Abstract
Background
PARP inhibitors are effective in treating ovarian cancer, especially for BRCA1/2 pathogenic variant carriers and those with HRD (homologous recombination deficiency). Concerns over toxicity and costs have led to the search for predictive biomarkers. We present an updated systematic review, expanding on a previous ESMO review on PARP inhibitor biomarkers.
Methods
Following ESMO’s 2020 review protocol, we extended our search to March 31, 2023, including PubMed and clinical trial data. We also reviewed the reference lists of review articles. We conducted a meta-analysis using a random-effects model to evaluate hazard ratios and assess the predictive potential of biomarkers and the effectiveness of PARP inhibitors in survival.
Results
We found 375 articles, 103 of which were included after screening (62 primary research, 41 reviews). HRD remained the primary biomarker (95%), particularly BRCA1/2 variants (77%). In the non-HRD category, six articles (10%) introduced innovative biomarkers, including ADP-ribosylation, HOXA9 promoter methylation, patient-derived organoids, KELIM, and SLFN11.
Discussion
Prospective assessment of real-time homologous recombination repair via nuclear RAD51 levels shows promise but needs validation. Emerging biomarkers like ADP-ribosylation, HOXA9 promoter methylation, patient-derived organoids, KELIM, and SLFN11 offer potential but require large-scale validation.
Similar content being viewed by others
Introduction
Ovarian cancer ranks as the fifth leading cause of cancer-related deaths among women in developed countries [1, 2], with epithelial ovarian cancer (EOC) accounting for 90% of cases [3]. Despite advances in therapy and surgery, EOC exhibits a high recurrence rate (up to 75% in advanced stages) [4]. Since 2011, survival was only marginally improved by the addition of bevacizumab to standard care [5, 6]. Over the past two decades, the overall 5-year survival rate for this condition has shown minimal improvement, with increases reported from around 31% to 34% in some regions [7].
Significant progress has been made with the advent of poly(ADP-ribose) polymerase (PARP) inhibitors, particularly in managing platinum-sensitive recurrent EOC [8, 9]. When used as maintenance therapy for patients responding to salvage chemotherapy, these inhibitors extend progression-free survival (PFS) by at least 5 months [10]. Notably, in the Phase 3 SOLO2 trial, a remarkable 19.1-month PFS benefit was reported for patients with BRCA1/2 pathogenic variants versus placebo (p < 0.0001) [9]. Other Phase 3 trials, such as NOVA and ARIEL3, also demonstrated similar PARP inhibitor benefits in platinum-sensitive recurrent EOC with BRCA1/2 pathogenic variants [8, 10]. Additionally, PARP inhibitors have shown efficacy as maintenance therapy in newly diagnosed, advanced-stage EOC patients [11, 12]. The efficacy of PARP inhibitors had also been affirmed in other cancer types, such as prostate cancer and breast cancer, particularly for genetically defined subgroups. In prostate cancer, the PROfound trial showed olaparib significantly improves outcomes in metastatic castration-resistant prostate cancer (mCRPC) patients with BRCA1/2 and ATM pathogenic variants compared to hormone therapy [13], while TRITON3 highlighted rucaparib’s effectiveness in delaying disease progression for those with BRCA1/2 pathogenic variants [14]. In breast cancer, OlympiAD demonstrated olaparib’s benefit in prolonging PFS for HER2-negative, BRCA1/2-mutated metastatic patients [15], and EMBRACA confirmed similar efficacy for talazoparib [16]. These findings underscore the value of PARP inhibitors in targeted treatment across cancer types.
The role of predictive biomarkers has been crucial in this context. The Phase 2 trial, Study 19, demonstrated the efficacy of olaparib for platinum-sensitive recurrent EOC, particularly in patients with BRCA1/2 pathogenic variants, who experienced a significantly longer PFS [17]. ARIEL3 introduced tumour homologous recombination deficiency (HRD) as an additional predictive marker for PARP inhibitors, with significant PFS benefits for both BRCA1/2 pathogenic variant carriers and patients with tumour HRD [10]. Other Phase 3 trials, including PAOLA-1, PRIMA, and VELIA, also demonstrated the benefits of PARP inhibitors in newly diagnosed advanced EOC patients with tumour HRD [12, 18]. BRCA1/2 pathogenic variants and HRD are candidate predictive markers for PARP inhibitor effectiveness due to their roles in the homologous recombination repair (HRR) pathway. HRD cells employ non-homologous end joining (NHEJ) for DNA repair, resulting in less precise repair and increased DNA insertions and deletions [19].
Microhomology-mediated end joining (MMEJ) is another error-prone mechanism for repairing DNA double-strand breaks. This process involves aligning microhomologous sequences at the break ends and is often associated with deletions adjacent to the original break. DNA polymerase θ (Polθ) is crucial for MMEJ. In cells with HRD, these imperfect repair mechanisms further compromise genomic stability. This vulnerability makes PARP inhibitors particularly effective, as they disrupt single-strand DNA repair even more. This disruption results in the accumulation of DNA damage, leading to synthetic lethality and cell death [20].
The selection of EOC patients for PARP inhibitor treatment is increasingly based on the presence of BRCA1/2 pathogenic variants and HRD status, with about 20% of EOC cases associated with BRCA1/2 pathogenic variants [21]. One study examined tumour samples from over four thousand EOC patients to determine the frequency of tumour HRD and showed that 41.4% of patients across all histologic types exhibited tumour HRD. Among specific histologic types, serous carcinoma had the highest proportion of patients with tumour HRD at 42.4%. The prevalence of tumour HRD was 37.6% in endometrioid carcinoma, 13.6% in clear cell carcinoma, and 8.1% in mucinous carcinoma [22]. However, the clinical application of PARP inhibitors is not without challenges, including adverse events and concerns regarding cost-effectiveness. Patients treated with PARP inhibitors often experience a range of adverse events. Common non-haematologic side effects include nausea, fatigue, vomiting, diarrhoea, constipation, abdominal pain, and headache. Haematologic adverse events frequently observed include anaemia, neutropenia, and thrombocytopenia. Notably, approximately half of the patients may develop significant adverse events, which can impact the tolerability and management of treatment [8, 18, 23,24,25,26,27,28]. Moreover, the high cost of PARP inhibitors raises concerns regarding cost-effectiveness. Biomarkers such as germline/tumour BRCA1/2 pathogenic variants or tumour HRD provide some patient stratification, potentially improving economic efficiency and patient safety [29, 30].
Establishing a universally accepted method or biomarker for patient stratification to maximise PARP inhibitor benefits is challenging. Approaches have focused on HRR genes like BRCA1/2, genomic instability markers such as genomic scars or mutational signatures, and functional assays such as RAD51 [31]. In view of the rapid advances in biomarkers and emerging non-HRD predictors of PARP inhibitor efficacy [32, 33] an updated systematic review is required.
Materials and methods
This systematic review adheres to the PRISMA guidelines and follows the study protocol of the original ESMO systematic review [31]. The updated protocol for this systemic review was registered in PROSPERO with the study ID: CRD42023449959. For this updated review, we used the same search terms like the ESMO systematic review with updated search dates until March 31, 2023. We conducted a comprehensive search on PubMed and the clinical trial database (https://clinicaltrials.gov/), identifying 430 articles initially. After removing duplicates using Endnote (version X7.8), 375 articles remained. The search terms used for finding relevant articles are detailed in the Appendix Table 1. Figure 1 illustrates the PRISMA workflow.
We imported the 375 articles into Rayyan, a systematic review article screening platform. Two independent reviewers screened articles based on titles and abstracts using predefined inclusion and exclusion criteria. We included primary research articles or review articles which addressed ovarian cancer, assays or biomarkers, and PARP inhibitors. We excluded Phase 1 trials, studies unrelated to biomarkers (e.g., focused on the quality of life, pharmacokinetics, etc), and studies involving non-human subjects.
After screening, reviewer assessments were compared, and major discrepancies were resolved with input from a third reviewer. Minor discrepancies were resolved through discussion and full-text review. After resolving all discrepancies, we comprehensively reviewed the included articles. We assessed the level of evidence (LOE) by considering the study design and biomarker methodology [34]. We evaluated the quality of genomics-based predictive biomarkers using the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) guidelines established by the US Centers for Disease Control and Prevention. This framework evaluates genomic assay’s analytic and clinical validity and utility [35]. Key information of articles included in this systematic review is presented in Appendix Table 2.
