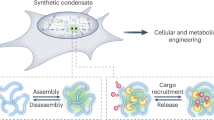
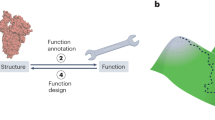
Abstract
Proteins are essential components in synthetic biology, providing multiple functions at the nanoscale. Newly developed protein optimization and design tools allow the generation of proteins with desired properties, offering new opportunities for the engineering of protein-based biological systems. In this Review, we explore how bottom-up synthetic biology, with its aim to construct synthetic cells, can use these tools to devise complex biological functions and functional systems from scratch. We provide an overview of current capabilities in protein optimization, de novo protein design and iterative system optimization, and discuss their potential in synthetic cell science with regard to standardization, the generation of missing functionality and integration. We conclude with the outline of an integrated pipeline that combines protein engineering, automated synthetic cell generation and active learning, which might allow the design of entirely new biological systems that do not rely on naturally evolved protein components.
Key points
-
Synthetic cell science aims to create minimal biological systems from individual protein-based modules; however, proteins often fail to show desired functions or show new functions when reconstituted in synthetic cells.
-
Protein optimization and design promise to resolve this problem, either by adapting natural proteins to the environment of the synthetic cell, or by designing entirely new proteins.
-
Zero-shot optimization can enhance stability and solubility of natural proteins, whereas de novo design enables the design of protein systems based on mechanistical theories from scratch.
-
To make complex synthetic cells, proteins must eventually be iteratively co-optimized with other components of the synthetic cell, such as lipids or buffers; this requires high-throughput generation and screening techniques.
-
International collaboration is necessary to establish a standardized workflow, integrating community-driven databases, replicable design methods and standardized functional screenings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ashburner, M. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000).
Baeshen, N. A. et al. Cell factories for insulin production. Microb. Cell Fact. 13, 141 (2014).
Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021).
Hutchison, C. A. et al. Design and synthesis of a minimal bacterial genome. Science 351, aad6253 (2016).
Hirschi, S., Ward, T. R., Meier, W. P., Müller, D. J. & Fotiadis, D. Synthetic biology: bottom-up assembly of molecular systems. Chem. Rev. 122, 16294–16328 (2022).
Schwille, P. Bottom-up synthetic biology: engineering in a tinkerer’s world. Science 333, 1252–1254 (2011).
Listov, D., Goverde, C. A., Correia, B. E. & Fleishman, S. J. Opportunities and challenges in design and optimization of protein function. Nat. Rev. Mol. Cell Biol. 25, 639–653 (2024).
Kortemme, T. De novo protein design—from new structures to programmable functions. Cell 187, 526–544 (2024).
Notin, P., Rollins, N., Gal, Y., Sander, C. & Marks, D. Machine learning for functional protein design. Nat. Biotechnol. 42, 216–228 (2024).
Wittmann, B. J., Johnston, K. E., Wu, Z. & Arnold, F. H. Advances in machine learning for directed evolution. Curr. Opin. Struct. Biol. 69, 11–18 (2021).
Chu, A. E., Lu, T. & Huang, P. S. Sparks of function by de novo protein design. Nat. Biotechnol. 42, 203–215 (2024).
Lu, H. et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 604, 662–667 (2022).
Jiang, K. et al. Rapid in silico directed evolution by a protein language model with EVOLVEpro. Science 387, eadr6006 (2025).
Gainza, P. et al. De novo design of protein interactions with learned surface fingerprints. Nature 617, 176–184 (2023).
Watson, J. L. et al. De novo design of protein structure and function with RFdiffusion. Nature 620, 1089–1100 (2023).
Pacesa, M. et al. BindCraft: one-shot design of functional protein binders. Preprint at bioRxiv https://doi.org/10.1101/2024.09.30.615802 (2024).
Huddy, T. F. et al. Blueprinting extendable nanomaterials with standardized protein blocks. Nature 627, 898–904 (2024).
Pillai, A. et al. De novo design of allosterically switchable protein assemblies. Nature 632, 911–920 (2024).
Huang, B. et al. Designed endocytosis-inducing proteins degrade targets and amplify signals. Nature 638, 796–804 (2025).
Edman, N. I. et al. Modulation of FGF pathway signaling and vascular differentiation using designed oligomeric assemblies. Cell 187, 3726–3740.e43 (2024).
