Abstract
Cytomegalovirus (CMV) infection is a common complication in newborns with severe combined immunodeficiency (SCID). Prolonged antiviral treatment in immunocompromised patients increases the risk of the emergence of drug resistance. We analyzed drug resistance in a newborn with SCID who developed neonatal CMV infection. Sequencing of viral DNA polymerase (DP; UL54), protein kinase (UL97), and terminase (UL51, UL56, UL89) genes identified ganciclovir (GCV) and foscarnet (PFA) resistance mutations in blood, but not cerebrospinal fluid. After treatment was shifted to cidofovir and letermovir (LMV), a LMV resistance mutation rapidly emerged in UL56 (C325F). Eventually, a multidrug-resistant genotype was established (DP-V781I and UL56-C325F). Whole-genome sequencing of CMV in clinical blood samples showed an otherwise stable genotype. This case describes a CMV infection complicated by compartmentalization and the emergence of resistance to GCV, PFA, and LMV. It highlights the need for further investigation into alternative antiviral strategies for the prevention and treatment of CMV.
Similar content being viewed by others
Introduction
Cytomegalovirus (CMV) infection can cause severe morbidity or mortality in infants with severe combined immunodeficiency (SCID)1,2. After maternal immunity wanes, infants with SCID are left vulnerable due to their impaired adaptive immunity, putting them at risk of severe disseminated CMV infection presenting, for example, as pneumonia, hepatitis, retinitis, neutropenia, thrombocytopenia, or central nervous system infection1.
SCID is a group of rare genetic disorders leading to a primary immunodeficiency (PID) that is characterized by the absence of functional T lymphocytes (T-), with possible disruption of B lymphocyte and/or natural killer (NK) cell function3. Currently, over 20 genetic defects are known to cause SCID and the incidence is estimated between 1:40,000 and 1:100,000 live births4,5,6,7. If left untreated, SCID is typically fatal in the first two years of life as a result of opportunistic infections stemming from the lack of a functional immune system. T-cell disorders are often characterized by pneumonia with Pneumocystis jirovecii or CMV, mycobacterial infection, recurrent skin candidiasis, or diarrhea.
The main curative treatment option for SCID is hematopoietic stem cell transplantation (HSCT)8. Hygiene measures are essential to prevent infection before HSCT, as active infections at the time of transplantation lower the chances of successful immune reconstitution and patient survival8,9. In newborns with SCID, CMV transmission through breast milk from CMV-seropositive mothers is a concern which can be addressed by advising mothers to withhold breastfeeding, although further research is needed to clearly define the risks10,11,12.
Antivirals against CMV include ganciclovir (GCV), its prodrug valganciclovir (VGCV), cidofovir (CDV), and foscarnet (PFA), all targeting the viral DNA polymerase (DP). Nucleoside or nucleotide analogs (GCV and CDV, respectively) inhibit DP through competitive inhibition resulting in chain termination during DNA replication, while PFA is a pyrophosphate analog that directly inhibits the DP through binding of the pyrophosphate binding site, disrupting chain elongation. Maribavir (MBV) competitively inhibits the viral protein kinase (PK), preventing its phosphorylating functions and thereby preventing DNA replication, encapsidation, and nuclear egress. Letermovir (LMV) disturbs viral DNA processing and packaging through binding of the UL56 subunit of the viral terminase complex. Prolonged antiviral treatment is often required for immunocompromised individuals, but this approach may facilitate the emergence of drug resistance when viral replication is incompletely suppressed. A 1998 study observed an earlier emergence of drug resistance in CMV infections in pediatric patients with primary combined immunodeficiencies than in other CMV-infected populations13. The authors stressed the need for resistance surveillance and prompt therapeutic response. A strategy for the prevention of antiviral drug resistance, well-established in the human immunodeficiency virus (HIV) field, is combination therapy. The combined administration of multiple antivirals with different mechanisms of action and/or different viral targets increases the barrier to resistance.
Traditionally, resistance screening consists of Sanger sequencing of genes where resistance mutations are known to appear: DP gene UL54 (GCV, CDV, PFA resistance), protein kinase (PK) gene UL97 (GCV, MBV resistance), UL27 (MBV resistance, in vitro), and terminase subunit genes UL56, UL89, UL51 (LMV resistance)14. Recent advances in next-generation sequencing (NGS) techniques have driven the consideration of NGS in resistance screening15. The increased sensitivity of NGS over Sanger sequencing allows for the detection of minor genetic variants, offering significant clinical benefits. A validated, standardized approach is required for the systematic implementation of NGS in resistance screening.
