitrogen-containing bicyclic heterocycles for use as antibacterials. WO Patent 2003/87098 (2003).
Sheldon, R. A. & Woodley, J. M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 118, 801–838 (2018).
Abdelraheem, E. M. M., Busch, H., Hanefeld, U. & Tonin, F. Biocatalysis explained: from pharmaceutical to bulk chemical production. React. Chem. Eng. 4, 1878–1894 (2019).
Wu, S., Snajdrova, R., Moore, J. C., Baldenius, K. & Bornscheuer, U. Biocatalysis: enzymatic synthesis for industrial applications. Angew. Chem. Int. Ed. Engl. 60, 88–119 (2020).
Fryszkowska, A. & Devine, P. N. Biocatalysis in drug discovery and development. Curr. Opin. Chem. Biol. 55, 151–160 (2020).
Savile, C. K. et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science 329, 305–309 (2010).
Huffman, M. A. et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir. Science 366, 1255–1259 (2019).
Li, J., Amatuni, A. & Renata, H. Recent advances in the chemoenzymatic synthesis of bioactive natural products. Curr. Opin. Chem. Biol. 55, 111–118 (2020).
Stout, C. N., Wasfy, N. M., Chen, F. & Renata, H. Charting the evolution of chemoenzymatic strategies in the syntheses of complex natural products. J. Am. Chem. Soc. 145, 18161–18181 (2023).
Casini, A. et al. A pressure test to make 10 molecules in 90 days: external evaluation of methods to engineer biology. J. Am. Chem. Soc. 140, 4302–4316 (2018).
Sokolova, N., Peng, B. & Haslinger, K. Design and engineering of artificial biosynthetic pathways — where do we stand and where do we go? FEBS Lett. 597, 2897–2907 (2023).
Maggiora, G., Vogt, M., Stumpfe, D. & Bajorath, J. Molecular similarity in medicinal chemistry. J. Med. Chem. 57, 3186–3204 (2013).
Stumpfe, D., Hu, H. & Bajorath, J. Advances in exploring activity cliffs. J. Comp. Aid. Mol. Des. 34, 929–942 (2020).
Martin, Y. C., Kofron, J. L. & Traphagen, L. M. Do structurally similar molecules have similar biological activity? J. Med. Chem. 45, 4350–4358 (2002).
Probst, D. et al. Biocatalysed synthesis planning using data-driven learning. Nat. Commun. 13, 964 (2022).
Sankaranarayanan, K. et al. Similarity based enzymatic retrosynthesis. Chem. Sci. 13, 6039–6053 (2022).
Delépine, B., Duigou, T., Carbonell, P. & Faulon, J.-L. RetroPath2.0: a retrosynthesis workflow for metabolic engineers. Metab. Eng. 45, 158–170 (2018).
Kim, D. I., Chae, T. U., Kim, H. U., Jang, W. D. & Lee, S. Y. Microbial production of multiple short-chain primary amines via retrobiosynthesis. Nat. Commun. 12, 173 (2021).
Cho, A., Yun, H., Park, J. H., Lee, S. Y. & Park, S. Prediction of novel synthetic pathways for the production of desired chemicals. BMC Syst. Biol. 4, 35 (2010).
Lang, M., Stelzer, M. & Schomburg, D. BKM-react, an integrated biochemical reaction database. BMC Biochem. 12, 42 (2011).
Chang, A. et al. BRENDA, the ELIXIR core data resource in 2021: new developments and updates. Nucleic Acids Res. 49, D498–D508 (2021).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Karp, P. D. et al. The BioCyc collection of microbial genomes and metabolic pathways. Brief. Bioinforma. 20, 1085–1093 (2019).
Wittig, U. et al. SABIO-RK-database for biochemical reaction kinetics. Nucleic Acids Res. 40, D790–D796 (2012).
Sun, D. et al. EnzyMine: a comprehensive database for enzyme function annotation with enzymatic reaction chemical feature. Database 2023, baaa065 (2023).
Mou, Z. et al. Machine learning-based prediction of enzyme substrate scope: application to bacterial nitrilases. Proteins Struct. Funct. Bioinf. 89, 336–347 (2021).
Yang, M. et al. Functional and informatics analysis enables glycosyltransferase activity prediction. Nat. Chem. Biol. 14, 1109–1117 (2018).
Kroll, A. et al. A general model to predict small molecule substrates of enzymes based on machine and deep learning. Nat. Commun. 14, 2787 (2023).
Beker, W., Gajewska, E. P., Badowski, T. & Grzybowski, B. A. Prediction of major regio-, site-, and diastereoisomers in Diels-Alder reactions by using machine-learning: the importance of physically meaningful descriptors. Angew. Chem. Int. Ed. Engl. 58, 4515–4519 (2019).
Moskal, M., Beker, W., Szymkuć, S. & Grzybowski, B. A. Scaffold-directed face selectivity machine-learned from vectors of non-covalent interactions. Angew. Chem. Int. Ed. Engl. 2021, 15230–15235 (2021).