Piraner, D. I. et al. Engineered receptors for soluble cellular communication and disease sensing. Nature 638, 805–813 (2025).
Rideau, E., Dimova, R., Schwille, P., Wurm, F. R. & Landfester, K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem. Soc. Rev. 47, 8572–8610 (2018).
Percec, V. et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science 328, 1009–1014 (2010).
Samanta, A., Baranda Pellejero, L., Masukawa, M. & Walther, A. DNA-empowered synthetic cells as minimalistic life forms. Nat. Rev. Chem. 8, 454–470 (2024).
Huang, X. et al. Interfacial assembly of protein–polymer nano-conjugates into stimulus-responsive biomimetic protocells. Nat. Commun. 4, 2239 (2013).
Dimova, R. & Marques, C. M. (eds) The Giant Vesicle Book (CRC, 2019).
Litschel, T. & Schwille, P. Protein reconstitution inside giant unilamellar vesicles. Annu. Rev. Biophys. 50, 525–548 (2021).
Czogalla, A., Franquelim, H. G. & Schwille, P. Biophysical perspective DNA nanostructures on membranes as tools for synthetic biology. Biophys J. 110, 1698–1707 (2016).
Franquelim, H. G., Khmelinskaia, A., Sobczak, J. P., Dietz, H. & Schwille, P. Membrane sculpting by curved DNA origami scaffolds. Nat. Commun. 9, 811 (2018).
Tran, M. P. et al. Genetic encoding and expression of RNA origami cytoskeletons in synthetic cells. Nat. Nanotechnol. https://doi.org/10.1038/s41565-025-01879-3 (2025).
Yagüe Relimpio, A. et al. Bottom-up assembled synthetic SARS-CoV-2 miniviruses reveal lipid membrane affinity of omicron variant spike glycoprotein. ACS Nano 17, 23913–23923 (2023).
Chen, Z. et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 14, 86–93 (2018).
Schwille, P. et al. MaxSynBio: avenues towards creating cells from the bottom up. Angew. Chem. Int. Ed. 57, 13382–13392 (2018).
Ramirez-Diaz, D. A. et al. FtsZ induces membrane deformations via torsional stress upon GTP hydrolysis. Nat. Commun. 12, 3310 (2021).
Ramirez-Diaz, D. A. et al. Treadmilling analysis reveals new insights into dynamic FtsZ ring architecture. PLoS Biol. 16, e2004845 (2018).
Pontani, L. L. et al. Reconstitution of an actin cortex inside a liposome. Biophys. J. 96, 192–198 (2009).
Miyazaki, M., Chiba, M., Eguchi, H., Ohki, T. & Ishiwata, S. Cell-sized spherical confinement induces the spontaneous formation of contractile actomyosin rings in vitro. Nat. Cell Biol. 17, 480–489 (2015).
Sciortino, A. et al. Active membrane deformations of a minimal synthetic cell. Nat. Phys. https://doi.org/10.1038/s41567-025-02839-3 (2025).
Hovijitra, N. T., Wuu, J. J., Peaker, B. & Swartz, J. R. Cell-free synthesis of functional aquaporin Z in synthetic liposomes. Biotechnol. Bioeng. 104, 40–49 (2009).
Fujii, S., Matsuura, T., Sunami, T., Kazuta, Y. & Yomo, T. In vitro evolution of α-hemolysin using a liposome display. Proc. Natl Acad. Sci. USA 110, 16796–16801 (2013).
Dannhauser, P. N. & Ungewickell, E. J. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat. Cell Biol. 14, 634–639 (2012).
Takei, K. et al. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94, 131–141 (1998).
Kohyama, S., Yoshinaga, N., Yanagisawa, M., Fujiwara, K. & Doi, N. Cell-sized confinement controls generation and stability of a protein wave for spatiotemporal regulation in cells. eLife 8, e44591 (2019).
Litschel, T., Ramm, B., Maas, R., Heymann, M. & Schwille, P. Beating vesicles: encapsulated protein oscillations cause dynamic membrane deformations. Angew. Chem. Int. Ed. 57, 16286–16290 (2018).
Kohyama, S., Frohn, B. P., Babl, L. & Schwille, P. Machine learning-aided design and screening of an emergent protein function in synthetic cells. Nat. Commun. 15, 2010 (2024).
Lussier, F., Staufer, O., Platzman, I. & Spatz, J. P. Can bottom-up synthetic biology generate advanced drug-delivery systems? Trends Biotechnol. 39, 445–459 (2021).