In this study, we performed a longitudinal analysis of a CMV infection in a pediatric SCID patient treated at the Children’s Hospital (HUDERF) of the Brussels University Hospital and followed under our translational research platform RegaVir (www.regavir.org). Longitudinal sampling enabled the analysis of viral responses to treatment and the emergence of resistance. We prospectively studied antiviral resistance through Sanger sequencing to guide clinical decisions. Retrospective targeted NGS and whole-genome sequencing enabled the further analysis of viral adaptation in response to antiviral treatment.
Results
Case presentation and viral genotyping
The patient was born to a CMV-seropositive mother at 39 weeks gestation. The patient’s parents were 2nd degree consanguineous, and her family history included several unexplained miscarriages and two siblings who died at a young age (4.5 and 9 months) from infectious complications. The patient was admitted to the hospital 56 days after birth for thrush with ulcerated oral lesions and pyrexia. Immuno-hematological examinations were strongly suggestive of a constitutional immunodeficiency. T-B-NK+ SCID diagnosis was confirmed when the patient was found to be homozygous for a mutation in the gene encoding the RAG2 protein. P. jirovecii prophylaxis with trimethoprim-sulfamethoxazole was started.
CMV infection was first detected in urine, likely transmitted through breast milk as CMV PCR from the Guthrie test was negative. The child was weaned from breast milk and GCV treatment was administered from day 71 until day 83 after birth (5 mg/kg 2×/day; Fig. 1). The doses and timing of antiviral drug administration are described in detail in Supplementary Table 1. No intrafamilial human leukocyte antigen (HLA) matched donor was available for HSCT and the severity of the condition did not allow for the delay related to identifying an HLA-matched donor in the registry. Instead, a haploidentical peripheral blood stem cell transplantation (PBSCT) without prior conditioning was performed on day 83 after birth, with the patient’s mother as donor. The donor was stimulated with granulocyte colony-stimulating factor (G-CSF) three days prior to PBSCT, and the graft was T-cell depleted by selection of CD34+ cells.
With a CMV viral load of 6 log copies/mL, antiviral treatment was shifted from GCV to PFA (dose varied from 87 mg/kg 2×/day to 97 mg/kg 2×/day) on day 84 to prevent graft failure due to GCV-related hematological toxicity during the engraftment period. Blood CMV viral load had decreased to 5 log at 94 days after birth and the patient showed no clinical symptoms of infection. CMV was first detected in the cerebrospinal fluid (CSF) on day 111 after birth (3 log), indicating CMV meningitis. Three blood samples (B-94, B-108, B-111) and two CSF samples (CSF-111, CSF-117) were prospectively analyzed for genotypical antiviral resistance, showing no known resistance mutations in the UL54 (DP) and UL97 (PK) genes (Table 1).
Refractory CMV infection warranted combination therapy of GCV (4.5 mg/kg 2×/day and later 5.1 mg/kg 2×/day) and PFA (82 mg/kg 2×/day and later 89 mg/kg 2×/day) from day 117 after birth. Two doses of G-CSF were administered for GCV-related neutropenia and GCV was discontinued after one month due to leuko-neutropenia. PFA treatment was continued at a dose of 90 mg/kg 2×/day. The patient received a donor lymphocyte infusion (DLI) and later received CMV-specific cytotoxic T lymphocytes twice. Nevertheless, CMV viral load in blood gradually increased to 6.88 log on day 178 after birth, with febrile recurrences. CSF viral load remained relatively stable. GCV treatment was reinstated on day 167 at a reduced dose (2.8 mg/kg 2×/day), while awaiting resistance genotyping.
Sample B-164 carried two known resistance mutations in the DP gene: V715M (29.56%; PFA resistance) and A809V (36.01%; GCV and PFA resistance) (Table 1). GCV and PFA were discontinued and replaced with a combination therapy of CDV (5 mg/kg 1×/week; day 173) and LMV (70 mg; day 174). The following blood samples (B-178, B-185, B-188, B-192) showed the persistence of the previously identified mutations DP-V715M, DP-A809V, and the emergence of DP-V781I, known to confer GCV and PFA resistance, as mixed populations. A CSF sample (CSF-182) taken in this period showed no emergence of known resistance mutations, indicative of compartmentalized CMV infection.
