Abstract
Background
Breast cancer (BC) poses a significant health challenge globally. While high-income countries benefit from robust healthcare systems, sub-Saharan Africa (SSA) faces elevated BC mortality rates. Despite extensive research on BC survival, rural populations in SSA, including Uganda, remain underrepresented in scientific literature.
Methods
We performed a cohort study aiming to bridge this gap by investigating BC survival among the rural population of the Busoga Region, Uganda, leveraging data from patient registers of Rays of Hope Hospice Jinja. Using a retrospective survival study design, we estimated 1-, 3-, and 5-year survival rates (SRs) for BC cases from 2016 to 2022 via Kaplan-Meier plots.
Results
Our compiled diagnosis model found a 1-year SR of 57.7% (95%-CI: 51.6–64.4), a 3-year SR of 19.1% (95%-CI: 13.9–26.1) and a 5-year SR of 16.3% (95%-CI: 11.4–23.4). Our biopsy-confirmed documented date model finds a 1-year SR of 66.3% (95%-CI: 56.3–78.1) and a 3-year SR of 31.3% (95%-CI: 20.6–47.6). We found age ≥50 and higher education to be positively correlated with survival and a clinical presentation of advanced-stage disease to be negatively correlated.
Conclusions
This cohort study implies that rural populations have lower BC survival and hold implications for interventions to improve BC outcomes in SSA.
Similar content being viewed by others
Background
Breast cancer (BC) is one of the most prevalent and deadly cancers globally, affecting millions of women each year. With over 2.31 million new cases annually, BC is the second most common cancer and the most common of all female cancer diagnoses, making it a substantial health challenge worldwide [1, 2]. BC is the most frequent and the most deadly cancer in Africa [3]. Regional disparities in incidence and mortality rates imply varied burdens faced by different populations. While high-income countries (HICs) exhibit high incidence rates, Africa grapples with disproportionately high mortality rates [2]. Given that a significant proportion of BC patients are mothers, the loss of the primary caregiver often perpetuates suffering into the subsequent generation. Africa accounts for approximately 36% of the global population of maternal orphans due to cancer [4]. Consequently, orphaned children experience reduced survival prospects, potentially leading to an estimated 30% rise in mortality rates for sub-Saharan Africa (SSA) when assessing the next generation, further increasing the burden of BC [5].
In 2022, Africa accounted for 8.6% of global BC incidences, but 13.7% of global BC mortality [1]. This disparity indicates higher mortality rates from BC in Africa compared to the global average. However, these figures may be underestimated due to unreported cases, particularly in rural areas with insufficient cancer registries [6].
The elevated mortality rates observed in Africa, especially SSA, can be attributed to many different obstacles confronting patients before accessing appropriate treatment and care. A considerable proportion of BC cases are diagnosed at advanced stages, thereby limiting treatability [7]. This emphasizes the importance of early detection for mitigating cancer-related fatalities [8, 9].
Studies have investigated the barriers and enablers affecting BC care in low- and lower-middle income countries (LLMICs), revealing a nuanced healthcare pathway [10]. Factors at the individual level, such as cancer awareness, familial responsibilities, and geographical access to healthcare facilities, contribute to the delayed presentation of patients at advanced disease stages [11,12,13]. Additionally, inter-individual influences like cultural norms, gender dynamics, and societal stigma further prolong the time taken for patients to initiate healthcare-seeking behaviors and subsequently access timely interventions [14, 15].
Survival rates (SRs) for BC further illustrate the disparities in healthcare outcomes across global regions, with significant differences in both HICs vs. LLMICs and urban vs. rural settings [16, 17]. HICs boast increasing SRs, while SSA have not experienced the same improvements in survival during the past decades, with late diagnosis and treatment as the most important determinants of survival [18,19,20]. In Uganda, BC constitutes a significant health burden, ranking as the third most common cancer and the second most common cancer in women [21]. Despite the high prevalence of BC, survival data from rural regions, where healthcare accessibility and awareness might be limited, remain scarce.
We conducted a register-based retrospective survival analysis including 1-, 3-, and 5-year SRs in the rural Busoga Region in Uganda. Utilizing patient registers from the outreach palliative care NGO Rays of Hope Hospice Jinja (RHHJ), this study illuminates factors influencing BC survival among rural-living Ugandans. Through analysis of sociodemographic factors, HIV status and clinical presentation, the study endeavors to uncover determinants of SRs. Additionally, this study explores possible barriers to the disease trajectory and healthcare delivery in rural Uganda. By shedding light on the survival dynamics and healthcare practices of rural BC patients, this study aims to contribute to a deeper understanding of the disease burden in Uganda and be a basis for future studies that can identify points of intervention to improve patient outcomes and healthcare access in rural Uganda and SSA.
Methods
Study design
This cohort study is a register-based retrospective survival analysis, based on patient care sheets and home-visit notes from RHHJ archives.
All BC cases among RHHJ patients were drawn from the archives and included if they were enrolled between 01/01/2016 and 31/12/2022. If they were enrolled later than 31/12/2022 patients were included if they had an earlier clinical or biopsy-confirmed diagnosis from before 31/12/2022. The patient care sheets were updated and systematized at the beginning of 2016, why this period of data collection was chosen. Data was collected in February and March 2024, securing a minimum of one year follow-up time for all patients included.
The included patient care sheets were examined by the investigators. Patients were excluded if a BC diagnosis was later disproven. Clinical and biopsy-confirmed BC cases were included in the study [22].