Boyd, M. A. & Kamat, N. P. Designing artificial cells towards a new generation of biosensors. Trends Biotechnol. 39, 927–939 (2021).
Mukwaya, V., Mann, S. & Dou, H. Chemical communication at the synthetic cell/living cell interface. Commun. Chem. 4, 161 (2021).
Sela, M. et al. Brain-targeted liposomes loaded with monoclonal antibodies reduce alpha-synuclein aggregation and improve behavioral symptoms in Parkinson’s disease. Adv. Mater. 35, e2304654 (2023).
Toparlak, Ö. D. et al. Artificial cells drive neural differentiation. Sci. Adv. 6, eabb4920 (2020).
Lee, K. Y. et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat. Biotechnol. 36, 530–535 (2018).
Adamala, K. P. et al. Present and future of synthetic cell development. Nat. Rev. Mol. Cell Biol. 25, 162–167 (2023).
Schwille, P. & Frohn, B. P. Hidden protein functions and what they may teach us synthesizing from the bottom-up. Trends Cell Biol. 32, 102–109 (2022).
Gierasch, L. M. & Gershenson, A. Post-reductionist protein science, or putting Humpty Dumpty back together again. Nat. Chem. Biol. 5, 774 (2009).
Fu, M. et al. Mechanochemical feedback loop drives persistent motion of liposomes. Nat. Phys. 19, 1211–1218 (2023).
Ramm, B. et al. A diffusiophoretic mechanism for ATP-driven transport without motor proteins. Nat. Phys. 17, 850–858 (2021).
Reverte-López, M. et al. Self-organized spatial targeting of contractile actomyosin rings for synthetic cell division. Nat. Commun. 15, 10415 (2024).
Sternke, M., Tripp, K. W. & Barrick, D. Consensus sequence design as a general strategy to create hyperstable, biologically active proteins. Proc. Natl Acad. Sci. USA 116, 11275–11284 (2019).
Rives, A. et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc. Natl Acad. Sci. USA 118, e2016239118 (2021).
Elnaggar, A. et al. ProtTrans: towards cracking the language of life through self-supervised learning. IEEE Trans. Pattern Anal. Mach. Intell. 44, 7112–7127 (2022).
Heinzinger, M. et al. Bilingual language model for protein sequence and structure. Preprint at bioRxiv https://doi.org/10.1101/2023.07.23.550085 (2024).
Hayes, T. et al. Simulating 500 million years of evolution with a language model. Science 387, 850–858 (2025).
Zhang, Z. et al. Protein language models learn evolutionary statistics of interacting sequence motifs. Proc. Natl Acad. Sci. USA 121, e2406285121 (2024).
Shroff, R. et al. Discovery of novel gain-of-function mutations guided by structure-based deep learning. ACS Synth. Biol. 9, 2927–2935 (2020).
Hie, B. L. et al. nature biotechnology efficient evolution of human antibodies from general protein language models. Nat. Biotechnol. 42, 275–283 (2024).
Dauparas, J. et al. Robust deep learning–based protein sequence design using ProteinMPNN. Science 378, 49–56 (2022).
Sumida, K. H. et al. Improving protein expression, stability, and function with ProteinMPNN. J. Am. Chem. Soc. 146, 2054–2061 (2024).
Garenne, D. et al. Cell-free gene expression. Nat. Rev. Methods Primers 1, 49 (2021).
Kai, L. & Schwille, P. Cell-free protein synthesis and its perspectives for assembling cells from the bottom-up. Adv. Biosyst. 3, e1800322 (2019).
Hettiaratchi, M. H. et al. Reengineering biocatalysts: computational redesign of chondroitinase ABC improves efficacy and stability. Sci. Adv. 6, eabc6378 (2020).
Benegas, G., Ye, C., Albors, C., Li, J. C. & Song, Y. S. Genomic language models: opportunities and challenges. Preprint at https://doi.org/10.48550/arXiv.2407.11435 (2024).
Nguyen, E. et al. Sequence modeling and design from molecular to genome scale with Evo. Science 386, eado9336 (2024).
Brixi, G. et al. Genome modeling and design across all domains of life with Evo 2. Preprint at bioRxiv https://doi.org/10.1101/2025.02.18.638918 (2025).