Our meta-analysis focused on Phase 2 and Phase 3 trials that provided hazard ratios (HR) of PFS for PARP inhibitors in ovarian cancer. This approach ensured consistency and comparability across studies. We observed that most biomarkers identified in the literature predict the survival benefit of PARP inhibitors. However, a subset of studies, specifically Phase 2 single-arm trials on newly diagnosed or recurrent EOC [36, 37], were excluded from the meta-analyses due to their lack of HR data on survival and divergent study designs, such as heterogeneity in patient enrolment or small sample sizes. The meta-analysis was conducted using the “metagen” function in RStudio. We anticipated the true effect sizes to vary between studies because of the heterogeneity in intervention effects and methodological approaches; therefore, the random-effects model was used to pool the effect sizes of the included studies. The DerSimonian-Laird estimator was utilised to assess between-study variance, and the I-squared was calculated to estimate the between-study heterogeneity. Detailed information on studies included in the meta-analyses can be found in the Appendix Table 3.
Previous reviews often presented germline and somatic BRCA1/2 pathogenic variants as separate entities. However, due to their similar predictive capabilities and the widespread use of tumour BRCA1/2 assays in clinical practice [12, 18, 38], there has been a noticeable shift towards amalgamating the results [8, 12, 18]. In prostate cancer, both germline and somatic BRCA1/2 pathogenic variants similarly predict a favourable response to PARP inhibitors, as shown in pivotal trials like PROfound (olaparib) [13] and TRITON3 (rucaparib) [14]. In breast cancer, while germline and somatic BRCA1/2 pathogenic variants also suggest potential sensitivity to PARP inhibitors, germline pathogenic variants are generally considered stronger predictors of efficacy. Major trials, such as OlympiAD [15] and EMBRACA [16], have primarily focused on patients with germline pathogenic variants. Although somatic BRCA1/2 pathogenic variants are less common in breast cancer, they are potentially predictive but not as strongly linked to PARP inhibitor efficacy as germline pathogenic variants. Reflecting on this evolving approach, our review has been structured to provide a comprehensive assessment of the predictive capabilities of germline and somatic BRCA1/2 pathogenic variants together.
Results
The systematic review included 62 primary research articles, comprising 50 articles selected through screening in Rayyan and an additional 12 articles identified by screening the references of review articles. The predictive biomarkers proposed by these studies are summarised in Table 1.
HRR-related gene-level test
Of 62 articles in this review, 55 (89%) used assays to assess genes related to HRR as a primary method for detecting patients with HRD. The biomarkers suggested in the context of HRR-related genes can be grouped into three main categories: (1) germline or somatic BRCA1/2 pathogenic variants, (2) pathogenic variants of HRR-related genes other than BRCA1/2, and (3) HRR-related gene promoter methylation. The majority of the studies were focused on detecting germline or somatic BRCA1/2 pathogenic variants.
More than a hundred genes contribute to HRR, either through direct involvement or indirect mechanisms [39]. Central to the HRR pathway are the BRCA1 and BRCA2 proteins, which are critical because of their complex interactions with components like PALB2 and CHEK2 [40]. Additionally, significant roles are played by PALB2, ATM, ATR, CHEK1, CHEK2, RAD51, and genes linked to Fanconi anaemia [40]. BRCA1 assists in the initial processing of DNA double-strand breaks and interacts with PALB2. The combined action of BRCA1 and PALB2 aids BRCA2 in identifying processed DNA overhangs at the site of DNA double-strand break [40]. Once BRCA2 identifies the location of the single-strand DNA overhangs, it promotes the assembly of RAD51 recombinase, ensuring the progression of the subsequent HRR process [40].
Germline or somatic BRCA1/2 gene pathogenic variants
The primary and most widely employed predictive biomarker for assessing the effectiveness of PARP inhibitors is using assays to detect germline or somatic BRCA1/2 pathogenic variants. Of the 62 studies reviewed, 77% utilised this particular assay as their predictive biomarker of choice. It is worth noting that these assays were predominantly commercial, with the Myriad MyChoice® CDx kit being the most prevalent at 46%, followed closely by Myriad BRACAnalysis® CDx at 40%, FoundationOne® CDx at 17%, and the BROCA Cancer Risk Panel at 6% of the studies that looked at BRCA1/2.
A key point of interest is that around 20% of EOC cases are associated with BRCA1/2 pathogenic variants [21]. In the 2020 ESMO review, the results of major randomised clinical trials were summarised, clearly indicating that patients with BRCA1/2 pathogenic variants derive the greatest benefits from PARP inhibitors [31]. In this updated review, additional Phase 2/3 studies published after 2020 have been included. These additional data solidify the status of BRCA1/2 pathogenic variants as an established predictive biomarker for assessing the efficacy of PARP inhibitors. The key findings of studies utilising BRCA1/2 pathogenic variants to predict PARP inhibitor efficacy are illustrated in Appendix Table 4A.
Pathogenic variants of HRR-related genes other than BRCA1/2
Of 55 primary research articles focusing on HRD predictability through HRR-related gene-level tests, 15 (27%) examined pathogenic variants in HRR-related genes other than BRCA1/2 (Table 1). There is increasing interest in HRR genes beyond BRCA1/2, exemplified by studies involving hMOB2 [41], CCDC6 [42] and EMSY [43]. This was highlighted in the 2020 ESMO review, which underscored their varying impacts on synthetic lethality with PARP inhibitors [31]. Subsequent research has supported this concept. In ovarian cancer, pathogenic variants in BRCA1, BRCA2, RAD51C, RAD51D, and PALB2 predict PARP inhibitor efficacy, but this has not been established for other HRR-related genes [44, 45].
Regarding the predictability of individual HRR-related genes and PARP inhibitor efficacy, the results of the ARIEL3 Phase 3 trial indicated that RAD51C/D pathogenic variants were predictive of the efficacy of rucaparib in platinum-sensitive recurrent EOC patients [45]. Other studies suggest associations between the expression of EMSY, hMOB2, CCDC6-PP4, and PARP inhibitor efficacy based on retrospective and in vitro findings that require further validation [41, 43]. On the other hand, some studies have explored HRD predictability using different HRR-related gene panels, but none have effectively predicted PARP inhibitor efficacy for EOC patients [46, 47]. These gene panels, designed to identify mutations within a select group of genes, do not adequately capture the multifaceted nature of HRD. Key findings from studies that investigated HRR-related genes other than BRCA1/2 to predict the efficacy of PARP inhibitors are summarised in Appendix Table 4B.
When interpreting assay results detecting variants in BRCA1/2 or other HRR-related genes, the impact on the HRR pathway is linked to their pathogenicity. Benign or likely benign variants are typically associated with a proficient HRR pathway, while likely pathogenic or pathogenic variants are linked to HRD. For variants classified as variants of unknown significance (VUS), the impact on the HRR pathway remains uncertain. While VUS rates specifically for BRCA1/2 genes have decreased due to advances in classification and data collection, the overall VUS rate tends to increase with larger gene panels. This is because expanded panels include more genes, many of which are less well-characterised, leading to a higher likelihood of identifying VUS [48]. Kurian et al. investigated trends in genetic testing among women with breast or ovarian cancer and found that the VUS rate increased from 8.1% in 2013 to 28.3% in 2017 with the use of broader HRR-related gene panels [49].
HRR-related gene promoter methylation
Genetic alterations and epigenetic changes, such as DNA methylation, can influence HRR capacity by modulating gene regulatory elements. DNA methylation can either silence (hypermethylation) or activate (hypomethylation) genes [50]. The ESMO review emphasised the need for stronger evidence to validate HRR-related gene promoter methylation as a predictor of PARP inhibitor efficacy due to doubts about earlier research reliability [31]. In this updated review of 55 articles using gene-level HRD assays, only 7 (13%) addressed HRR-related gene promoter methylation.
While BRCA1 promoter methylation is associated with HRD and is present in a subset of high-grade serous ovarian cancer (HGSOC) cases, findings from the TCGA study [21], indicate that BRCA1 promoter methylation was not highlighted as an independent prognostic marker for patient outcomes. This aligns with the observation that while BRCA1 promoter methylation contributes to the molecular characterisation of HGSOC, its role as a prognostic indicator remains uncertain.
