Abstract
Background
Hepatocellular carcinoma (HCC) remains a deadly cancer in the UK despite advancements in curative therapies. Societal conditions and health inequalities influence the development of chronic liver disease and outcomes from complications including HCC. Scoping this emergent evidence-base is required to inform research and solutions for the NHS.
Methods
A PRISMA scoping review was performed up to September 2023. Articles exploring health inequalities in HCC involving the UK population were included.
Results
This review has characterised axes of health inequality and their impact across the HCC care continuum in the UK. Studies predominantly employed a cohort design or population-based analyses, with meta-analyses of surveillance utilisation including only a single UK study. These methodologies provided an appropriate lens to understand longitudinal trends and identify disadvantaged groups. However, important evidence gaps remain, including exploration of patient perspectives, intersectional analyses, and statistical measures of socioeconomic inequity in HCC.
Conclusions
HCC is a rapidly growing cause of cancer mortality and disproportionally affects underserved groups, presenting a major public health concern. Further research is required to innovate and evaluate surveillance and management pathways to reduce systemic inequities. Direction is needed at the national level to improve prevention, early diagnosis and access to curative treatment.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) remains a deadly cancer in the UK despite advancements in curative therapies [1]. Due to the growing burden of chronic liver disease (CLD) in the general population, HCC is becoming one of the fastest growing causes of cancer mortality [2, 3]. Concern around this evolving public health problem has stimulated national efforts to improve standards of surveillance and management of HCC [4,5,6,7]. Social determinants and health inequalities are closely linked to the development of CLD, this extends to complications of chronic inflammation and fibrosis such as HCC [8, 9]. The World Health Organization [10]) defines health inequalities as differences in health status or in the distribution of health resources between different population groups, arising from the social conditions in which people are born, grow, live, work and age. In CLD, there is a multi-layered interaction between the aetiological causes and access to the appropriate healthcare [11]. This combines with geographical variations in socio-economic deprivation, provision of liver services and burden of disease, which all contribute to inequitable clinical outcomes [12,13,14]. The seminal Marmot Review [15] asserts that reducing health inequalities is a matter of fairness and social justice, and must focus on reducing the social gradient in health. A better understanding of how health inequalities interact with HCC outcomes for the UK population is urgently needed to inform future research and improvements to liver disease care pathways. Thus, striving for improved and equitable access to curative treatment for patients in line with the NHS Long Term Plan and Core20PLUS5 initiative [16, 17]. A scoping review was chosen to map this emergent body of literature and identify knowledge gaps [18]. The aim is to determine the extent of research undertaken, how well subgroups and regions have been represented, the methodologies used and whether they are sufficient in characterising health inequalities and their impact on outcomes across the HCC care continuum.
Methods
A scoping review was conducted in line with the updated Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) standards, and extension for scoping reviews [19, 20]. A comprehensive database search included MedLINE, EMBASE, CINAHL and Cochrane Library to identify studies published from inception until September 2023 (when the search was conducted). A search strategy using Boolean logic and MeSH terms was developed to identify studies which focused on a population of HCC or primary liver cancer (PLC); and explored the phenomenon of interest, the impact of health inequalities on outcomes including surveillance, diagnosis, treatment, and survival. To ensure inclusivity, the search strategy was not narrowed for study type or design, therefore formal use of the PICOS or SPIDER criteria was not required [21]. No limits on time, language or type of article facilitated inclusive evidence mapping. After identification and removal of duplicates, records underwent title and abstract screening, full text for reports were then assessed for eligibility. Subsequently, citation searching was performed on all included studies to identify further relevant reports. No automation tools were used. Peer-reviewed original articles were eligible for inclusion if they involved the UK population, defined as studies where the study population included individuals from the UK, even if the population also included individuals from other countries (e.g., in meta-analyses). Articles which focused on liver disease more broadly and abstracts were excluded. An R package and Shiny app was used to produce a PRISMA 2020 compliant flow diagram [22]. Study design, cohort, setting, period, dimensions of HCC care, axes of health inequality, key findings and implications were recorded and organised in a literature matrix table to facilitate data charting and synthesis. Critical appraisal was performed for all included articles with key limitations recorded in implications in the table and an appraisal of the evidence included in the discussion. The full search strategy and eligibility criteria are included in the supplementary material.
Results
The results section has been presented in terms of the study selection and relevant design parameters, followed by the axes of health inequality identified and their impact on clinical outcomes across the HCC care continuum.
Study selection
This scoping review identified 1264 records, after removal of duplicates 704 records underwent title and abstract screening. The predominant reason for screening fail was unsuitability, as per the eligibility criteria. A single report was not retrievable to assess. Subsequently, 50 full reports were assessed for eligibility and 16 new studies, and 3 reports of new studies were included (Fig. 1).