Hwang, Y., Cornman, A. L., Kellogg, E. H., Ovchinnikov, S. & Girguis, P. R. Genomic language model predicts protein co-regulation and function. Nat. Commun. 15, 2880 (2024).
Goverde, C. A. et al. Computational design of soluble and functional membrane protein analogues. Nature 631, 449–458 (2024).
Shimizu, Y. et al. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 19, 751–755 (2001).
Koblan, L. W. et al. Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat. Biotechnol. 36, 843–846 (2018).
Zakas, P. M. et al. Enhancing the pharmaceutical properties of protein drugs by ancestral sequence reconstruction. Nat. Biotechnol. 35, 35–37 (2017).
Khersonsky, O. et al. Automated design of efficient and functionally diverse enzyme repertoires. Mol. Cell 72, 178–186.e5 (2018).
Funk, J. et al. ProteusAI: an open-source and user-friendly platform for machine learning-guided protein design and engineering. Preprint at bioRxiv https://doi.org/10.1101/2024.10.01.616114 (2024).
Cabré, E. J. et al. Bacterial division proteins FtsZ and ZipA induce vesicle shrinkage and cell membrane invagination. J. Biol. Chem. 288, 26625–26634 (2013).
Fan, S. et al. Morphology remodelling and membrane channel formation in synthetic cells via reconfigurable DNA nanorafts. Nat. Mater. 24, 278–286 (2025).
Woolfson, D. N. A brief history of de novo protein design: minimal, rational, and computational. J. Mol. Biol. 433, 167160 (2021).
Korendovych, I. V. & DeGrado, W. F. De novo protein design, a retrospective. Q. Rev. Biophys. 53, e3 (2020).
Ovchinnikov, S. & Huang, P. S. Structure-based protein design with deep learning. Curr. Opin. Chem. Biol. 65, 136–144 (2021).
Ferruz, N. et al. From sequence to function through structure: deep learning for protein design. Comput. Struct. Biotechnol. J. 21, 238–250 (2023).
Mallik, B. B., Stanislaw, J., Alawathurage, T. M. & Khmelinskaia, A. De novo design of polyhedral protein assemblies: before and after the AI revolution. ChemBioChem 24, e202300117 (2023).
Ingraham, J. B. et al. Illuminating protein space with a programmable generative model. Nature 623, 1070–1078 (2023).
Krishna, R. et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 384, eadl2528 (2024).
Harteveld, Z. et al. Exploring “dark matter” protein folds using deep learning. Cell Syst. 15, 898–910 (2024).
Frank, C. et al. Scalable protein design using optimization in a relaxed sequence space. Science 386, 439–445 (2024).
Dauparas, J. et al. Atomic context-conditioned protein sequence design using LigandMPNN. Preprint at bioRxiv https://doi.org/10.1101/2023.12.22.573103 (2023).
Akpinaroglu, D., Seki, K., Zhu, E. & Kortemme, T. Frame2seq: structure-conditioned masked language modeling for protein sequence design. In Machine Learning for Structural Biology Workshop (NeurIPS, 2023).
Bennett, N. R. et al. Improving de novo protein binder design with deep learning. Nat. Commun. 14, 2625 (2023).
Johnson, S. R. et al. Computational scoring and experimental evaluation of enzymes generated by neural networks. Nat. Biotechnol. 43, 396–405 (2025).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Lisanza, S. L. et al. Multistate and functional protein design using RoseTTAFold sequence space diffusion. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02395-w (2024).
Chu, A. E. et al. An all-atom protein generative model. Proc. Natl Acad. Sci. USA 121, e2311500121 (2024).
Wang, J. et al. Scaffolding protein functional sites using deep learning. Science 377, 387–394 (2022).
Yeh, A. H. W. et al. De novo design of luciferases using deep learning. Nature 614, 774–780 (2023).
Gainza, P. et al. Deciphering interaction fingerprints from protein molecular surfaces using geometric deep learning. Nat. Methods 17, 184–192 (2020).
Marchand, A. et al. Targeting protein–ligand neosurfaces with a generalizable deep learning tool. Nature 639, 522–531 (2025).
Wicky, B. I. M. et al. Hallucinating symmetric protein assemblies. Science 378, 56–61 (2022).
Praetorius, F. et al. Design of stimulus-responsive two-state hinge proteins. Science 381, 754 (2023).