Recent evidence has highlighted that an additional 10% of EOC patients exhibit “BRCAness”, which refers to tumours that share molecular features with tumours possessing pathogenic variants of BRCA1/2, such as those identified through HRR-related gene promoter methylation, beyond what genetic testing alone can reveal [51]. Further analysis of the Phase 2 ARIEL2 trial showed that BRCA1 promoter hypermethylation is associated with improved PFS in recurrent EOC patients with wild-type BRCA1/2 (p = 0.01) [52]. Some studies suggest extended overall survival and heightened sensitivity to platinum and PARP inhibitors among patients with BRCA1/2 promoter methylation [53]. These findings are retrospective and preclinical and thus require validation in larger prospective studies [53,54,55]. In summary, even with new studies exploring HRR-related gene promoter methylation as a predictive biomarker for PARP inhibitor efficacy, its predictability remains inconclusive. The key findings of reviewed studies utilising HRR-related gene promoter methylation to predict the efficacy of PARP inhibitors are summarised in Appendix Table 4C.
Genomic scars and mutational signatures
Genomic scars
Genomic scars, or mutational signatures resulting from HRD, provide essential markers for HRD detection. These signatures encompass various DNA copy number alterations, including Loss of Heterozygosity (LOH), Telomeric Allelic Imbalance (TAI), and Large-Scale State Transitions (LST).
LOH, a genomic phenotype, often emerges following HRD [56]. Abkevich et al. classified LOH into small, intermediate, or large categories, observing a significantly higher prevalence of intermediate LOH regions in tumours with BRCA1 or BRCA2 deficiency (p = 10−11) [56]. TAI refers to the unequal distribution of alleles at chromosome ends, which is also indicative of HRD and is associated with a positive response to DNA-damaging agents like cisplatin [57]. This relationship was confirmed in breast cancer patients, with cisplatin-sensitive individuals showing higher TAI levels compared to resistant individuals (median TAI: 24 vs. 17.5, p = 0.047) [57]. Tumorigenesis often leads to increased DNA breaks and genomic fragmentation, resulting in LST, which are large chromosome rearrangements characterised by breaks spanning at least 10 Mb between neighbouring regions, excluding the centromere. LST is a marker of genomic instability and indicates BRCA1/2 deficiency [58]. In their study, Popova et al. examined breast cancer cells and found that an elevated number of LST events effectively distinguished cells with defective BRCA1/2 genes from those with proficient BRCA1/2 genes (p < 10−6) [58].
The clinical validity and utility of genomic scar assays have been affirmed in the ESMO review, summarising their predictive value in Phase 3 studies like ARIEL3, PRIMA, VELIA, and PAOLA-1. The most commonly utilised assays are MyChoice® CDx and FoundationOne® CDx, which have evolved from the concept of genomic scars mentioned earlier [31]. In this updated review, out of 62 primary research articles, 33 (53%) used genomic scars or mutational signatures to detect HRD in cancer cells. Most articles focused on genomic scars, with two utilising the mutational signature approach [46, 59]. Beyond the Phase 3 studies covered in the ESMO review, the predictability of genomic scar assays has received additional support from subsequent clinical trials, such as OPINION [60], LIGHT [36], MITO16A [61], OVARIO [37] and ATHENA-MONO [62]. The key findings of the included studies that utilise genomic scars to predict the efficacy of PARP inhibitors are summarised in Appendix Table 4D.
When interpreting the results of genomic scar assays, the uncertain predictability of these assays for the survival benefits of PARP inhibitors is worth considering. The HRD score, primarily derived from FoundationOne® CDx and Myriad MyChoice® CDx, offers an approach to quantify genomic scars within the cancer genome and predict the efficacy of DNA-damaging agents in ovarian cancer. As introduced by Telli et al., the HRD score compiles three genomic scars—LOH, TAI, and LST—into an unweighted sum. An HRD score of 42 or higher indicates increased sensitivity to DNA-damaging agents, such as platinum and PARP inhibitors [63]. However, clinical trials have elucidated the complex nature of predicting treatment outcomes using HRD scores. For example, the Phase 3 PRIMA trial, focusing on niraparib as maintenance therapy for newly diagnosed advanced EOC, demonstrated survival benefits of using niraparib for patients with proficient HRR pathways (HRD score < 42), resulting in a hazard ratio of 0.68 with a 95% CI of 0.49–0.94 compared to the control group [38]. However, long-term follow-up data from PRIMA, published recently, indicated no difference in OS between the treatment and control arms (HR 0.93, 95% CI: 0.69–1.26) after a median follow-up of 73.9 months [64]. The Phase 3 ATHENA-MONO trial supported the effectiveness of rucaparib monotherapy as a first-line maintenance option across various subgroups of newly diagnosed advanced EOC, including those with different levels of LOH and BRCA1/2 wild-type status. Specifically, for patients with BRCA1/2 wild-type and low LOH, the hazard ratio of rucaparib compared to the control was 0.65 (95% CI: 0.45 to 0.95), for those with intermediate LOH, it was 0.39 (95% CI: 0.20 to 0.78), and for those with high LOH, it was 0.58 (95% CI: 0.33 to 1.01) [62]. Furthermore, the Phase 3 ARIEL3 trial for platinum-sensitive recurrent EOC revealed that patients without germline BRCA1/2 pathogenic variants and with low LOH also benefited from rucaparib, with a hazard ratio of 0.58 (95% CI: 0.40–0.85, p-value = 0.0049) [10]. This finding indicates that even those with wild-type BRCA1/2 genes and low HRD scores can derive survival advantages from PARP inhibitors compared to the control group.
Mutational signatures
Genomic scars, which are primarily derived from chromosome microarrays, provide a broad view of genomic instability by detecting large-scale DNA alterations. In contrast, mutational signatures offer a more detailed snapshot of the genome. These signatures detect pathogenic variants associated with tumorigenesis and encompass single nucleotide substitutions, small insertions/deletions, and large-scale structural rearrangements through whole exome or whole genome sequencing. Among single nucleotide substitution mutational signatures, researchers have proposed 21 major types [65]. Signature 3, for example, found in 14.4% of pan-cancer samples, is mainly associated with BRCA1/2 pathogenic variants and pathogenic variants in other HRR-related genes, making it a potential biomarker for HRD [66]. However, it is important to note that various HRR-related genes have different impacts on mutational signatures. BRCA1/2 pathogenic variants predominantly contribute to signature 3 and, to a lesser extent, signature 8 of base substitution [66]. HRD resulting from BRCA1/2, RAD51C, or PALB2 inactivation often exhibits dominance of signature 3, while HRD due to CHEK2 or ATM pathogenic variants may not [67].
To address the complexity of HRR-related genes and limitations in individual mutational signatures for predicting PARP inhibitor efficacy, HRDetect integrates multiple features of mutational signatures. These features include base-substitution signatures 3 and 8, rearrangement signatures with increased large deletions (>3 bp) and microhomology at junctions, rearrangement signature 5, specific copy number variations, and LOH. HRDetect excels with an AUC of 0.98 and 98.7% sensitivity for BRCA1/2 deficiency and HRD identification (cutoff: 0.7). Its performance is optimal with whole genome sequencing and diminishes with exome or targeted panel sequencing [68].
The ESMO review found no significant evidence to confirm the reliability of mutational signatures from whole genome sequencing as predictors of PARP inhibitor effectiveness. In this updated review, three subsequent studies (Färkkilä et al. along with two preclinical studies [46, 54, 55]) used mutational signatures or HRDetect as biomarkers for PARP inhibitor efficacy in EOC. In the TOPACIO trial, Färkkilä et al. found that only mutational signature 3 significantly correlated with a longer median PFS (5.0 months vs. 2.2 months, p = 0.0005) in recurrent EOC patients [46]. However, the two preclinical studies did not yield conclusive results for HRDetect and mutational signature 3 [54, 55]. A summary of key findings from studies using mutational signatures to predict PARP inhibitor efficacy is provided in Appendix Table 4D.
Interpreting genomic scars and mutational signatures has its limitations as mutational signatures can vary based on the duration and extent of mutagen exposure, reflecting the evolving complexity of tumorigenesis. Additionally, mutational signatures can emerge during the precancerous stage. Genomic scar assays offer a “snapshot” of the cancer genome, potentially misrepresenting the current tumour status [69]. For instance, cells can regain HRR capability through BRCA1/2 mutation reversion and develop resistance to PARP inhibitors, even when their genomic scar score indicates HRD [69].
Functional assays for detecting HRD
Functional assays for HRD provide real-time insights into the capacity of HRR, offering advantages over “genomic scars” and “mutational signatures.” Unlike these other methods, which reflect accumulated historical damage and may not indicate the current state of HRR, functional assays assess the active repair capacity of tumours. This dynamic assessment allows for more accurate identification of HRD-positive tumours and can detect cases where HRR proficiency has been restored through mechanisms like reversion mutations. Additionally, functional assays may offer better predictive power for treatment response by measuring actual repair activity, enhancing their utility for guiding therapy decisions.