Demonstrating the flow of sources through the different phases of identification, screening, and inclusion. Reasons for report exclusion after full-text assessment are included. 19 original articles were included in this scoping review. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, UK United Kingdom, HCC hepatocellular carcinoma.
Study design parameters
The new studies (Table 1) predominantly adopted a retrospective cohort design or nationwide population-based analysis; single studies used comparative retrospective and prospective cohorts, a prospective longitudinal design, and projected future disease burden using an age-period-cohort model respectively. Cohorts of new PLC or HCC cases were obtained through regional hepato-pancreato-biliary (HPB) multi-disciplinary meeting (MDM) outcomes or from national cancer registries with linked hospital episode statistics (HES) and mortality data. A single study utilised a large primary care database [12]. The methods for identifying cohorts of patients with CLD active in HCC surveillance included screening patient records for “cirrhosis”, interrogating radiology ultrasound requests and using the Hepatitis C Research UK database linked with national cancer registry data. One study generated a cohort listed for or in receipt of liver transplantation from a national registry [23]. The setting varied from single-centre to wider regions covered by a tertiary Hepatology service, to national registries and population-based data. In the latter, different combinations of nations within the UK were represented. The period across studies included data from 1968 to 2021. The study characteristics, dimensions of HCC care, axes of health inequality, key findings, and implications of new studies are presented in Table 1.
The reports of new studies (Table 2) were all systematic reviews with meta-analysis of surveillance utilisation across studies internationally, and all included a single UK retrospective cohort study [24] within the synthesis. These were therefore deemed relevant to the UK population. However, it should be noted the included studies were predominantly conducted in the USA, with fewer studies from Europe and Asia. Included studies were predominantly retrospective or prospective cohort design, with a single randomised-control trial. Cohorts were mostly of cirrhosis, but non-cirrhotic chronic hepatitis B (HBV) was also represented. Study periods spanned from 1985 to 2020. Similarly, the characteristics, dimensions of HCC care, axes of health inequality, key findings, and implications of the reports of new studies are presented in Table 2.
Axes of Health Inequality in HCC
The findings are presented as ‘axes of health inequality’, this term has been used to refer to the dimensions of individual identity, status and social position that influence health outcomes. These axes help to identify the marginalised groups that are affected by health inequalities. This concept is closely related to ‘social determinants of health’, which refers to the wider societal conditions that also shape and sustain unequal health outcomes. There are references to the wider literature where appropriate for context.
Age
There is an inverse relationship as HCC incidence increases with age but access to curative treatment diminishes [13]. Older adults are also more likely to be diagnosed with HCC through emergency presentation and in late stages, with associated reduced survival [12, 13]. In cirrhosis with cured chronic hepatitis C (HCV), increasing age may be associated with better surveillance adherence despite potential reducing benefit [25]. Across the cancer care continuum more broadly, clinical outcomes for older adults are often inferior. Treatment is complicated by the need to adapt to baseline health and performance status, which can vary widely. Higher rates of socio-economic deprivation with advancing age and barriers to accessing care, further compound health inequalities [26].
Sex
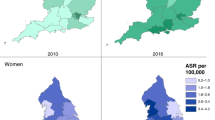
Higher incidence and mortality in males has been observed longitudinally across the UK [1, 12, 27,28,29,30,31]. This reflects global patterns of disease [32], and is largely explained by clustering of risk factors in men and differences in sex hormones [33]. In addition, hepatic iron levels are a co-factor in fibrosis progression and the relative iron deficiency observed in menstruating women appears protective, although this advantage is lost post-menopause [34].
Ethnicity
Asian and Black Caribbean ethnic minority groups are disproportionately affected with higher incidence and mortality [28, 29, 31], this is largely explained by higher viral hepatitis incidence in migrants from endemic countries but genetic differences probably have a role [35]. However, there is also a growing burden of metabolic dysfunction-associated steatotic liver disease (MASLD) across diverse ethnicities worldwide [36]. In terms of the patient journey, higher rates of emergency presentation and late stage diagnosis, and reduced access to curative treatment are observed for ethnic minority groups in the UK [12]. This may be partly due to higher rates of socio-economic deprivation [37] and greater barriers to accessing services such as cancer screening [38].
Socioeconomic status
Local socioeconomic deprivation levels are strong predictors of inequalities in health [39] and deprivation has now been linked to liver cancer mortality in the UK [40]. Ranked indices of deprivation have been used as a proxy for income level or socioeconomic status. Increasing deprivation is associated with a higher incidence of HCC and late-stage diagnosis through symptomatic routes [12, 13, 27, 29]. Variations in the HCC disease burden have been observed with higher rates in Greater Manchester and London [13], which are presumed due to a combination of higher deprivation and ethnically diverse urban populations. However, deprivation in rural communities is also well established. The highest incidence and mortality affects men in Scotland [1], which could reflect increased exposure to risk factors such as drug and alcohol use [41]. These associations tell only part of the story, the wider societal context is clearly highly relevant but has not been captured by the evidence included in this review.