Genotyping of samples B-206 and B-209 showed the emergence of the pUL56-C325F (94.50% and 99.10%, respectively), known to confer resistance to LMV. The DP gene now only carried the V781I mutation (91.27% and 98.49%, respectively), as the virus established a stable genotype. Retrospectively, pUL56-C325F was detected in the previous sample B-192 at a frequency of 6.48% due to the increased sensitivity of targeted NGS. At the time, this knowledge would have allowed an earlier evaluation of clinical decisions. Blood viral load increased again to 6.49 log on day 213 after birth, when the parents were informed of graft failure and antiviral escape and palliative supportive care was started before the patient was discharged. Sample B-234 showed the persistent presence of DP-V781I (97.21%) and UL56-C325F (96.91%). The patient died 251 days after birth.
Polymorphisms were shared between CMV detected in blood and in CSF: UL54 carried S655L, F669L, N685S, L897S, and N898D and UL56 carried V425A, M442T, T452I, S454N, A476V, N586D, and V778A.
Whole-genome sequencing
Whole-genome sequencing using the target enrichment technique based on RNA probes was performed for all blood samples except B-185, B-188, B-206 (insufficient quantity). The remaining eight samples were sequenced with successful target enrichment and high sequencing coverage (Table 2). Throughout the infection, consensus sequences showed no longitudinal variation over the entire genome except for the emergence of the previously described drug resistance mutations. Manual evaluation of minor variants (5–50% frequency) showed no longitudinal variation in known hypervariable genes [RL5A, UL1, UL9, UL11, UL120, UL123, UL139, UL146, UL148D] and genes encoding glycoproteins [UL55 (glycoprotein B; gB), UL73 (gN), UL74 (gO), UL75 (gH), UL100 (gM), UL115 (gL)]16,17.
Discussion
The longitudinal follow-up of a severely immunocompromised newborn with SCID who developed neonatal CMV infection in the present study enabled the investigation of infection dynamics and development of drug resistance throughout the course of infection. This case highlights the challenge of managing CMV infections and the complexity of antiviral treatment in the pediatric SCID population. Pediatric patients with the primary immune deficiency SCID are extremely susceptible to infection. In this case, the newborn contracted CMV through breastfeeding before HSCT. The active CMV infection at the time of HSCT possibly affected the success of immune reconstitution8,9. Antiviral treatment was repeatedly adapted due to toxicity, resulting in an increasingly complex treatment regimen (Fig. 1).
Combination therapies of GCV + PFA and LMV + CDV were employed to address refractory/resistant CMV infection. The clinical application of combination therapy in the treatment of CMV has been described sporadically18,19. The 10th European Conference on Infections in Leukaemia (ECIL) supported the consideration of combination therapy for second or third line pre-emptive therapy in the pediatric population, as in the ECIL 7 guidelines20. A publication describing the ECIL 10 recommendations is anticipated. Combination therapy is most effective when different viral proteins are targeted. While the range of treatment options has expanded with the recent approval of novel anti-CMV drugs targeting viral proteins other than the DP, their safety and efficacy in the pediatric population require further investigation21,22,23. In HSV-1, the combination of GCV and trifluridine, and of GCV or PFA with pritelivir, inhibitors of the viral helicase-primase complex, prevented the emergence of drug resistance mutations in vitro24,25. Further research is required to validate this principle in the context of CMV.
In this case, resistance mutations emerged in the genes encoding the viral DP and pUL56. DP-V715M confers PFA resistance and DP-A809V confers PFA- and low-level GCV resistance, but both mutations cause viral growth attenuation26,27,28. In contrast, DP-V781I confers PFA and GCV resistance with no effect on viral fitness29. The lack of fitness cost might explain the preferential selection of DP-V781I in this infection. Based on the similar pattern in variant frequencies, the pUL56-C325F substitution appeared to have been selected in the virus carrying the DP-V781I, after which positive selection allowed these mutations to reach fixation in the population as the major viral variant (Table 1).