Among these assays, the RAD51 assay is a prominent method for assessing HRD. This assay evaluates the role of RAD51 in repairing DNA double-strand breaks through the HRR pathway, which involves essential components like BRCA1, BRCA2, and PALB2. RAD51 plays a crucial role in assembling recombinase on single-strand DNA overhangs, a critical step in HRR [40]. In 2010, Graeser et al. demonstrated its predictive value in breast cancer patients undergoing neoadjuvant chemotherapy, showing that tumours with a complete pathological response had lower RAD51 scores compared to non-responders (median RAD51 score: 2.6% vs 44%, p = 0.028) [70]. Initially validated in preclinical studies and smaller patient groups, the assay is now gaining clinical recognition for HRD diagnosis and predicting PARP inhibitor response.
The ESMO review found insufficient evidence to support the clinical validity of RAD51 functional assays in predicting PARP inhibitor responses. In this updated review of 62 studies, 7 articles (11%) utilised the RAD51 assay. Among these, four clinical studies provided valuable insights: Two clinical studies indicated improved survival for patients with low RAD51 levels [71, 72]. A post-hoc analysis of the MITO16A Phase 4 study suggested comparable predictability between the RAD51 assay and the Myriad MyChoice® CDx assay in predicting the efficacy of platinum-based chemotherapy [61]. However, in the TOPACIO Phase 2 trial, RAD51 did not consistently predict treatment response in platinum-resistant recurrent EOC patients receiving niraparib and pembrolizumab [46]. Apart from these clinical findings, additional case series and preclinical studies [54, 55, 73] have also contributed to evidence regarding the predictive value of RAD51.
Assessing nuclear RAD51 recombinase levels provides a functional evaluation of the HRR pathway. However, the absence of RAD51 recombinase formation does not always indicate defects in the later stages of HRR [74]. Downstream impairments that can affect HRR include defects in RAD54, which disrupt the resolution of repair intermediates [75], and alterations in endonucleases such as MUS81-EME1, which impact the final resolution of DNA structures [76]. These defects can result in partial, error-prone repair and contribute to genomic instability. Key findings from studies using the RAD51 functional assay to predict PARP inhibitor efficacy are summarised in Appendix Table 4E.
Other biomarkers
Six articles were included that explore alternative biomarkers that are not directly linked to the HRR pathway but hold promise in predicting the effectiveness of PARP inhibitors. A concise overview of the key findings from studies employing these non-HRR pathway biomarkers to predict PARP inhibitor efficacy is provided in Appendix Table 4F.
ADP-ribosylation
One study introduces ADP-ribosylation as a potential predictor for PARP inhibitor efficacy. ADP-ribosylation, a posttranslational modification affecting protein function, has been associated with cellular processes such as stress response and metabolism [77]. Conrad et al. analysed tumour samples from high-grade serous carcinoma (HGSC) patients and identified distinct molecular phenotypes based on ADP-ribosylation levels. These patterns correlated with RNA expression profiles and clinical outcomes. Elevated PARP-1 levels were also found to drive increased ADP-ribosylation in ovarian cancers, with olaparib sensitivity relating to ADP-ribosylation levels [32].
Methylation status of HOXA9 promoter
The Phase 2 study conducted by Rusan et al. suggests that changes in HOXA9 promoter methylation may serve as a predictor of PARP inhibitor efficacy. HOXA9, a member of the HOX gene family, plays a crucial role in solid tumour development [78]. Rusan et al. investigated the response to veliparib in patients with platinum-resistant recurrent BRCA1/2-mutated EOC, monitoring HOXA9 promoter methylation in circulating tumour DNA. While initial results did not reach statistical significance, post-veliparib treatment changes in HOXA9 methylation correlated with survival outcomes, suggesting its potential as a predictive biomarker. However, these findings are preliminary and should be interpreted with caution, as further investigation is necessary to confirm their clinical relevance [78].
Patient-derived organoids
Two studies explored the use of patient-derived organoids to assess PARP inhibitor sensitivity in ovarian cancer patients. Organoids provide a more realistic representation of organ complexity compared to traditional in vitro cell line studies. Tao et al. demonstrated the feasibility of using ovarian cancer organoids to predict PARP inhibitor responses in a small patient cohort [73], while Sheta et al. generated 3D organoids from cancer cells of HGSC patients and identified distinct gene expression differences between PARP-sensitive and PARP-resistant organoids [79].
KELIM (CA-125 ELIMination Rate Constant K)
One study proposes using the KELIM score, initially designed for predicting chemosensitivity in ovarian cancer, as a predictor of PARP inhibitor efficacy. Based on the CA-125 decline over the initial cycles of platinum-based chemotherapy, the KELIM score has shown reliability in assessing chemosensitivity. An integrated analysis of two Phase 2 trials evaluating rucaparib’s efficacy in recurrent EOC demonstrated that patients with a favourable KELIM score experienced a significant reduction in the risk of disease progression or death when treated with rucaparib, with a HR of 0.67 (95% CI: 0.50–0.91) compared to those with an unfavourable KELIM score. These findings suggest that the KELIM score may serve as a valuable tool in identifying patients who are more likely to benefit from rucaparib treatment [33].
Schlafen 11 (SLFN11)
SLFN11, known for inducing irreversible replication block, is emerging as a predictive biomarker in various cancer types, including ovarian cancer. A retrospective analysis showed that patients with high SLFN11 expression had a median OS of 80 months (95% CI: 55–105) compared to 49 months (95% CI: 38–60) for those with low expression (p = 0.016), which suggested a positive correlation between SLFN11 levels and the effectiveness of DNA-damaging agents such as platinum and PARP inhibitors [80]. The other retrospective study demonstrated that higher SLFN11 expression was associated with platinum sensitivity and extended PFS [81]. These findings underscore SLFN11’s potential as a predictive biomarker for guiding treatment strategies.
In Table 2, we present all the new or updated papers in comparison with the 2020 ESMO systematic review.
Results of the meta-analyses
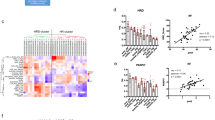
Figure 2 presents the findings of our meta-analyses. Figure 2a consistently demonstrates that both BRCA1/2 pathogenic variants and tumour HRD serve as robust indicators of the effectiveness of PARP inhibitors. In newly diagnosed advanced EOC (frontline) and recurrent EOC, the combined hazard ratio (pooled HR) falls below 1, indicating a substantial treatment advantage (pooled HR = 0.4552; 95% CI: 0.3387; 0.5717). The examination of heterogeneity across studies shows minimal variation (I² = 0.0%, p = 0.9375), affirming the reliability of these results. Figure 2b and c provide separate meta-analyses for BRCA1/2 and HRD in the context of frontline and recurrent treatments, respectively. Details of all articles included in the meta-analysis are presented in Appendix Table 3.
Discussion
This review extends the ESMO search period from September 25, 2019, to March 31, 2023, providing updated evidence. It includes Phase 2 and 3 trials such as SOLO3, OPINION, NORA, ARIEL4, and ATHENA [27, 60, 62, 82, 83], expanding the understanding of biomarker predictability for PARP inhibitor effectiveness. This updated review also covers biomarkers beyond the HRR pathway, such as ADP-Ribosylation [32], HOXA9 promoter methylation [78], patient-derived organoids [73, 79], KELIM [33], and SLFN11 [81].
The ESMO review featured 68 articles (66 primary research articles and 2 review articles), while this updated review included 103 articles (62 primary research articles and 41 review articles). Notably, only three research articles were included in both reviews due to the overlapping of certain clinical trials that were published in November and December 2019. The ESMO review primarily focused on early evidence concerning the predictive potential of biomarkers for platinum and PARP inhibitors. About one-third of the articles covered in the ESMO reviews centred on predictive biomarkers for platinum efficacy, with the remaining two-thirds concentrating on PARP inhibitors. Additionally, a third of the articles discussed other types of cancer. The ESMO review encompassed articles explaining concepts of testing assays (such as genomic scars and HRDetect scores). In contrast, this updated review places a stronger emphasis on primary research articles, predominantly concerning ovarian cancer and predictive biomarkers for PARP inhibitors, as detailed in Table 2 for a comparative summary.