Lifestyle factors and aetiology
An epidemic of lifestyle related liver disease and subsequent HCC is being driven by behaviours including high alcohol consumption, eating low quality diets, and limited physical inactivity [42, 43]. There is conflicting evidence on surveillance adherence across different CLD aetiologies [44], but particularly poor adherence has been observed in cirrhosis with cured HCV [25]. There is a concern that MASLD is underrepresented in surveillance due to a large burden of undetected disease in the community, despite being associated with developing HCC in the absence of cirrhosis [45]. In addition, ultrasound inadequacy is a growing challenge with the increasing prevalence of people living with obesity [46], and data is awaited on the use of abbreviated MRI as an alternative. Alcohol-related liver disease (ARLD) appears associated with reduced access to treatment [14], which could represent the intersection between ongoing alcohol use and systemic inequities experienced by this group.
Access to healthcare services
The geographical provision of Hepatology services across the UK is inequitable. Higher uptake of surveillance appears associated with attending Level 3 Hepatology centres [25]. In contrast, increasing travel time is associated with increased death after listing and reduced likelihood of liver transplantation or recovery [23]; it remains debated where would be the optimum site for an additional UK transplant centre to mitigate this effect. London appears to be an outlier with better access to curative treatment and improved survival [14]. This may be due to improved access to Level 3 centres and a relatively younger population with a greater viral hepatitis predominance. The barriers faced by marginalised groups in accessing HCC care pathways remain underexplored and poorly understood.
Impact on outcomes across the HCC care continuum
The impact of the identified axes of health inequality on outcomes has been presented across the HCC care continuum, from surveillance and diagnosis to treatment and survival. Findings pertinent to the UK population and healthcare system are explored in the context of the wider literature, including research performed in different healthcare systems and cultures.
Surveillance
Surveillance appears poorly targeted, inefficient, and inequitable [24, 25, 47]. This reflects a lack of resources and infrastructure nationally despite evidence demonstrating its effectiveness in improving access to curative treatment and reducing mortality [48, 49]. Poor adherence is undoubtedly undermining these benefits, but there is a lack of good quality data and monitoring in the UK [50, 51]. A limited number of single-centre retrospective studies have reported 19–76% adherence to bi-annual surveillance [24, 25, 47], and appears particularly patchy in people with cirrhosis and cured HCV, 9% across all follow-up [25]. This aligns with meta-analyses reporting 24–52% adherence across studies internationally [52,53,54]. However, the definition of adherence is heterogenous and measurement often fraught with methodological limitations; the quality of data is low and comprehensive subgroup analyses are missing.
A combination of provider factors, such as doubting effectiveness and inappropriate requesting of tests [24, 47, 50, 51], health system factors including limited capacity and complex pathways [55], and patient factors such as non-attendance and related barriers [47, 56] contribute to poor adherence with surveillance standards. Patient-reported barriers and attitudes have not been explored in a UK population but there are emerging learnings on barriers to cancer screening more widely for underserved and marginalised groups, which could be applied [38]. There can be significant misconceptions, such as surveillance not being necessary in the absence of symptoms or after normal tests [52]. Understanding of the importance of timely surveillance is likely suboptimal and requires improved patient communications [50, 51, 55]. In the USA, a strong predictor of continued surveillance is having multiple visits with a liver specialist, and there appears to be an inverse relationship between ultrasound lead time (difference between the dates it was ordered and subsequently performed) and adherence [57].
In the UK, a radiology-led automatic recall system was trialled with no benefit [24]. More widely, other interventions have been explored including focused patient education [58], and interventional surveillance programmes which employ automatic recall, mail outreach/reminders and pathway navigators [52, 53]. All demonstrate potential to improve adherence. Further efforts to design and evaluate interventional surveillance programmes are required.
Diagnosis
HCC incidence is growing in the UK [1, 27, 28, 30] and projected to have the highest average annual increase of all cancers over the next 15 years [2]. A large proportion of cases are diagnosed outside of surveillance (60%) [44], the exact reasons are unknown and require further investigation. However, there is a growing burden of undetected CLD in the general population, which requires co-ordinated efforts to improve screening and diagnosis [43]. Symptomatic presentation of HCC is common via emergency (35.6%), GP referral (31.1%) and two-week wait (11.5%) pathways [13], which are associated with a more advanced stage at diagnosis [12]. The Covid-19 pandemic had a negative impact with higher rates of emergency presentation and larger tumour diameter [59]. One study reported an early detection rate of 25.6% for Barcelona Clinic Liver Cancer stage 0-A [44]. However, there is a lack of available national data on cancer stage, liver function and performance status at diagnosis [14].