LMV is currently approved by the European Medicines Agency (EMA) for CMV prophylaxis in adult CMV-seropositive recipients of allogeneic HSCT. Its use as salvage therapy in either adults or the pediatric population is therefore off-label30,31. Resistance screening using Sanger sequencing identified the LMV resistance mutation pUL56-C325F in a sample taken 32 days after treatment onset in this patient. Retrospective NGS could already detect this mutation in a sample taken 18 days after treatment onset at 6.48% frequency, illustrating the benefit of the increased sensitivity of NGS. The implementation of NGS in routine resistance screening would require validated thresholds for variant prevalence and a more comprehensive understanding of the clinical relevance of low-frequency variants32,33,34. Until standardized NGS protocols with clearly defined thresholds for clinically relevant low-frequency variants are established, the detection of minor variants (<10%) in clinical samples should not warrant treatment adaptation, but encourage a closer monitoring of potential drug resistance. Further research is critical to understanding the impact of minor viral subpopulations on drug susceptibility and clinical outcomes.
LMV is known to have a low genetic barrier to resistance, which is further confirmed by the rapid onset of resistance in this case35. Addressing the prevention of drug resistance, Alain and colleagues discuss the possible benefit of therapeutic drug monitoring to verify LMV concentrations in blood, as well as the possible role of standing variation in CMV breakthrough viremia during LMV treatment36. LMV does not inhibit viral DNA replication, but prevents the packaging of viral DNA and the maturation of infectious virions37. Especially in highly immunocompromised SCID patients, this mechanism of action might allow for low-level viral replication where a mutation could silently emerge, allowing rapid expansion under antiviral pressure.
As the case described here shows, pediatric patients with SCID who develop a severe CMV infection and receive long-term, often complex regimens of antiviral therapy are at a high risk for the development of drug resistance. Resistance screening is crucial to prevent severe outcomes in this vulnerable population, and should be performed when antiviral treatment fails to achieve significant viral load reduction after two weeks. Repeated screening is necessary for a prompt clinical response to emerging resistance, as viral drug susceptibility can be impacted by a rapidly changing composition of the viral population.
Most samples were whole blood, while four of the fifteen samples were CSF. In contrast to the blood samples, no known CMV antiviral resistance mutations were detected in the CSF. The same genetic polymorphisms were carried in both blood and CSF, indicating that the viral populations originated from the same strain. Compartmentalization has previously been described in CMV infection38,39,40. Differences in antiviral drug distribution between body compartments could explain the divergent viral adaptation. The blood-brain barrier (BBB) limits the penetration of certain antivirals, such as CDV and LMV, into the central nervous system41. However, herpesvirus infections have been shown to affect the integrity of the BBB and increase permeability, possibly affecting drug distribution42,43. The most plausible hypothesis for the compartmentalization between the viral populations in CSF and blood is the existence of a common viral origin, followed by independent viral adaptation in each compartment. Alternatively, suboptimal antiviral concentrations may have promoted the emergence of minor drug-resistant viral subpopulations in the CSF, which then redistributed into the systemic circulation, where positive selection may have driven the expansion of the drug-resistant viral population in the blood. Unfortunately, our data cannot confirm either hypothesis, as we were unable to analyze the low-frequency viral variants in the CSF using NGS due to low viral load.
Although recent studies have investigated the pharmacokinetics and pharmacodynamics of LMV in the pediatric population, many aspects, including the drug distribution within the body, remain poorly understood23,31,44,45,46. A phase 2b study (NCT03940586) investigating the pharmacokinetics, safety and efficacy of LMV in adolescent HSCT recipients showed that the administration of adult LMV doses (480 mg, 240 mg if co-administered with cyclosporin A) to adolescent HSCT recipients resulted in comparable exposures as in the adult population, with a similar efficacy and safety profile47. A phase 3 trial studying LMV prophylaxis after HSCT in pediatric patients is currently ongoing (NCT05711667).
Whole-genome sequencing of blood samples demonstrated the stability of the viral genome. No intrapatient variation was seen apart from the mutations conferring drug resistance, considering both whole-genome consensus sequences and minor variants (5–50% frequency) in highly variable genes and genes encoding glycoproteins. Unfortunately, a comparison with the CSF viral population could not be conducted due to an insufficient quantity of DNA to perform whole-genome sequencing.
CMV infection poses a major concern in immunocompromised newborns. There is an urgent need for further research into pharmacokinetics and -dynamics of existing antivirals, as well as alternative treatment options such as combination therapy and novel antivirals in the pediatric population. This case illustrates the often underestimated, but at times severe burden of CMV infections in the immunocompromised host and emphasizes the importance of awareness, screening, and the need for effective and safe antiviral treatment strategies.
Methods
Ethics
This study was approved by the local Ethics Committee of KU Leuven (S61201).