Among the various biomarkers used to assess the effectiveness of PARP inhibitors in treating ovarian cancer, commercial genomic assays such as FoundationOne® CDx, and Myriad MyChoice® CDx are the primary ones employed in both clinical trials and clinical practice. Despite the binary classification system established around a threshold of 42, analysis has shown that patients with an HRD score ≥42 include a small proportion of patients with intact BRCA1/2 genes, whereas those scoring <42 may also include some patients who exhibit BRCA1/2 deficiencies. This finding helps explain why some ovarian cancer patients, even with an HRD score below 42, respond to PARP inhibitors [63]. Given the complexity of selecting PARP inhibitors for ovarian cancer patients, we propose a decision-making framework (Fig. 3) for newly diagnosed advanced EOC and platinum-sensitive EOC, based on evidence from Phase 3 randomised trials, to aid clinicians in understanding PARP inhibitor efficacy.
Current evidence underscores the clinical utility of HRR-related gene promoter methylation as a predictor of PARP inhibitor effectiveness [51, 52, 54, 55], yet larger prospective studies are vital for confirmation. Similarly, HRDetect, which utilises specific mutational signatures from cancer genome sequencing, shows promise in predictability [46, 54, 55] but requires further validation through extensive studies. The potential of real-time HRR pathway assessment, primarily through the RAD51 functional assay, in predicting PARP inhibitor efficacy is emerging from clinical research [46, 54, 55, 61, 73]; however, robust evidence from larger patient cohorts remains a prerequisite. While novel biomarkers outside the HRR pathway appear promising [32, 33, 73, 81], their validation in clinical studies is essential. Notably, the KELIM biomarker, already supported by post-hoc clinical trial analyses, necessitates prospective study validation to establish its utility.
The absence of a standardised approach for diagnosing HRD when comparing biomarkers remains a significant challenge. These biomarkers were originally developed to assist clinicians in stratifying patients for PARP inhibitor efficacy rather than precisely diagnosing HRD. Consequently, establishing a reference testing method to compare these predictive biomarkers’ performance effectively is complex. Rather than focusing solely on the detailed mechanisms of the HRR pathway, phenotypic outcomes following HRD, such as specific genomic scars and mutational signatures, show potential as standard diagnostic approaches. Additionally, functional studies, such as assessing RAD51 activity, can provide real-time information on the status of the HRR pathway. Together, these approaches may promise future incorporation as standard strategies for diagnosing HRD in the cancer genome.
In this systematic review, we adopted a similar approach to the original ESMO review (2020), conducting our literature search exclusively in the PubMed database. Expanding the search to additional medical databases, such as Embase and Scopus, could have provided a broader scope, potentially capturing further relevant evidence and ensuring a more comprehensive analysis. Compared to the ESMO review, which included 68 articles (66 primary research articles and 2 review articles), our updated review incorporates 103 articles, of which 62 are primary research articles and 41 are review articles. This increase in the number of review articles reflects an evolving and expanding body of literature on this topic, underscoring the ongoing research interest and the need for continuous updates in this field.
Conclusion
Genomic assays for detecting BRCA1/2 pathogenic variants and genomic scars are widely utilised as primary predictive biomarkers for assessing the efficacy of PARP inhibitors in ovarian cancer patients. While these assays provide valuable insights into HRD, the complexity of gene pathogenic variants and the multifaceted nature of genomic scars require further refinement in interpretation. Assessing the real-time functionality of HRR through nuclear RAD51 levels shows promise but necessitates additional investigation. Furthermore, novel biomarkers unrelated to HRD, such as ADP-ribosylation, HOXA9 promoter methylation, patient-derived organoids, KELIM scores, and SLFN11 expression, offer potential. Still, their clinical applicability and reliability require validation in large-scale studies. The quest for improved biomarkers to enhance PARP inhibitor response prediction in ovarian cancer patients continues, emphasising the need for standardised methodologies and comprehensive clinical validation to advance precision medicine in ovarian cancer treatment.
Data availability
No datasets were generated or analysed during the current study.
References
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–87.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–96.
Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–53.
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83.
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A Phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96.
Timmermans M, Sonke GS, Van de Vijver KK, van der Aa MA, Kruitwagen RFPM. No improvement in long-term survival for epithelial ovarian cancer patients: a population-based study between 1989 and 2014 in the Netherlands. Eur J Cancer. 2018;88:31–7.
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–64.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, Phase 3 trial. Lancet Oncol. 2017;18:1274–84.
Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet. 2017;390:1949–61.
Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505.
Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019;381:2416–28.
Bono JD, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091–102.
Fizazi K, Piulats JM, Reaume MN, Ostler P, McDermott R, Gingerich JR, et al. Rucaparib or physician’s choice in metastatic prostate cancer. N Engl J Med. 2023;388:719–32.
Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–63.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised Phase 2 trial. Lancet Oncol. 2014;15:852–61.
Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–15.
Mao Z, Bozzella M, Seluanov A, Gorbunova V. DNA repair by nonhomologous end joining and homologous recombination during cell cycle in human cells. Cell Cycle. 2008;7:2902–6.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–8.
Bell D, Berchuck A, Birrer M, Chien J, Cramer DW, Dao F, et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15.
Elvin JA, He Y, Sun J, Odunsi K, Szender JB, Moore KN, et al. Comprehensive genomic profiling (CGP) with loss of heterozygosity (LOH) to identify therapeutically relevant subsets of ovarian cancer (OC). J Clin Oncol. 2017;35:5512.
Ray-Coquard I, Leary A, Pignata S, Cropet C, González-Martín A, Marth C, et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 2023;34:681–92.
González-Martín A, Pothuri B, Vergote I, Graybill W, Lorusso D, McCormick CC, et al. Progression-free survival and safety at 3.5 years of follow-up: results from the randomised Phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur J Cancer. 2023;189:112908.
Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, Phase 3 trial. Lancet Oncol. 2021;22:620–31.
Banerjee S, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, Phase 3 trial. Lancet Oncol. 2021;22:1721–31.
Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA 3rd, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized Phase III trial. J Clin Oncol. 2020;38:1164–74.
Ledermann JA, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib for patients with platinum-sensitive, recurrent ovarian carcinoma (ARIEL3): post-progression outcomes and updated safety results from a randomised, placebo-controlled, Phase 3 trial. Lancet Oncol. 2020;21:710–22.
Penn CA, Wong MS, Walsh CS. Cost-effectiveness of maintenance therapy based on molecular classification following treatment of primary epithelial ovarian cancer in the United States. JAMA Netw Open. 2020;3:e2028620.
Gonzalez R, Havrilesky LJ, Myers ER, Secord AA, Dottino JA, Berchuck A, et al. Cost-effectiveness analysis comparing “PARP inhibitors-for-all” to the biomarker-directed use of PARP inhibitor maintenance therapy for newly diagnosed advanced stage ovarian cancer. Gynecol Oncol. 2020;159:483–90.
Miller RE, Leary A, Scott CL, Serra V, Lord CJ, Bowtell D, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol. 2020;31:1606–22.
Conrad LB, Lin KY, Nandu T, Gibson BA, Lea JS, Kraus WL. ADP-ribosylation levels and patterns correlate with gene expression and clinical outcomes in ovarian cancers. Mol Cancer Ther. 2020;19:282–91.
Colomban O, Swisher EM, Kristeleit R, McNeish I, Shapira-Frommer R, Goble S, et al. Mathematical modeling of the early modeled CA-125 longitudinal kinetics (KELIM-PARP) as a pragmatic indicator of rucaparib efficacy in patients with recurrent ovarian carcinoma in ARIEL2 & STUDY 10. EBioMedicine. 2023;89:104477.
Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52.
Teutsch SM, Bradley LA, Palomaki GE, Haddow JE, Piper M, Calonge N, et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Initiative: methods of the EGAPP Working Group. Genet Med. 2009;11:3–14.
Cadoo K, Simpkins F, Mathews C, Liu YL, Provencher D, McCormick C, et al. Olaparib treatment for platinum-sensitive relapsed ovarian cancer by BRCA mutation and homologous recombination deficiency status: Phase II LIGHT study primary analysis. Gynecol Oncol. 2022;166:425–31.
Hardesty MM, Krivak TC, Wright GS, Hamilton E, Fleming EL, Belotte J, et al. OVARIO Phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol Oncol. 2022;166:219–29.
González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–402.
Riaz N, Blecua P, Lim RS, Shen R, Higginson DS, Weinhold N, et al. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat Commun. 2017;8:857.
Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015;7:a016600.
Gundogdu R, Erdogan MK, Ditsiou A, Spanswick V, Garcia-Gomez JJ, Hartley JA, et al. hMOB2 deficiency impairs homologous recombination-mediated DNA repair and sensitises cancer cells to PARP inhibitors. Cell Signal. 2021;87:110106.