The reporting method for imaging remains largely unstandardised across UK centres [50], despite the development of tools such as LI-RADS [60, 61] which could help standardise our approach to management [4]. Liver biopsy and histopathological assessment is increasingly required to verify diagnosis in favour of reliance on radiological evidence [28]. This shift may improve access to clinical trials, experimental treatment options and personalised therapy, an area which has been lacking compared to other cancers [62].
Treatment and survival
According to a single study with data encompassing 2001–2007, access to curative treatment (ablation, resection, transplantation) appears limited (24.4%), with the majority of patients receiving no treatment at all (58.4%), and only a small proportion undergoing transplantation at any stage (5.5%) [14]. Therapeutic advances such as loco-regional and systemic therapies over the last couple of decades have resulted in only modest improvements and 5-year survival remains low (18.3%) [1]. Intersecting axes of inequality including increasing age and deprivation, ARLD, and black Caribbean and Asian ethnic groups are associated with reduced access to treatment and survival [12,13,14]. However, rates of treatment utilisation across subgroups and reasons for non-utilisation have not been established in the UK. In addition, stage-specific survival data are not available from HES or cancer registries due to a lack of granularity. Within cancer more widely, increased travel time is associated with more advanced stage at diagnosis, inappropriate treatment and reduced survival [63]. HCC treatment is centralised at specialist centres, therefore travel time may have a significant role in treatment and outcomes and needs further exploration.
Discussion
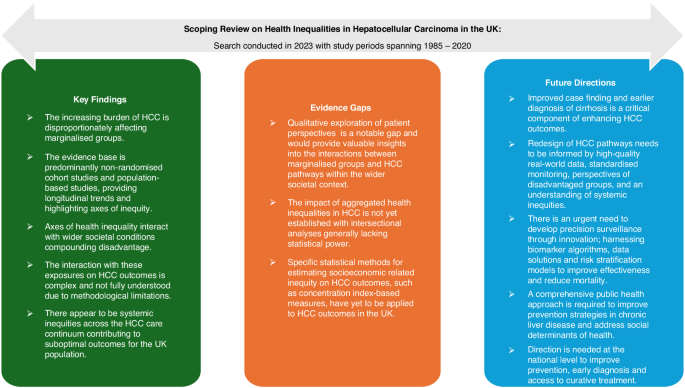
The discussion provides a synthesis of key findings, appraises the evidence – including its strengths and limitations – and outlines future directions with implications for practice, policy and research, as illustrated in Fig. 2.
Summary of key findings
This is the first scoping review to characterise axes of health inequality in HCC and their impact across the care continuum in the UK. The incidence and mortality of HCC are increasing and disproportionally affecting marginalised groups who remain underserved by the current healthcare system, presenting a major public health concern. The relationship between exposures and outcomes is complex. There is an interplay between individual axes of health inequality and wider societal conditions, compounded by the increasing burden of liver disease and systemic inequities in HCC care pathways. The fairness of health inequalities is nuanced, acknowledging the distinction of causal variables into ‘circumstances’ beyond individual responsibility (e.g. biological sex and socioeconomic status) and ‘efforts’ for which individuals are responsible (e.g. lifestyle factors) [64]. Regardless, the axes of inequality at play here appear to have a considerable impact with low adherence to HCC surveillance standards, late-stage HCC diagnosis, limited access to curative treatment, and low survival rates common outcomes faced by individuals. Outcomes in the UK are suboptimal in comparison to other leading healthcare systems such as Japan [65]. The axes of health inequality identified in this review have highlighted marginalised groups that experience disproportionately poor HCC outcomes. These findings have underscored the need for targeted consultation and consideration of these groups when redesigning care pathways.
Appraisal of the evidence
The evidence base is largely comprised of non-randomised cohort studies and observational epidemiological data. This has traditionally been considered a lower quality of evidence. However, it provides an appropriate lens to understand longitudinal trends and identify axes of health inequality in HCC. Several studies have attempted to explore the impact of aggregated axes of health inequality on outcomes through multivariate regression modelling [12, 13, 25, 44, 47]. However, their statistical power was generally insufficient to effectively demonstrate these intersectionalities. Statistical methods for estimating socioeconomic related inequality in health, such as concentration index based measures, have yet to be applied to HCC outcomes in the UK [66]. Qualitative studies exploring patient perspectives are a notable gap at present and would provide valuable insights into the factors that influence interactions between marginalised groups and HCC care pathways within their broader societal context. Furthermore, the meta-analyses on surveillance utilisation predominantly consider studies of non-UK populations, highlighting the paucity of UK data [52,53,54]. Therefore, differences in ethnic diversity, liver disease aetiology, and healthcare systems (such as a health-insurance model), reduce the transferability of these findings. A scoping review was adopted to map evidence and identify gaps for future research rather than answer a specific question related to HCC care. Therefore, assessing the risk of bias and certainty of evidence were not required.