Eguchi, R. R. et al. Deep generative design of epitope-specific binding proteins by latent conformation optimization. Preprint at bioRxiv https://doi.org/10.1101/2022.12.22.521698 (2022).
Bennett, N. R. et al. Atomically accurate de novo design of antibodies with RFdiffusion. Preprint at bioRxiv https://doi.org/10.1101/2024.03.14.585103 (2025).
Schweke, H. et al. An atlas of protein homo-oligomerization across domains of life. Cell 187, 999–1010.e15 (2024).
Iacovache, I., Bischofberger, M. & van der Goot, F. G. Structure and assembly of pore-forming proteins. Curr. Opin. Struct. Biol. 20, 241–246 (2010).
Huber, F. et al. Emergent complexity of the cytoskeleton: from single filaments to tissue. Adv. Phys. 62, 1–112 (2013).
Kirchhausen, T. Bending membranes. Nat. Cell Biol. 14, 906–908 (2012).
Xu, C. et al. Computational design of transmembrane pores. Nature 585, 129–134 (2020).
Berhanu, S. et al. Sculpting conducting nanopore size and shape through de novo protein design. Science 385, 282–288 (2024).
Joh, N. H. et al. De novo design of a transmembrane Zn2+-transporting four-helix bundle. Science 346, 1520 (2014).
Mahendran, K. R. et al. A monodisperse transmembrane α-helical peptide barrel. Nat. Chem. 9, 411–419 (2017).
Lu, P. et al. Accurate computational design of multipass transmembrane proteins. Science 359, 1042–1046 (2018).
Votteler, J. et al. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature 540, 292–295 (2016).
Svitkina, T. The actin cytoskeleton and actin-based motility. CSH Perspect. Biol. 10, a018267 (2018).
Robert-Paganin, J., Pylypenko, O., Kikuti, C., Sweeney, H. L. & Houdusse, A. Force generation by myosin motors: a structural perspective. Chem. Rev. 120, 5–35 (2020).
Thomas, C. & Tampé, R. Structural and mechanistic principles of ABC transporters. Annu. Rev. Biochem. 89, 605–636 (2020).
Rudden, L. S. P., Hijazi, M. & Barth, P. Deep learning approaches for conformational flexibility and switching properties in protein design. Front. Mol. Biosci. 9, 928534 (2022).
Harrington, L., Fletcher, J. M., Heermann, T., Woolfson, D. N. & Schwille, P. De novo design of a reversible phosphorylation-dependent switch for membrane targeting. Nat. Commun. 12, 1472 (2021).
Courbet, A. et al. Computational design of mechanically coupled axle-rotor protein assemblies. Science 376, 383–390 (2022).
Ng, A. H. et al. Modular and tunable biological feedback control using a de novo protein switch. Nature 572, 265–269 (2019).
Langan, R. A. et al. De novo design of bioactive protein switches. Nature 572, 205–210 (2019).
Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. Science 369, 1637–1643 (2020).
Shui, S. et al. A rational blueprint for the design of chemically-controlled protein switches. Nat. Commun. 12, 5754 (2021).
Glasgow, A. A. et al. Computational design of a modular protein sense-response system. Science 366, 1024–1028 (2019).
Gantz, M., Neun, S., Medcalf, E. J., van Vliet, L. D. & Hollfelder, F. Ultrahigh-throughput enzyme engineering and discovery in in vitro compartments. Chem. Rev. 123, 5571–5611 (2023).
Utada, A. S. et al. Monodisperse double emulsions generated from a microcapillary device. Science 308, 537–541 (2005).
Lamborg, M. R. & Zamecnik, P. C. Amino acid incorporation into protein by extracts of E. coli. Biochim. Biophys. Acta 42, 206–211 (1960).
Abil, Z. et al. Darwinian evolution of self-replicating DNA in a synthetic protocell. Nat. Commun. 15, 9091 (2024).
Okauchi, H. & Ichihashi, N. Continuous cell-free replication and evolution of artificial genomic DNA in a compartmentalized gene expression system. ACS Synth. Biol. 10, 3507–3517 (2021).
van Nies, P. et al. Self-replication of DNA by its encoded proteins in liposome-based synthetic cells. Nat. Commun. 9, 1583 (2018).
Holstein, J. M., Gylstorff, C. & Hollfelder, F. Cell-free directed evolution of a protease in microdroplets at ultrahigh throughput. ACS Synth. Biol. 10, 252–257 (2021).