Morra F, Merolla F, Damia G, Ricci F, Varricchio S, Ilardi G, et al. The disruption of the CCDC6–PP4 axis induces a BRCAness like phenotype and sensitivity to PARP inhibitors in high-grade serous ovarian carcinoma. J Exp Clin Cancer Res. 2022;41:245.
Hollis RL, Churchman M, Michie CO, Rye T, Knight L, McCavigan A, et al. High EMSY expression defines a BRCA-like subgroup of high-grade serous ovarian carcinoma with prolonged survival and hypersensitivity to platinum. Cancer. 2019;125:2772–81.
Abida W, Campbell D, Patnaik A, Shapiro JD, Sautois B, Vogelzang NJ, et al. Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: analysis from the Phase II TRITON2 study. Clin Cancer Res. 2020;26:2487–96.
O’Malley DM, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Clinical and molecular characteristics of ARIEL3 patients who derived exceptional benefit from rucaparib maintenance treatment for high-grade ovarian carcinoma. Gynecol Oncol. 2022;167:404–13.
Färkkilä A, Gulhan DC, Casado J, Jacobson CA, Nguyen H, Kochupurakkal B, et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat Commun. 2020;11:1459.
Pujade-Lauraine E, Brown J, Barnicle A, Wessen J, Lao-Sirieix P, Criscione SW, et al. Homologous recombination repair gene mutations to predict olaparib plus bevacizumab efficacy in the first-line ovarian cancer PAOLA-1/ENGOT-ov25 trial. JCO Precis Oncol. 2023;7:e2200258.
Eccles DM, Mitchell G, Monteiro ANA, Schmutzler R, Couch FJ, Spurdle AB, et al. BRCA1 and BRCA2 genetic testing—pitfalls and recommendations for managing variants of uncertain clinical significance. Ann Oncol. 2015;26:2057–65.
Kurian AW, Ward KC, Abrahamse P, Bondarenko I, Hamilton AS, Deapen D, et al. Time trends in receipt of germline genetic testing and results for women diagnosed with breast cancer or ovarian cancer, 2012–2019. J Clin Oncol. 2021;39:1631–40.
Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–42.
Aref-Eshghi E, McGee JD, Pedro VP, Kerkhof J, Stuart A, Ainsworth PJ, et al. Genetic and epigenetic profiling of BRCA1/2 in ovarian tumors reveals additive diagnostic yield and evidence of a genomic BRCA1/2 DNA methylation signature. J Hum Genet. 2020;65:865–73.
Swisher EM, Kwan TT, Oza AM, Tinker AV, Ray-Coquard I, Oaknin A, et al. Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (Parts 1 and 2). Nat Commun. 2021;12:2487.
Sahnane N, Carnevali I, Formenti G, Casarin J, Facchi S, Bombelli R, et al. BRCA methylation testing identifies a subset of ovarian carcinomas without germline variants that can benefit from PARP inhibitor. Int J Mol Sci. 2020;21:9708.
Guffanti F, Alvisi MF, Anastasia A, Ricci F, Chiappa M, Llop-Guevara A, et al. Basal expression of RAD51 foci predicts olaparib response in patient-derived ovarian cancer xenografts. Br J Cancer. 2022;126:120–8.
Pellegrino B, Herencia-Ropero A, Llop-Guevara A, Pedretti F, Moles-Fernández A, Viaplana C, et al. Preclinical in vivo validation of the RAD51 test for identification of homologous recombination-deficient tumors and patient stratification. Cancer Res. 2022;82:1646–57.
Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–82.
Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2:366–75.
Popova T, Manié E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–62.
da Costa A, do Canto LM, Larsen SJ, Ribeiro ARG, Stecca CE, Petersen AH, et al. Genomic profiling in ovarian cancer retreated with platinum based chemotherapy presented homologous recombination deficiency and copy number imbalances of CCNE1 and RB1 genes. BMC Cancer. 2019;19:422.
Poveda A, Lheureux S, Colombo N, Cibula D, Lindemann K, Weberpals J, et al. Olaparib maintenance monotherapy in platinum-sensitive relapsed ovarian cancer patients without a germline BRCA1/BRCA2 mutation: OPINION primary analysis. Gynecol Oncol. 2022;164:498–504.
Capoluongo ED, Pellegrino B, Arenare L, Califano D, Scambia G, Beltrame L, et al. Alternative academic approaches for testing homologous recombination deficiency in ovarian cancer in the MITO16A/MaNGO-OV2 trial. ESMO Open. 2022;7:100585.
Monk BJ, Parkinson C, Lim MC, O’Malley DM, Oaknin A, Wilson MK, et al. A randomized, Phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952–64.
Telli ML, Timms KM, Reid J, Hennessy B, Mills GB, Jensen KC, et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin Cancer Res. 2016;22:3764–73.
Monk BJ, Barretina-Ginesta MP, Pothuri B, Vergote I, Graybill W, Mirza MR, et al. Niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer: final overall survival results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann Oncol. 2024;35:981–92.
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21.
Nik-Zainal S, Davies H, Staaf J, Ramakrishna M, Glodzik D, Zou X, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534:47–54.
Póti Á, Gyergyák H, Németh E, Rusz O, Tóth S, Kovácsházi C, et al. Correlation of homologous recombination deficiency induced mutational signatures with sensitivity to PARP inhibitors and cytotoxic agents. Genome Biol. 2019;20:240.
Davies H, Glodzik D, Morganella S, Yates LR, Staaf J, Zou X, et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med. 2017;23:517–25.
Patel JN, Braicu I, Timms KM, Solimeno C, Tshiaba P, Reid J, et al. Characterisation of homologous recombination deficiency in paired primary and recurrent high-grade serous ovarian cancer. Br J Cancer. 2018;119:1060–6.
Graeser M, McCarthy A, Lord CJ, Savage K, Hills M, Salter J, et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2010;16:6159–68.
van Wijk LM, Vermeulen S, Meijers M, van Diest MF, Ter Haar NT, de Jonge MM, et al. The RECAP test rapidly and reliably identifies homologous recombination-deficient ovarian carcinomas. Cancers. 2020;12:2805.
Blanc-Durand F, Yaniz-Galende E, Llop-Guevara A, Genestie C, Serra V, Herencia-Ropero A, et al. A RAD51 functional assay as a candidate test for homologous recombination deficiency in ovarian cancer. Gynecol Oncol. 2023;171:106–13.
Tao M, Sun F, Wang J, Wang Y, Zhu H, Chen M, et al. Developing patient-derived organoids to predict PARP inhibitor response and explore resistance overcoming strategies in ovarian cancer. Pharm Res. 2022;179:106232.
Fuh K, Mullen M, Blachut B, Stover E, Konstantinopoulos P, Liu J, et al. Homologous recombination deficiency real-time clinical assays, ready or not? Gynecol Oncol. 2020;159:877–86.
Mazin AV, Mazina OM, Bugreev DV, Rossi MJ. Rad54, the motor of homologous recombination. DNA Repair. 2010;9:286–302.
Osman F, Whitby MC. Exploring the roles of Mus81-Eme1/Mms4 at perturbed replication forks. DNA Repair. 2007;6:1004–17.
Lüscher B, Bütepage M, Eckei L, Krieg S, Verheugd P, Shilton BH. ADP-ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem Rev. 2018;118:1092–136.
Rusan M, Andersen RF, Jakobsen A, Steffensen KD. Circulating HOXA9-methylated tumour DNA: a novel biomarker of response to poly (ADP-ribose) polymerase inhibition in BRCA-mutated epithelial ovarian cancer. Eur J Cancer. 2020;125:121–9.
Sheta R, Bachvarova M, Plante M, Renaud MC, Sebastianelli A, Gregoire J, et al. Development of a 3D functional assay and identification of biomarkers, predictive for response of high-grade serous ovarian cancer (HGSOC) patients to poly-ADP ribose polymerase inhibitors (PARPis): targeted therapy. J Transl Med. 2020;18:439.
Zoppoli G, Regairaz M, Leo E, Reinhold WC, Varma S, Ballestrero A, et al. Putative DNA/RNA helicase Schlafen-11 (SLFN11) sensitizes cancer cells to DNA-damaging agents. Proc Natl Acad Sci USA. 2012;109:15030–5.
Winkler C, King M, Berthe J, Ferraioli D, Garuti A, Grillo F, et al. SLFN11 captures cancer-immunity interactions associated with platinum sensitivity in high-grade serous ovarian cancer. JCI Insight. 2021;6:e146098.