Future directions
Improved case finding and earlier diagnosis of cirrhosis in the general population is a critical component of enhancing HCC outcomes. Further research is needed to evaluate the performance of current HCC surveillance and treatment pathways. High-quality data and standardised monitoring are needed to generate real-world evidence that can guide resource allocation to underserved regions and patient groups. Current surveillance strategies fail to account for individual HCC risk or competing outcomes, such as liver decompensation and death. A key priority is to develop robust, risk-based models that enable precision surveillance, enhance cost-effectiveness and reduce mortality [67]. Emerging surveillance tools such as GAAD/GALAD [68,69,70] and risk stratification models [71,72,73] move us in the right direction by incorporating age, sex and novel biomarkers within their algorithms. However, significant statistical and clinical challenges remain, and these models require external validation in the UK population before translation into routine practice [74]. To improve HCC care pathways, future efforts must prioritise incorporating the patient perspective and ensuring equitable access across underserved groups. From a policy and practice perspective, a comprehensive public health approach is critical to developing effective prevention strategies for CLD. Organisation and leadership at the national level are required to redesign HCC care pathways, reduce systemic inequities, and meet the urgent need for earlier diagnosis and more equitable access to curative treatments.
Data Availability
No datasets were generated or analysed during the current study.
References
Burton A, Tataru D, Driver RJ, Bird TG, Huws D, Wallace D, et al. Primary liver cancer in the UK: Incidence, incidence-based mortality, and survival by subtype, sex, and nation. JHEP Rep. 2021;3:100232. https://doi.org/10.1016/j.jhepr.2021.100232
Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115:1147–55. https://doi.org/10.1038/bjc.2016.304
Shelton J, Zotow E, Smith L, Johnson SA, Thomson CS, Ahmad A, et al. 25 year trends in cancer incidence and mortality among adults aged 35-69 years in the UK, 1993-2018: retrospective secondary analysis. BMJ. 2024;384:e076962 https://doi.org/10.1136/bmj-2023-076962
Suddle A, Reeves H, Hubner R, Marshall A, Rowe I, Tiniakos D, et al. British Society of Gastroenterology guidelines for the management of hepatocellular carcinoma in adults. Gut. 2024;73:1235–68.
NHS England. Hepatocellular carcinoma: delivering quality ultrasound surveillance 2024 [Available from: https://www.england.nhs.uk/long-read/hepatocellular-carcinoma-delivering-quality-ultrasound-surveillance/#guidance-statements.
Liver Cancer UK. Liver Cancer - A Call to action. British Liver Trust; 2023.
NHS England. Hepatocellular carcinoma surveillance: minimum standards 2024 [Available from: https://www.england.nhs.uk/long-read/hepatocellular-carcinoma-surveillance-minimum-standards/.
Jones PD, Lai JC, Bajaj JS, Kanwal F. Actionable Solutions to Achieve Health Equity in Chronic Liver Disease. Clin Gastroenterol Hepatol. 2023;21:1992–2000. https://doi.org/10.1016/j.cgh.2023.03.043
Kondili LA, Lazarus JV, Jepsen P, Murray F, Schattenberg JM, Korenjak M, et al. Inequities in primary liver cancer in Europe: The state of play. J Hepatol. 2024;80:645–60. https://doi.org/10.1016/j.jhep.2023.12.031
World Health Organization. Health inequalities and their causes 2018 [Available from: www.who.int/news-room/facts-in-pictures/detail/health-inequities-and-their-causes.
Ventura-Cots M, Bataller R, Lazarus JV, Benach J, Pericàs JM. Applying an equity lens to liver health and research in Europe. J Hepatol. 2022;77:1699–710. https://doi.org/10.1016/j.jhep.2022.07.021
Liao W, Coupland CAC, Innes H, Jepsen P, Matthews PC, Campbell C, et al. Disparities in care and outcomes for primary liver cancer in England during 2008-2018: a cohort study of 8.52 million primary care population using the QResearch database. eClinicalMed. 2023;59. https://doi.org/10.1016/j.eclinm.2023.101969.