Fallah-Araghi, A., Baret, J. C., Ryckelynck, M. & Griffiths, A. D. A completely in vitro ultrahigh-throughput droplet-based microfluidic screening system for protein engineering and directed evolution. Lab Chip 12, 882–891 (2012).
Mazutis, L. et al. Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis. Anal. Chem. 81, 4813–4821 (2009).
Packer, M. S. & Liu, D. R. Methods for the directed evolution of proteins. Nat. Rev. Genet. 16, 379–394 (2015).
Romero, P. A. & Arnold, F. H. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 10, 866–876 (2009).
Yang, G. & Withers, S. G. Ultrahigh-throughput FACS-based screening for directed enzyme evolution. ChemBioChem 10, 2704–2715 (2009).
Goto, H. et al. Microfluidic screening system based on boron-doped diamond electrodes and dielectrophoretic sorting for directed evolution of NAD(P)-dependent oxidoreductases. Lab Chip 20, 852–861 (2020).
Rees, P., Summers, H. D., Filby, A., Carpenter, A. E. & Doan, M. Imaging flow cytometry. Nat. Rev. Methods Primers 2, 86 (2022).
Godino, E., Restrepo Sierra, A. M. & Danelon, C. Imaging flow cytometry for high-throughput phenotyping of synthetic cells. ACS Synth. Biol. 12, 2015–2028 (2023).
Yang, K. K., Wu, Z. & Arnold, F. H. Machine-learning-guided directed evolution for protein engineering. Nat. Methods 16, 687–694 (2019).
Biswas, S., Khimulya, G., Alley, E. C., Esvelt, K. M. & Church, G. M. Low-N protein engineering with data-efficient deep learning. Nat. Methods 18, 389–396 (2021).
Pandi, A. et al. A versatile active learning workflow for optimization of genetic and metabolic networks. Nat. Commun. 13, 3876 (2022).
Boiko, D. A., MacKnight, R., Kline, B. & Gomes, G. Autonomous chemical research with large language models. Nature 624, 570–578 (2023).
Abolhasani, M. & Kumacheva, E. The rise of self-driving labs in chemical and materials sciences. Nat. Synth. 2, 483–492 (2023).
Szymanski, N. J. et al. An autonomous laboratory for the accelerated synthesis of novel materials. Nature 624, 86–91 (2023).
Rapp, J. T., Bremer, B. J. & Romero, P. A. Self-driving laboratories to autonomously navigate the protein fitness landscape. Nat. Chem. Eng. 1, 97–107 (2024).
Munsamy, G. et al. Conditional language models enable the efficient design of proficient enzymes. Preprint at bioRxiv https://doi.org/10.1101/2024.05.03.592223 (2024).
Braun, M. et al. Computational design of highly active de novo enzymes. Preprint at bioRxiv https://doi.org/10.1101/2024.08.02.606416 (2024).
Kim, D. et al. Computational design of metallohydrolases. Preprint at bioRxiv https://doi.org/10.1101/2024.11.13.623507 (2024).
Lauko, A. et al. Computational design of serine hydrolases. Science 388, eadu2454 (2025).
Ferruz, N., Schmidt, S. & Höcker, B. ProtGPT2 is a deep unsupervised language model for protein design. Nat. Commun. 13, 4348 (2022).
Madani, A. et al. Large language models generate functional protein sequences across diverse families. Nat. Biotechnol. 41, 1099–1106 (2023).
Smolke, C. D. Building outside of the box: iGEM and the BioBricks foundation. Nat. Biotechnol. 27, 1099–1102 (2009).
Kryshtafovych, A., Schwede, T., Topf, M., Fidelis, K. & Moult, J. Critical assessment of methods of protein structure prediction (CASP)—Round XV. Proteins 91, 1539–1549 (2023).
Castelvecchi, D. Drivers gear up for world’s first nanocar race. Nature 544, 278–279 (2017).
Fu, M., Li, X. & Zhao, W. The Asian synthetic cell initiative: highlights from the first SynCell Asia workshop. Natl Sci. Rev. 12, nwae377 (2024).
Otim, G. et al. SynBio Africa’s story from the grassroots, the present, and the future. Biotechnol. Notes 4, 1–6 (2023).
Noireaux, V. & Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc. Natl Acad. Sci. USA 101, 17669–17674 (2004).