Prospective resistance analysis: Sanger sequencing
After DNA was extracted from clinical blood and cerebrospinal fluid (CSF) samples (QIAamp DNA Blood Mini kit, Qiagen), Sanger sequencing was performed of the DP (UL54), PK (UL97), and viral terminase complex (UL51, UL56, UL89) genes, following previously established protocols (ABI3730 sequencer, Thermo Fisher Scientific)48. Sequencing reads were mapped to the reference strain Merlin (NCBI GenBank accession NC_006273.2).
Retrospective variant detection: Illumina sequencing
For samples where resistance mutations were discovered during prospective resistance analysis, targeted, amplicon-based NGS of the UL54 DP and UL56 terminase subunit genes was performed retrospectively on the MiSeq v2 DNA sequencer (Illumina), as described previously48. CLC Genomics Workbench (v21.0.5, Qiagen) was used to map reads to the Merlin reference and identify variants using the Low Frequency Variant Detection tool.
Whole-genome sequencing and data analysis
Whole-genome sequencing was performed as outlined in a previous study, using the SureSelect XT HS library preparation kit with the SureSelect XT CD CMV RNA probe library for target enrichment (Agilent)49,50. Multiplexed samples were prepared for sequencing on a MiSeq v2 DNA sequencer (MiSeq Reagent Kit v2, 500 cycles, Illumina).
CLC Genomics Workbench was used to trim and map reads to the Merlin reference strain. Trimming parameters set the quality limit at 0.05 and short (<15 bp) and long (>1000 bp) reads were discarded. Target enrichment was also verified by mapping reads to the human reference genome (GRCh38/hg38). The Low Frequency Variant Detection tool identified variants with a minimum coverage of 15 reads.
Data availability
Targeted Illumina sequencing reads have been deposited in the NCBI Sequence Read Archive (SRA) under BioProject number PRJNA1193001. Whole-genome sequences of CMV samples are available at NCBI GenBank (accession numbers PQ851837- PQ851844).
References
Bateman, C. M., Kesson, A., Powys, M., Wong, M. & Blyth, E. Cytomegalovirus infections in children with primary and secondary immune deficiencies. Viruses 13, 2001 (2021).
Vora, S. B. & Englund, J. A. Cytomegalovirus in immunocompromised children. Curr. Opin. Infect. Dis. 28, 323–329 (2015).
Justiz-Vaillant, A. A., Gopaul, D., Akpaka, P. E., Soodeen, S. & Arozarena Fundora, R. Severe combined immunodeficiency-classification, microbiology association and treatment. Microorganisms 11, 1589 (2023).
Chinn, I. K. & Shearer, W. T. Severe combined immunodeficiency disorders. Immunol. Allergy Clin. North Am. 35, 671–694 (2015).
Chien, Y. H. et al. Newborn screening for severe combined immunodeficiency in Taiwan. Int J. Neonatal Screen. 3, 16 (2017).
Argudo-Ramírez, A. et al. First universal newborn screening program for severe combined immunodeficiency in Europe. Two-years’ experience in Catalonia (Spain). Front Immunol. 10, 2406 (2019).
Kumrah, R. et al. Genetics of severe combined immunodeficiency. Genes Dis. 7, 52–61 (2020).
Pai, S. Y. et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N. Engl. J. Med. 371, 434–446 (2014).
Heimall, J. et al. Immune reconstitution and survival of 100 SCID patients post–hematopoietic cell transplant: a PIDTC natural history study. Blood 130, 2718–2727 (2017).
Dorsey, M. J., Dvorak, C. C., Cowan, M. J. & Puck, J. M. Treatment of infants identified as having severe combined immunodeficiency by means of newborn screening. J. Allergy Clin. Immunol. 139, 733–742 (2017).
Kelty, W. J. et al. The role of breast-feeding in cytomegalovirus transmission and hematopoietic stem cell transplant outcomes in infants with severe combined immunodeficiency. J. Allergy Clin. Immunol. Pract. 7, 2863–2865.e3 (2019).
Walter, J. E. & Heimall, J. CMV-seropositive mothers of SCID: to breastfeed or not?. J. Allergy Clin. Immunol. Pract. 7, 2866–2867 (2019).
Wolf, D. G. et al. Early emergence of ganciclovir-resistant human cytomegalovirus strains in children with primary combined immunodeficiency. J. Infect. Dis. 178, 535–538 (1998).