Burton A, Balachandrakumar VK, Driver RJ, Tataru D, Paley L, Marshall A, et al. Regional variations in hepatocellular carcinoma incidence, routes to diagnosis, treatment and survival in England. Br J Cancer. 2022;126:804–14. https://doi.org/10.1038/s41416-021-01509-4
Beecroft S, O’Connell M, Nassar A, Noon K, Pollock KG, Palmer D, et al. Major variation in hepatocellular carcinoma treatment and outcomes in England: A retrospective cohort study. Frontline Gastroenterol. 2022;14:19–24. https://doi.org/10.1136/flgastro-2022-102142
Marmot M Fair Society, Healthy Lives. 2010
Alderwick H, Dixon J The NHS long term plan. British Medical Journal Publishing Group; 2019.
NHS England. Core20PLUS5 (adults) – an approach to reducing healthcare inequalities 2021 [Available from: https://www.england.nhs.uk/about/equality/equality-hub/national-healthcare-inequalities-improvement-programme/core20plus5/.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143 https://doi.org/10.1186/s12874-018-0611-x
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579 https://doi.org/10.1186/s12913-014-0579-0
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev. 2022;18:e1230.
Webb GJ, Hodson J, Chauhan A, O’Grady J, Neuberger JM, Hirschfield GM, et al. Proximity to transplant center and outcome among liver transplant patients. Am J Transpl. 2019;19:208–20. https://doi.org/10.1111/ajt.15004
Farrell C, Halpen A, Cross TJ, Richardson PD, Johnson P, Joekes EC. Ultrasound surveillance for hepatocellular carcinoma: service evaluation of a radiology-led recall system in a tertiary-referral centre for liver diseases in the UK. Clin Radio. 2017;72:338.e11–.e17. https://doi.org/10.1016/j.crad.2016.10.019
Hamill V, Gelson W, MacDonald D, Richardson P, Ryder SD, Aldersley M, et al. Delivery of biannual ultrasound surveillance for individuals with cirrhosis and cured hepatitis C in the UK. Liver Int. 2023;43:917–27. https://doi.org/10.1111/liv.15528
Dharmarajan KV, Presley CJ, Wyld L. Care Disparities Across the Health Care Continuum for Older Adults: Lessons From Multidisciplinary Perspectives. Am Soc Clin Oncol Educ Book. 2021;41:e215–e24. https://doi.org/10.1200/edbk_319841
Konfortion J, Coupland VH, Kocher HM, Allum W, Grocock MJ, Jack RH. Time and deprivation trends in incidence of primary liver cancer subtypes in England. J Evaluation Clin Pr. 2014;20:498–504. https://doi.org/10.1111/jep.12188
Ladep NG, Khan SA, Crossey MM, Thillainayagam AV, Taylor-Robinson SD, Toledano MB. Incidence and mortality of primary liver cancer in England and Wales: changing patterns and ethnic variations. World J Gastroenterol. 2014;20:1544–53. https://doi.org/10.3748/wjg.v20.i6.1544
Jack RH, Konfortion J, Coupland VH, Kocher HM, Berry DP, Allum W, et al. Primary liver cancer incidence and survival in ethnic groups in England, 2001–2007. Cancer Epidemiol. 2013;37:34–8. https://doi.org/10.1016/j.canep.2012.10.008
West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751–8. https://doi.org/10.1038/sj.bjc.6603127
Haworth EA, Soni Raleigh V, Balarajan R. Cirrhosis and Primary Liver Cancer Amongst First Generation Migrants in England and Wales. Ethnicity Health. 1999;4:93–9. https://doi.org/10.1080/13557859998227
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7:6 https://doi.org/10.1038/s41572-020-00240-3
Rigamonti C, Andorno S, Maduli E, Capelli F, Boldorini R, Sartori M. Gender and liver fibrosis in chronic hepatitis: the role of iron status. Alimentary Pharm therapeutics. 2005;21:1445–51.
Chavda V, Zajac KK, Gunn JL, Balar P, Khadela A, Vaghela D, et al. Ethnic differences in hepatocellular carcinoma prevalence and therapeutic outcomes. Cancer Reports. 2023;6 (no pagination) https://doi.org/10.1002/cnr2.1821.
Yip TCF, Vilar‐Gomez E, Petta S, Yilmaz Y, Wong GLH, Adams LA, et al. Geographical similarity and differences in the burden and genetic predisposition of NAFLD. Hepatology. 2023;77:1404–27. https://doi.org/10.1002/hep.32774
Ministry of Housing Communities & Local Government. People living in deprived neighbourhoods 2020 [Available from: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/people-living-in-deprived-neighbourhoods/latest/#data-sources.
Okolie C, Hookway A, Wale A, Everitt J, Shaw H, Lewis R, et al. A rapid review of barriers and facilitators to cancer screening uptake (breast, cervical and bowel) in underserved populations. medRxiv. 2022:2022.08.11.22278362 https://doi.org/10.1101/2022.08.11.22278362.