Wu XH, Zhu JQ, Yin RT, Yang JX, Liu JH, Wang J, et al. Niraparib maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer using an individualized starting dose (NORA): a randomized, double-blind, placebo-controlled Phase III trial. Ann Oncol. 2021;32:512–21.
Kristeleit R, Lisyanskaya A, Fedenko A, Dvorkin M, de Melo AC, Shparyk Y, et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, Phase 3 trial. Lancet Oncol. 2022;23:465–78.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92.
Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140:199–203.
Ledermann JA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: an updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17:1579–89.
Matulonis UA, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive, relapsed serous ovarian cancer and a BRCA mutation: overall survival adjusted for postprogression poly(adenosine diphosphate ribose) polymerase inhibitor therapy. Cancer. 2016;122:1844–52.
Dougherty BA, Lai Z, Hodgson DR, Orr MCM, Hawryluk M, Sun J, et al. Biological and clinical evidence for somatic mutations in BRCA1 and BRCA2 as predictive markers for olaparib response in high-grade serous ovarian cancers in the maintenance setting. Oncotarget. 2017;8:43653–61.
Lheureux S, Lai Z, Dougherty BA, Runswick S, Hodgson DR, Timms KM, et al. Long-term responders on olaparib maintenance in high-grade serous ovarian cancer: clinical and molecular characterization. Clin Cancer Res. 2017;23:4086–94.
Hodgson DR, Dougherty BA, Lai Z, Fielding A, Grinsted L, Spencer S, et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br J Cancer. 2018;119:1401–9.
Friedlander M, Matulonis U, Gourley C, du Bois A, Vergote I, Rustin G, et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy. Br J Cancer. 2018;119:1075–1085.
Del Campo JM, Matulonis UA, Malander S, Provencher D, Mahner S, Follana P, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial. J Clin Oncol. 2019;37:2968–73.
Mirza MR, Åvall Lundqvist E, Birrer MJ, dePont Christensen R, Nyvang GB, Malander S, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol. 2019;20:1409–19.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87.
Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7:984–98.
Kristeleit RS, Oaknin A, Ray-Coquard I, Leary A, Balmaña J, Drew Y, et al. Antitumor activity of the poly(ADP-ribose) polymerase inhibitor rucaparib as monotherapy in patients with platinum-sensitive, relapsed, BRCA-mutated, high-grade ovarian cancer, and an update on safety. Int J Gynecol Cancer. 2019;29:1396–404.
Kristeleit RS, Drew Y, Oza AM, Domchek SM, Banerjee S, Glasspool RM, et al. Efficacy and safety of rucaparib treatment in patients with BRCA-mutated, relapsed ovarian cancer: final results from Study 10. Br J Cancer. 2023;128:255–65.
Clamp AR, Lorusso D, Oza AM, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma: the effects of progression-free interval and prior therapies on efficacy and safety in the randomized phase III trial ARIEL3. Int J Gynecol Cancer. 2021;31:949–58.
Oaknin A, Oza AM, Lorusso D, Aghajanian C, Dean A, Colombo N, et al. Maintenance treatment with rucaparib for recurrent ovarian carcinoma in ARIEL3, a randomized phase 3 trial: the effects of best response to last platinum-based regimen and disease at baseline on efficacy and safety. Cancer Med. 2021;10:7162–73.
Frenel JS, Kim JW, Aryal N, Asher R, Berton D, Vidal L, et al. Efficacy of subsequent chemotherapy for patients with BRCA1/2-mutated recurrent epithelial ovarian cancer progressing on olaparib versus placebo maintenance: post-hoc analyses of the SOLO2/ENGOT Ov-21 trial. Ann Oncol. 2022;33:1021–8.
Liu J, Yin R, Wu L, Zhu J, Lou G, Wu X, et al. Olaparib maintenance monotherapy in Chinese patients with platinum-sensitive relapsed ovarian cancer: China cohort from the phase III SOLO2 trial. Asia Pac J Clin Oncol. 2022;18:714–22.
DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial. J Clin Oncol. 2023;41:609–17.
Aghajanian C, Swisher EM, Okamoto A, Steffensen KD, Bookman MA, Fleming GF, et al. Impact of veliparib, paclitaxel dosing regimen, and germline BRCA status on the primary treatment of serous ovarian cancer - an ancillary data analysis of the VELIA trial. Gynecol Oncol. 2022;164:278–87.
Swisher EM, Aghajanian C, O'Malley DM, Fleming GF, Kaufmann SH, Levine DA, et al. Impact of homologous recombination status and responses with veliparib combined with first-line chemotherapy in ovarian cancer in the Phase 3 VELIA/GOG-3005 study. Gynecol Oncol. 2022;164:245–53.
Mizuno M, Ito K, Nakai H, Kato H, Kamiura S, Ushijima K, et al. Veliparib with frontline chemotherapy and as maintenance in Japanese women with ovarian cancer: a subanalysis of efficacy, safety, and antiemetic use in the phase 3 VELIA trial. Int J Clin Oncol. 2023;28:163–74.
Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–48.
Okamoto A, Kondo E, Nakamura T, Yanagida S, Hamanishi J, Harano K, et al. Phase 2 single-arm study on the efficacy and safety of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer. J Gynecol Oncol. 2021;32:e16.
Fujiwara K, Fujiwara H, Yoshida H, Satoh T, Yonemori K, Nagao S, et al. Olaparib plus bevacizumab as maintenance therapy in patients with newly diagnosed, advanced ovarian cancer: Japan subset from the PAOLA-1/ENGOT-ov25 trial. J Gynecol Oncol. 2021;32:e82.
Harter P, Mouret-Reynier MA, Pignata S, Cropet C, González-Martín A, Bogner G, et al. Efficacy of maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed, advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Gynecol Oncol. 2022;164:254–64.
Callens C, Vaur D, Soubeyran I, Rouleau E, Just PA, Guillerm E, et al. Concordance between tumor and germline BRCA status in high-grade ovarian carcinoma patients in the phase III PAOLA-1/ENGOT-ov25 trial. J Natl Cancer Inst. 2021;113:917–23.
Loverix L, Vergote I, Busschaert P, Vanderstichele A, Venken T, Boeckx B, et al. PARP inhibitor predictive value of the Leuven HRD test compared with Myriad MyChoice CDx PLUS HRD on 468 ovarian cancer patients from the PAOLA-1/ENGOT-ov25 trial. Eur J Cancer. 2023;188:131–9.
Christinat Y, Ho L, Clément S, Genestie C, Sehouli J, Cinieri S, et al. Normalized LST is an efficient biomarker for homologous recombination deficiency and olaparib response in ovarian carcinoma. JCO Precis Oncol. 2023;7:e2200555.
Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5:1141–1149.
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12:852–61.
Kummar S, Oza AM, Fleming GF, Sullivan DM, Gandara DR, Naughton MJ, et al. Randomized trial of oral cyclophosphamide and veliparib in high-grade serous ovarian, primary peritoneal, or fallopian tube cancers, or BRCA-mutant ovarian cancer. Clin Cancer Res. 2015;21:1574–82.
Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RHJ, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87–97.
Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer. 2016;114:e21.
Liu JF, Herold C, Gray KP, Penson RT, Horowitz N, Konstantinopoulos PA, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5:1731–8.
Lee JY, Kim BG, Kim JW, Lee JB, Park E, Joung JG, et al. Biomarker-guided targeted therapy in platinum-resistant ovarian cancer (AMBITION; KGOG 3045): a multicentre, open-label, five-arm, uncontrolled, umbrella trial. J Gynecol Oncol. 2022;33:e45.
Chen AP, Kummar S, Moore N, Rubinstein LV, Zhao Y, Williams PM, et al. Molecular profiling-based assignment of cancer therapy (NCI-MPACT): a randomized multicenter phase II trial. JCO Precis Oncol. 2021;5:PO.20.00372.
Poveda AM, Davidson R, Blakeley C, Milner A. Olaparib maintenance monotherapy in platinum-sensitive, relapsed ovarian cancer without germline BRCA mutations: OPINION Phase IIIb study design. Future Oncol. 2019;15:3651–63.
Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM, et al. Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): a randomized, open-label, phase III trial. J Clin Oncol. 2022;40:2138–47.
Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann Oncol. 2019;30:551–7.
Lips EH, Mulder L, Hannemann J, Laddach N, Vrancken Peeters M, van de Vijver MJ, et al. Indicators of homologous recombination deficiency in breast cancer and association with response to neoadjuvant chemotherapy. Ann Oncol. 2011;22:870–6.