Chou, S. Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance. Antivir. Res. 176, 104711 (2020).
Mostafa, H. H. Next-generation sequencing for cytomegalovirus genotypic antiviral resistance testing. J. Clin. Microbiol. 61, e01302-23 (2023).
Renzette, N., Bhattacharjee, B., Jensen, J. D., Gibson, L. & Kowalik, T. F. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog. 7, e1001344 (2011).
Suárez, N. M. et al. Human cytomegalovirus genomes sequenced directly from clinical material: variation, multiple-strain infection, recombination, and gene loss. J. Infect. Dis. 220, 781–791 (2019).
Mylonakis, E., Kallas, W. M. & Fishman, J. A. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin. Infect. Dis. 34, 1337–1341 (2002).
Vora, S. B., Brothers, A. W., Waghmare, A. & Englund, J. A. Antiviral combination therapy for cytomegalovirus infection in high-risk infants. Antivir. Ther. 23, 505–511 (2018).
Ljungman, P. et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect. Dis. 19, e260–e272 (2019).
Pfeiffer, T. et al. Letermovir as cytomegalovirus prophylaxis in children undergoing allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 59, 1193–1195 (2024).
Doshi, S., Dassner, A. & Hanisch, B. Maribavir use in pediatric immunocompromised hosts: A case series to talk about real-world experience. J. Pediatr. Infect. Dis. Soc. 13, 26–27 (2024).
Pai, V. B., Tansmore, J. & Song, E. Pharmacokinetic analysis driven letermovir dosing in pediatric hematopoietic cell transplantation patients with resistant CMV disease. Pediatr. Transplant. 28, e14580 (2024).
Schalkwijk, H. H., Shewakramani, N. R., Das, K., Andrei, G. & Snoeck, R. Combination of ganciclovir and trifluridine prevents drug-resistance emergence in HSV-1. Antimicrob. Agents Chemother. 15, e0011024 (2024).
Schalkwijk, H. H., Andrei, G. & Snoeck, R. Combined use of pritelivir with acyclovir or foscarnet suppresses evolution of HSV-1 drug resistance. Virus Evol. 10, veae101 (2024).
Baldanti, F. et al. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J. Virol. 70, 1390–1395 (1996).
Chou, S. et al. Mutation in region III of the DNA polymerase gene conferring foscarnet resistance in cytomegalovirus isolates from 3 subjects receiving prolonged antiviral therapy. J. Infect. Dis. 178, 526–530 (1998).
Lurain, N. S. & Chou, S. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 23, 689–712 (2010).
Mousavi-Jazi, M. et al. Variations in the cytomegalovirus DNA polymerase and phosphotransferase genes in relation to foscarnet and ganciclovir sensitivity. J. Clin. Virol. 23, 1–15 (2001).
Kilgore, J. T. et al. Use of letermovir for salvage therapy for resistant cytomegalovirus in a pediatric hematopoietic stem cell transplant recipient. J. Pediatr. Infect. Dis. Soc. 9, 486–489 (2020).
Kuhn, A. et al. Letermovir as cytomegalovirus prophylaxis in a pediatric cohort: a retrospective analysis. Transpl. Cell Ther. 29, 62.e1–62.e4 (2023).
Koboldt, D. C. Best practices for variant calling in clinical sequencing. Genome Med. 12, 91 (2020).
Streck, N. T. et al. Use of next-generation sequencing to detect mutations associated with antiviral drug resistance in cytomegalovirus. J. Clin. Microbiol. 61, e0042923 (2023).
Mallory, M. A. et al. Development and validation of a next-generation sequencing assay with open-access analysis software for detecting resistance-associated mutations. J. Clin. Microbiol. 61, e00829–23 (2023).
Hofmann, E. et al. Emergence of letermovir resistance in solid organ transplant recipients with ganciclovir resistant cytomegalovirus infection: a case series and review of the literature. Transpl. Infect. Dis. J. Transpl. Soc. 23, e13515 (2021).
Alain, S. et al. Letermovir breakthroughs during the French Named Patient Programme: interest of monitoring blood concentration in clinical practice. J. Antimicrob. Chemother. 75, 2253–2257 (2020).
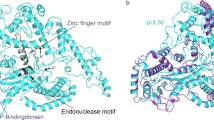
Ligat, G., Cazal, R., Hantz, S. & Alain, S. The human cytomegalovirus terminase complex as an antiviral target: a close-up view. FEMS Microbiol Rev. 42, 137–145 (2018).