Marmot M. Health equity in England: the Marmot review 10 years on. BMJ. 2020:m693 https://doi.org/10.1136/bmj.m693.
Rashid T, Bennett JE, Muller DC, Cross AJ, Pearson-Stuttard J, Asaria P, et al. Mortality from leading cancers in districts of England from 2002 to 2019: a population-based, spatiotemporal study. Lancet Oncol. 2024;25:86–98. https://doi.org/10.1016/S1470-2045(23)00530-2
Curran C, Stanley AJ, Barclay ST, Priest M, Graham J. The association between deprivation and the incidence and survival of patients with hepatocellular carcinoma in the West of Scotland. Expert Rev Gastroenterol Hepatol. 2021;15:1427–33. https://doi.org/10.1080/17474124.2021.1997586
Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105:S77–81. https://doi.org/10.1038/bjc.2011.489
Williams R, Aspinall R, Bellis M, Camps-Walsh G, Cramp M, Dhawan A, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–97. https://doi.org/10.1016/S0140-6736(14)61838-9
Haq MI, Drake TM, Goh TL, Ahmed A, Forrest E, Barclay S, et al. Effect of Hepatocellular Carcinoma Surveillance Programmes on Overall Survival in a Mixed Cirrhotic UK Population: A Prospective, Longitudinal Cohort Study. J Clin Med. 2021;10:2770.
Tan DJH, Ng CH, Lin SY, Pan XH, Tay P, Lim WH, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol. 2022;23:521–30. https://doi.org/10.1016/s1470-2045(22)00078-x
Hydes TJ, Cuthbertson DJ, Palmer DH, Elshaarawy O, Johnson PJ, Fernando R, et al. Ultrasonography in surveillance for hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Hepatoma Res. 2023;9:12. https://doi.org/10.20517/2394-5079.2022.97
Selvapatt N, House H, Brown A. Hepatocellular Carcinoma Surveillance: Are We Utilizing It? J Clin Gastroenterol. 2016;50:e8–e12. https://doi.org/10.1097/MCG.0000000000000344
Singal AG, Pillai A, Tiro J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLOS Med. 2014;11:e1001624. https://doi.org/10.1371/journal.pmed.1001624
van Meer S, de Man RA, Coenraad MJ, Sprengers D, van Nieuwkerk KM, Klümpen HJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: Results from a large cohort in the Netherlands. J Hepatol. 2015;63:1156–63. https://doi.org/10.1016/j.jhep.2015.06.012
Scott RA, Cross TJS, Clarke C, Khan SA, Ryder SD, Franklin J, et al. Outcomes of National Survey of the Practice of Hepatocellular Carcinoma Surveillance. J Hepatocell Carcinoma. 2023;10:725–31. https://doi.org/10.2147/jhc.s403702
Cross TJS, Villanueva A, Shetty S, Wilkes E, Collins P, Adair A, et al. A national survey of the provision of ultrasound surveillance for the detection of hepatocellular carcinoma. Frontline Gastroenterol. 2016;7:82–9. https://doi.org/10.1136/flgastro-2015-100617
Ramai D, Singh J, Chandan S, Tartaglia N, Ambrosi A, Khan SR, et al. Utilization of Hepatocellular Carcinoma Surveillance Programs in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2023;57:198–203. https://doi.org/10.1097/MCG.0000000000001668
Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of Hepatocellular Carcinoma Surveillance in Patients With Cirrhosis: A Systematic Review and Meta‐Analysis. Hepatology. 2021;73:713–25. https://doi.org/10.1002/hep.31309
Zhao, Jin M, Le RH, Le MH, Chen VL, Jin M, et al. Poor adherence to hepatocellular carcinoma surveillance: A systematic review and meta-analysis of a complex issue. Liver Int. 2018;38:503–14. https://doi.org/10.1111/liv.13555
Ladhani S, Ohri A, Wong RJ. Disparities in Hepatocellular Carcinoma Surveillance: Dissecting the Roles of Patient, Provider, and Health System Factors. J Clin Gastroenterol. 2020;54:218–26. https://doi.org/10.1097/MCG.0000000000001313
Singal AG, Tiro JA, Murphy CC, Blackwell JM, Kramer JR, Khan A, et al. Patient-Reported Barriers Are Associated With Receipt of Hepatocellular Carcinoma Surveillance in a Multicenter Cohort of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2021;19:987–95. https://doi.org/10.1016/j.cgh.2020.06.049.
Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65:864–74. https://doi.org/10.1002/hep.28765
Shaw J, Patidar KR, Reuter B, Hajezifar N, Dharel N, Wade JB, et al. Focused Education Increases Hepatocellular Cancer Screening in Patients with Cirrhosis Regardless of Functional Health Literacy. Digestive Dis Sci. 2021;66:2603–9. https://doi.org/10.1007/s10620-020-06583-x
Geh D, Watson R, Sen G, French JJ, Hammond J, Turner P, et al. COVID-19 and liver cancer: lost patients and larger tumours. BMJ Open Gastroenterol. 2022;9:e000794. https://doi.org/10.1136/bmjgast-2021-000794
American College of Radiology. LI-RADS® US Surveillance v2024 Core2024. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-US-Surveillance-v2024-Core.pdf.
American College of Radiology. CT/MRI LI-RADS® v2018 CORE2018. Available from: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf.
Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2016;2:16018. https://doi.org/10.1038/nrdp.2016.18
Ambroggi M, Biasini C, Giovane CD, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: Review of the literature. Oncologist. 2015;20:1378–85. https://doi.org/10.1634/theoncologist.2015-0110
Fleurbaey M, Schokkaert E. Unfair inequalities in health and health care. J Health Econ. 2009;28:73–90. https://doi.org/10.1016/j.jhealeco.2008.07.016
Kudo M. Management of Hepatocellular Carcinoma in Japan as a World-Leading Model. Liver Cancer. 2018;7:134–47. https://doi.org/10.1159/000484619
Erreygers G, Van Ourti T. Measuring socioeconomic inequality in health, health care and health financing by means of rank-dependent indices: a recipe for good practice. J Health Econ. 2011;30:685–94. https://doi.org/10.1016/j.jhealeco.2011.04.004
European Association for the Study of the Liver. Policy Statement Risk-based surveillance for hepatocellular carcinoma among patients with cirrhosis 2023 [Available from: https://easl.eu/wp-content/uploads/2023/04/Policy-Statement-Liver-Cancer-Screening_VFF.pdf.
Chan H, Vogel A, Berg T, De Toni E, Kudo M, Trojan J, et al. A comparative analysis of Elecsys GALAD and Elecsys GAAD score to detect early-stage hepatocellular carcinoma in an international cohort. J Hepatol. 2022;77:S937. https://doi.org/10.1016/S0168-8278(22)02154-7
Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as Biomarkers for Detection of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin Gastroenterol Hepatol. 2023;21:415–23. https://doi.org/10.1016/j.cgh.2022.01.047.
Singal AG, Tayob N, Mehta A, Marrero JA, El‐Serag H, Jin Q, et al. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology. 2022;75:541–9. https://doi.org/10.1002/hep.32185
Fan R, Papatheodoridis G, Sun J, Innes H, Toyoda H, Xie Q, et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J Hepatol. 2020;73:1368–78. https://doi.org/10.1016/j.jhep.2020.07.025
El-Serag H, Kanwal F, Ning J, Powell H, Khaderi S, Singal AG, et al. Serum biomarker signature is predictive of the risk of hepatocellular cancer in patients with cirrhosis. Gut. 2024. https://doi.org/10.1136/gutjnl-2024-332034.
Kanwal F, Khaderi S, Singal AG, Marrero JA, Asrani SK, Amos CI, et al. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023;21:3296–304.e3. https://doi.org/10.1016/j.cgh.2023.04.019.
Innes H, Nahon P. Statistical perspectives on using hepatocellular carcinoma risk models to inform surveillance decisions. J Hepatol. 2023;79:1332–7. https://doi.org/10.1016/j.jhep.2023.05.005
Acknowledgements
Nil.
Funding
CM and VSA are funded by the NHS Cancer Programme (NCPC02013) and supported by Small Business Research Initiative (SBRI) Healthcare.
Author information
Authors and Affiliations
Contributions
VSA conceived the idea for this paper and VSA, KPH, NH and AJA secured the funding. The scoping review was conducted by CM and verified by VSA. All authors contributed to the interpretation of the findings. CM drafted the manuscript. A plain English summary was co-developed by patient advisors GL and SR, and CM. All authors read and commented on the earlier drafts, contributed to the revision of the manuscript, approved the final version of the manuscript, and had final responsibility for the decision to submit for publication. CM is supervised by VSA, KPH and NH.
Corresponding author
Ethics declarations
Competing interests
A. Joy Allen is an employee of Roche Diagnostics Limited. The remaining authors have no competing interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mysko, C., Landi, S., Purssell, H. et al. Health inequalities in hepatocellular carcinoma surveillance, diagnosis, treatment, and survival in the United Kingdom: a scoping review. BJC Rep 3, 13 (2025). https://doi.org/10.1038/s44276-025-00126-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44276-025-00126-5
This article is cited by
-
Inequalities in Gastrointestinal Care Provision in the United Kingdom
Digestive Diseases and Sciences (2025)