Mukhopadhyay A, Plummer ER, Elattar A, Soohoo S, Uzir B, Quinn JE, et al. Clinicopathological features of homologous recombination-deficient epithelial ovarian cancers: sensitivity to PARP inhibitors, platinum, and survival. Cancer Res. 2012;72:5675–82.
Shah MM, Dobbin ZC, Nowsheen S, Wielgos M, Katre AA, Alvarez RD, et al. An ex vivo assay of XRT-induced Rad51 foci formation predicts response to PARP-inhibition in ovarian cancer. Gynecol Oncol. 2014;134:331–7.
Lee YH, Liu X, Qiu F, O'Connor TR, Yen Y, Ann DK. HP1β is a biomarker for breast cancer prognosis and PARP inhibitor therapy. PLoS ONE. 2015;10:e0121207.
O'Donnell RL, Kaufmann A, Woodhouse L, McCormick A, Cross PA, Edmondson RJ, et al. Advanced ovarian cancer displays functional intratumor heterogeneity that correlates to ex vivo drug sensitivity. Int J Gynecol Cancer. 2016;26:1004–11.
Zhao EY, Shen Y, Pleasance E, Kasaian K, Leelakumari S, Jones M, et al. Homologous recombination deficiency and platinum-based therapy outcomes in advanced breast cancer. Clin Cancer Res. 2017;23:7521–7530.
Cruz C, Castroviejo-Bermejo M, Gutiérrez-Enríquez S, Llop-Guevara A, Ibrahim YH, Gris-Oliver A, et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann Oncol. 2018;29:1203–1210.
Castroviejo-Bermejo M, Cruz C, Llop-Guevara A, Gutiérrez-Enríquez S, Ducy M, Ibrahim YH, et al. A RAD51 assay feasible in routine tumor samples calls PARP inhibitor response beyond BRCA mutation. EMBO Mol Med. 2018;10:e9172.
Kondrashova O, Topp M, Nesic K, Lieschke E, Ho GY, Harrell MI, et al. Methylation of all BRCA1 copies predicts response to the PARP inhibitor rucaparib in ovarian carcinoma. Nat Commun. 2018;9:3970.
Funnell T, Zhang AW, Grewal D, McKinney S, Bashashati A, Wang YK, et al. Integrated structural variation and point mutation signatures in cancer genomes using correlated topic models. PLoS Comput Biol. 2019;15:e1006799.
Coelho R, Tozzi A, Disler M, Lombardo F, Fedier A, López MN, et al. Overlapping gene dependencies for PARP inhibitors and carboplatin response identified by functional CRISPR-Cas9 screening in ovarian cancer. Cell Death Dis. 2022;13:909.
Agarwal N, Azad A, Shore ND, Carles J, Fay AP, Dunshee C, et al. Talazoparib plus enzalutamide in metastatic castration-resistant prostate cancer: TALAPRO-2 phase III study design. Future Oncol. 2022;18:425–36.
Norquist BM, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner S, et al. Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: an NRG Oncology/Gynecologic Oncology Group study. Clin Cancer Res. 2018;24:777–83.
Stronach EA, Paul J, Timms KM, Hughes E, Brown K, Neff C, et al. Biomarker assessment of HR deficiency, tumor BRCA1/2 mutations, and CCNE1 copy number in ovarian cancer: associations with clinical outcome following platinum monotherapy. Mol Cancer Res. 2018;16:1103–11.
Swisher EM, Gonzalez RM, Taniguchi T, Garcia RL, Walsh T, Goff BA, et al. Methylation and protein expression of DNA repair genes: association with chemotherapy exposure and survival in sporadic ovarian and peritoneal carcinomas. Mol Cancer. 2009;8:48.
Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30:2654–63.
Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–75.
Bernards SS, Pennington KP, Harrell MI, Agnew KJ, Garcia RL, Norquist BM, et al. Clinical characteristics and outcomes of patients with BRCA1 or RAD51C methylated versus mutated ovarian carcinoma. Gynecol Oncol. 2018;148:281–5.
Wen H, Feng Z, Ma Y, Liu R, Ou Q, Guo Q, et al. Homologous recombination deficiency in diverse cancer types and its correlation with platinum chemotherapy efficiency in ovarian cancer. BMC Cancer. 2022;22:550.
da Costa A, Dos Santos ES, Cotrim DP, Pandolfi NC, Cesca MG, Mantoan H, et al. Prognostic impact of platinum sensitivity in ovarian carcinoma patients with brain metastasis. BMC Cancer. 2019;19:1194.
Wijk LM, Vermeulen S, Meijers M, van Diest MF, Ter Haar NT, de Jonge MM, et al. The RECAP test rapidly and reliably identifies homologous recombination-deficient ovarian carcinomas. Cancers. 2020;12:2805.
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–708.
Goodall J, Mateo J, Yuan W, Mossop H, Porta N, Miranda S, et al. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 2017;7:1006–17.
Roviello G, Milani M, Gobbi A, Dester M, Cappelletti MR, Allevi G, et al. A Phase II study of olaparib in breast cancer patients: biological evaluation from a 'window of opportunity' trial. Future Oncol. 2016;12:2189–93.
Quigley D, Alumkal JJ, Wyatt AW, Kothari V, Foye A, Lloyd P, et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 2017;7:999–1005.
Mukhopadhyay A, Elattar A, Cerbinskaite A, Wilkinson SJ, Drew Y, Kyle S, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin Cancer Res. 2010;16:2344–51.
Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–31.
Zhang S, Yuan Y, Hao D. A genomic instability score in discriminating nonequivalent outcomes of BRCA1/2 mutations and in predicting outcomes of ovarian cancer treated with platinum-based chemotherapy. PLoS ONE. 2014;9:e113169.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7.
Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21.
Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–5.
Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–20.
Wang ZC, Birkbak NJ, Culhane AC, Drapkin R, Fatima A, Tian R, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012;18:5806–15.
Nomura H, Kataoka F, Aoki D, Jinno H, Kitagawa Y, Sato Y, et al. Expression of potential biomarkers associated with homologous recombination repair in patients with ovarian or triple-negative breast cancer. Cancer Biomark. 2016;16:145–52.
Maxwell KN, Wubbenhorst B, Wenz BM, De Sloover D, Pluta J, Emery L, et al. BRCA locus-specific loss of heterozygosity in germline BRCA1 and BRCA2 carriers. Nat Commun. 2017;8:319.
Prieske K, Prieske S, Joosse SA, Trillsch F, Grimm D, Burandt E, et al. Loss of BRCA1 promotor hypermethylation in recurrent high-grade ovarian cancer. Oncotarget. 2017;8:83063–74.
Wang YK, Bashashati A, Anglesio MS, Cochrane DR, Grewal DS, Ha G, et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. Nat Genet. 2017;49:856–65.
Deniz M, Romashova T, Kostezka S, Faul A, Gundelach T, Moreno-Villanueva M, et al. Increased single-strand annealing rather than non-homologous end-joining predicts hereditary ovarian carcinoma. Oncotarget. 2017;8:98660–76.
Weigelt B, Comino-Méndez I, de Bruijn I, Tian L, Meisel JL, García-Murillas I, et al. Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian cancer. Clin Cancer Res. 2017;23:6708–20.
Kang J, Lee J, Lee A, Lee YS. Prediction of homologous recombination deficiency from cancer gene expression data. J Int Med Res. 2022;50:3000605221133655.
Funding
MT was supported by the NIHR Cambridge Biomedical Research Centre (NIHR203312). YWJ was supported by the CRUK Catalyst Award CanGene-CanVar (C61296/A27223).
Author information
Authors and Affiliations
Contributions
Conceptualization, YWW, YWJ, and MT; Methodology, YWW, YWJ, and IA; Data review and analysis, YWW, YWJ, and IA; Writing—Original Draft Preparation, YWW, YWJ, and MT; Writing—Review and Editing, YWW, YWJ, IA, GF, and MT; Supervision, YWJ, IA, GF, and MT. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
MT is the Editor in Chief of BJC Reports, he was not involved in any aspect of handling of this manuscript or any editorial decisions.
Ethics approval and consent to participate
Ethics approval was not required for this review.
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, YW., Allen, I., Funingana, G. et al. Predictive biomarkers for the efficacy of PARP inhibitors in ovarian cancer: an updated systematic review. BJC Rep 3, 14 (2025). https://doi.org/10.1038/s44276-025-00122-9
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44276-025-00122-9
This article is cited by
-
Personalisiertere Therapie mit Biomarkern
InFo Hämatologie + Onkologie (2025)