Miller, G. G. et al. Cytomegalovirus Ventriculoencephalitis in a Peripheral Blood Stem Cell Transplant Recipient. Clin. Infect. Dis. 42, e26–e29 (2006).
Frange, P. et al. Temporal and Spatial Compartmentalization of Drug-Resistant Cytomegalovirus (CMV) in a Child with CMV Meningoencephalitis: Implications for Sampling in Molecular Diagnosis. J. Clin. Microbiol. 51, 4266–4269 (2013).
Piret, J. et al. Compartmentalization of a multidrug-resistant cytomegalovirus UL54 mutant in a stem cell transplant recipient with encephalitis. J. Infect. Dis. 220, 1302–1306 (2019).
Pohlmann, C. et al. Cidofovir and foscarnet for treatment of human herpesvirus 6 encephalitis in a neutropenic stem cell transplant recipient. Clin. Infect. Dis. 44, e118–e120 (2007).
Liu, H., Qiu, K., He, Q., Lei, Q. & Lu, W. Mechanisms of blood-brain barrier disruption in herpes simplex encephalitis. J. Neuroimmune Pharmacol. 14, 157–172 (2019).
Harrison, M. A. A. et al. Intermittent cytomegalovirus infection alters neurobiological metabolism and induces cognitive deficits in mice. Brain Behav. Immun. 117, 36–50 (2024).
César, T. et al. Letermovir for CMV prophylaxis in very high-risk pediatric hematopoietic stem cell transplantation recipients for inborn errors of immunity. J. Clin. Immunol. 44, 6 (2024).
Chen, T. T., David, A. P., Barthelmess, E. K. & MacBrayne, C. E. Letermovir for Cytomegalovirus prophylaxis in pediatric hematopoietic stem cell transplantation. Pediatr. Blood Cancer 70, e30608 (2023).
Wang, Q. et al. Real-world safety and effectiveness of letermovir in pediatric patients undergoing allogenic hematopoietic stem cell transplantation. Blood 144, 7297–7297 (2024).
Groll, A. H. et al. Pharmacokinetics, safety, and efficacy of letermovir for cytomegalovirus prophylaxis in adolescent hematopoietic cell transplantation recipients. Pediatr. Infect. Dis. J. 43, 203–208 (2024).
Andrei, G. et al. Persistent primary cytomegalovirus infection in a kidney transplant recipient: Multi-drug resistant and compartmentalized infection leading to graft loss. Antivir. Res. 168, 203–209 (2019).
Williams, R. J. et al. Utilization of agilent sureselect target enrichment for whole genome sequencing of viruses and bacteria. Available from: http://www.agilent.com/cs/library/applications/infectious_APP5994-0909EN.pdf (2019).
Horsten, F. et al. Dynamics and evolution of donor-derived cytomegalovirus infection in 3 solid organ transplant recipients with the same multiorgan donor. Transplantation 109, 890–899 (2025).
Acknowledgements
We would like to thank Dr. Nadira Azzi for excellent patient care and Brecht Dirix, Wim Werckx, Hien Do, and Robbe Sinnesael for excellent technical assistance. This work was presented as a poster at the 26th annual conference of the European Society for Clinical Virology (ESCV) in September, 2024 in Frankfurt, Germany (abstract PP-118). This work was supported by Sciensano, the Belgian Institute for Health, whose funding supports the translational research platform RegaVir.
Author information
Authors and Affiliations
Contributions
Conceptualization, F.H., R.S. and G.A.; Methodology, F.H., S.G., R.S. and G.A.; Validation, F.H., S.G.; Formal analysis, F.H., S.G. and G.A.; Investigation, F.H. and S.G.; Resources, P.C. and P. Mazilier; Data Curation, F.H.; Writing – Original Draft Preparation, F.H.; Writing – Review & Editing, F.H., S.G., P.C., P. Mazilier, P. Maes, R.S. and G.A.; Visualization, F.H.; Supervision, P. Maes, R.S. and G.A.; Project Administration, R.S. and G.A.; Funding Acquisition, R.S. and G.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Horsten, F., Gillemot, S., Calò, P. et al. Cytomegalovirus infection and drug resistance emergence during letermovir salvage therapy in a pediatric SCID patient. npj Antimicrob Resist 3, 43 (2025). https://doi.org/10.1038/s44259-025-00118-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s44259-025-00118-y