Shi, X., Wu, T., Cole, C. M., Devaraj, N. K. & Joseph, S. Optimization of ClpXP activity and protein synthesis in an E. coli extract-based cell-free expression system. Sci. Rep. 8, 3488 (2018).
Pols, T. et al. A synthetic metabolic network for physicochemical homeostasis. Nat. Commun. 10, 4239 (2019).
Blanken, D., Foschepoth, D., Serrão, A. C. & Danelon, C. Genetically controlled membrane synthesis in liposomes. Nat. Commun. 11, 4317 (2020).
Eto, S. et al. Phospholipid synthesis inside phospholipid membrane vesicles. Commun. Biol. 5, 1016 (2022).
Kita, H. et al. Replication of genetic information with self-encoded replicase in liposomes. ChemBioChem 9, 2403–2410 (2008).
Zhao, J. & Han, X. Investigation of artificial cells containing the Par system for bacterial plasmid segregation and inheritance mimicry. Nat. Commun. 15, 4956 (2024).
Osawa, M., Anderson, D. E. & Erickson, H. P. Reconstitution of contractile FtsZ rings in liposomes. Science 320, 792–794 (2008).
Kohyama, S., Merino-Salomón, A. & Schwille, P. In vitro assembly, positioning and contraction of a division ring in minimal cells. Nat. Commun. 13, 6098 (2022).
De Franceschi, N., Barth, R., Meindlhumer, S., Fragasso, A. & Dekker, C. Dynamin A as a one-component division machinery for synthetic cells. Nat. Nanotechnol. 19, 70–76 (2024).
Ichihashi, N. et al. Darwinian evolution in a translation-coupled RNA replication system within a cell-like compartment. Nat. Commun. 4, 2494 (2013).
Adamala, K. P., Martin-Alarcon, D. A., Guthrie-Honea, K. R. & Boyden, E. S. Engineering genetic circuit interactions within and between synthetic minimal cells. Nat. Chem. 9, 431–439 (2016).
Buddingh, B. C., Elzinga, J. & van Hest, J. C. M. Intercellular communication between artificial cells by allosteric amplification of a molecular signal. Nat. Commun. 11, 1652 (2020).
Hindley, J. W. et al. Building a synthetic mechanosensitive signaling pathway in compartmentalized artificial cells. Proc. Natl Acad. Sci. USA 116, 16711–16716 (2019).
Shin, J. & Noireaux, V. E. coli cell-free expression toolbox: application to synthetic gene circuits and artificial cells. ACS Synth. Biol. 1, 29–41 (2012).
Nishimura, K. et al. Cell-free protein synthesis inside giant unilamellar vesicles analyzed by flow cytometry. Langmuir 28, 8426–8432 (2012).
Hüttemann, M. et al. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: from respiration to apoptosis. Mitochondrion 11, 369–381 (2011).
Callaway, E. Who will make AlphaFold3 open source? Scientists race to crack AI model. Nature 630, 14–15 (2024).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Acknowledgements
The authors thank L. Milles for helpful discussion and critical reading, V. Belousova and M. Reverte-López for input on functional protein modules, and K. Al Nahas for discussion on high-throughput synthetic cell generation and screening.
Author information
Authors and Affiliations
Contributions
B.P.F. conceptualized the manuscript, P.S. and B.P.F wrote the manuscript, and all authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Xin Zhou, who co-reviewed with Zhixing Ma, and the other, anonymous, reviewers(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BindCraft: https://github.com/martinpacesa/BindCraft
Build-A-Cell collaboration: https://www.buildacell.org/
ColabFold: https://github.com/sokrypton/ColabFold
ESM2: https://github.com/facebookresearch/esm
ESM3: https://github.com/evolutionaryscale/esm
iGEM competition: https://competition.igem.org/
List of papers about proteins design using deep learning: https://github.com/Peldom/papers_for_protein_design_using_DL
METIS: https://github.com/amirpandi/METIS
Nucleus: https://nucleus.bnext.bio/
ProteinMPNN: https://github.com/dauparas/ProteinMPNN
RFdiffusion: https://github.com/RosettaCommons/RFdiffusion
SynCellEU network: https://syntheticcell.eu/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frohn, B.P., Kohyama, S. & Schwille, P. Protein design and optimization for synthetic cells. Nat Rev Bioeng 3, 645–659 (2025). https://doi.org/10.1038/s44222-025-00318-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44222-025-00318-1