Kearnes, S. M. et al. The open reaction database. J. Am. Chem. Soc. 143, 18820–18826 (2021).
Angello, N. H. et al. Closed-loop optimization of general reaction conditions for heteroaryl Suzuki-Miyaura coupling. Science 378, 399–405 (2022).
Granda, J. M., Donina, L., Dragone, V., Long, D. L. & Cronin, L. Controlling an organic synthesis robot with machine learning to search for new reactivity. Nature 559, 377–381 (2018).
Rohrbach, S. et al. Digitization and validation of a chemical synthesis literature database in the ChemPU. Science 377, 172–180 (2022).
Lin, S. et al. Mapping the dark space of chemical reactions with extended nanomole synthesis and MALDI-TOF MS. Science 361, eaar6236 (2018).
Slattery, A. et al. Automated self-optimization, intensification, and scale-up of photocatalysis in flow. Science 383, eadj1817 (2024).
Szymanski, N. J. et al. An autonomous laboratory for the accelerated synthesis of novel materials. Nature 624, 86–91 (2023).
Mahjour, B., Shen, Y. & Cernak, T. Ultrahigh-throughput experimentation for information-rich chemical synthesis. ACC Chem. Res. 54, 2337–2346 (2021).
Mayr, A. et al. Large-scale comparison of machine learning methods for drug target prediction on ChEMBL. Chem. Sci. 9, 5441–5451 (2018).
Stokes, J. M. et al. A Deep Learning approach to antibiotic discovery. Cell 180, P688–702.E13 (2020).
Krishna, R. et al. Generalized biomolecular modeling and design with RoseTTAFold all-atom. Science 384, eadl2528 (2024).
Gallou, F., Gröger, H. & Lipshutz, B. H. Status check: biocatalysis; it’s use with and without chemocatalysis. How does the fine chemicals industry view this area? Green Chem. 25, 6092–6107 (2023).
Lowe, D. Predicting new small molecule binders. Science https://www.science.org/content/blog-post/predicting-new-small-molecule-binders (2024).
Quigley, I. BELKA results suggest computers can memorize, but not create, drugs. Leash https://leashbio.substack.com/p/belka-results-suggest-computers-can (2024).
Wang, X., Quinn, D., Moody, T. S. & Huang, M. ALDELE: all-purpose deep learning toolkits for predicting the biocatalytic activities of enzymes. J. Chem. Inf. Model. 64, 3123–3139 (2024).
Robinson, S. L., Smith, M. D., Richman, J. E., Aukema, K. G. & Wackett, L. P. Machine learning-based prediction of activity and substrate specificity for OleA enzymes in the thiolase superfamily. Synth. Biol. 5, ysaa004 (2020).
Xing, H. et al. High-throughput prediction of enzyme promiscuity based on substrate–product pairs. Brief. Bioinform. 25, bbae089 (2024).
Lyu, J. et al. Ultra-large library docking for discovering new chemotypes. Nature 566, 224–229 (2019).
Fink, E. A. et al. Structure-based discovery of nonopioid analgesics acting through the α2A-adrenergic receptor. Science 377, eabn7065 (2022).
Sadybekov, A. A. et al. Synthon-based ligand discovery in virtual libraries of over 11 billion compounds. Nature 601, 452–459 (2022).
Buttenschoen, M., Morris, G. M. & Deane, C. M. PoseBusters: AI-based docking methods fail to generate physically valid poses or generalise to novel sequences. Chem. Sci. 15, 3130–3139 (2024).
Luescher, M. U. & Gallou, F. Interactions of multiple metrics and environmental indicators to assess processes, detect environmental hotspots, and guide future development. Green Chem. 26, 5239–5252 (2024).
Lica, E. et al. The need to integrate mass- and energy-based metrics with life cycle impacts for sustainable chemicals manufacture. Green Chem. 26, 9300–9309 (2024).
Luescher, M. U., Gallou, F. & Lipshutz, B. H. The impact of earth-abundant metals as a replacement for Pd in cross coupling reactions. Chem. Sci. 15, 9016–9025 (2024).
Acknowledgements
B.A.G., N.O. and E.L. were supported by the Institute for Basic Science, Korea (project code IBS-R020-D1). A.Ż.-D. acknowledges support from the National Center of Science, NCN, Poland (award SONATA 2020/39/D/ST4/01890).
Author information
Authors and Affiliations
Contributions
B.A.G. wrote the manuscript with the help of other co-authors.
Corresponding author
Ethics declarations
Competing interests
B.A.G. is a stakeholder of Allchemy, Inc.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Vânia Zeidler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Biosecure act: https://www.pharmaceutical-technology.com/analyst-comment/biosecure-act-wuxi-drugs-us-market/
Living Foundries: https://www.darpa.mil/news-events/2021-12-08
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grzybowski, B.A., Żądło-Dobrowolska, A., Onishchenko, N. et al. Sustainable production of chemicals by algorithm-assisted (bio)synthesis. Nat Rev Bioeng 3, 791–803 (2025). https://doi.org/10.1038/s44222-025-00312-7
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44222-025-00312-7