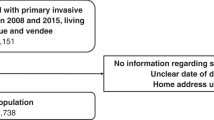
Complete data collection was done on 269 patients, 13 patients were excluded, see Fig. 1. Consequently, data analysis was done on the final 256 included patients making up the RHHJ-BC cohort.
Study setting
The investigation was carried out in partnership with RHHJ, an NGO specializing in palliative care situated in the Busoga Region of Uganda [23]. Palliative care services offered by RHHJ encompass home visits along with outreaches, roadside clinics, and hospice field offices, thus reaching rural populations that do not have health facilities in their vicinity. A significant majority of cancer patients referred to RHHJ present with advanced-stage disease [24]. In addition to delivering palliative care, RHHJ provides comprehensive treatment guidance, including referrals and post-treatment follow-ups, and financial assistance to poverty-stricken patients [24]. RHHJ covers all patients, notwithstanding religion, age, gender, or social affiliation. Cancer patients are enrolled regardless of disease severity. Thus, RHHJ offers a distinctive platform for investigating the SRs of BC patients in rural Uganda.
RHHJ’s patient care sheets contain extensive sociodemographic data alongside details concerning symptoms, clinical presentation, diagnosis, and treatment history of the patient’s disease. Around half of the enrolled patients are referred via Community Volunteers, designated by RHHJ to identify local individuals residing in rural areas in need of healthcare. The remaining patients are referred by healthcare professionals, via radio, other patients, friends, or relatives, contributing to a diverse referral network.
The Busoga region, spanning over 10.000 km2, encompasses vast rural areas with limited access to healthcare services [25]. Approximately 86% of the Busoga population resides in rural areas, necessitating extended travel to reach the nearest healthcare facility [25]. Furthermore, the population of Busoga faces notable economic challenges, with 24.3% living below the 2015 United Nations-defined international poverty line at $1,90 per day, and over 50% are classified as vulnerable to poverty in the event of adversities such as illness [26, 27].
Description of data
Data was collected from the patient care sheets, providing substantial information at enrollment and notes upon every revisit. A blank patient care sheet is available (see supplementary information). Information in patient care sheets and follow-up notes are collected through interviews conducted by an RHHJ clinical officer with relevant medical education. The sheets are updated every 14 to 30 days through home visits. The patient care sheets include information at enrollment in the RHHJ program covering comprehensive sociodemographic characteristics, BC symptom onset and development, HIV status and clinical presentation at enrollment. The clinical presentation at enrollment was found through a thorough objective examination of all enrolled patients. The physical examination aimed to assess the presence of lymphadenopathy in the axillary or internal mammary lymph nodes. Additionally, it evaluated whether the tumor had ulcerated the skin or infiltrated the chest wall, classifying it as ulcerating or infiltrative. Although no formal clinical staging of the BC was performed, patients presenting with lymphadenopathy and/or ulcerating/infiltrative tumors were most comparable to stage III or IV BC, as the tumors often exceeded 5 cm in size. The follow-up notes from home visits provide information on referral, diagnostic investigation, treatment and outcome.
Quantitative information was drawn directly from the patient care sheets, while qualitative information was transcribed into the note section of the data collection tool. Information concerning ‘history of illness’ often required interpretation or local knowledge, which was provided by the RHHJ clinical officers or other staff. This section in the patient care sheet also contained important information on the onset and development of BC symptoms and potential diagnosis and/or treatment before enrollment.
In this cohort study, we investigated all-cause survival time from different starting points. If the patient care sheet did not provide an exact date of a biopsy or clinical BC diagnosis the date was estimated systematically. If a month was mentioned the date was estimated to be the 15th. If a year was mentioned the date was estimated to be the 1st of July. If the patients already had a diagnosis before enrollment the starting point was the date of their clinical or biopsy-confirmed diagnosis. If the patients got a biopsy-confirmed diagnosis after enrollment, the date of the biopsy-confirmed diagnosis was chosen as the starting point. This way the starting point was sensibly chosen, so all individuals were as much on par as possible with their clinical or biopsy-confirmed diagnosis before, after or at enrollment. Initially, the symptom duration before enrollment was a numerical variable, but due to a lack of precise information from patients, the variable was changed to a categorical variable to increase reliability.
We examined causes for patients to be lost to follow-up (LTFU) and found various reasons. Some reasons were possibly associated with a negative disease state, such as patients moving to another part of Uganda, as they only had caregivers living there, or patients who did not want to be a part of the RHHJ program as they were afraid or did not believe in conventional medicine. Other reasons were possibly associated with a positive disease state, such as patients being removed from the RHHJ program, as they no longer required palliative care or assistance from other healthcare services. These were also considered LTFU, as they were not confirmed curatively treated.
Validation of information
To validate the quality of the information provided in the patient care sheets, we performed a random selection of re-interviews, similar to enrollment interviews, on active patients, supported by a translator. This was done to discover potential recall bias or other unforeseen information problems. The validation procedure revealed some recall bias, especially concerning dates of clinical diagnosis and biopsy, when no documentation was available. This was alleviated by only including exact dates of diagnosis when there were printed medical documents stating the exact date of diagnosis. The validation also revealed that the reported age is inaccurate as some of the women did not know their birthday or birth year. This led us to dichotomize the age group to </≥ the median of 50 years for the Cox proportional hazards model. We also observed an inaccuracy concerning educational level, as some would report having no education if they did not have papers or diplomas to prove it, leading us to compare wider grouped variables.
Data management
Data was drawn from the records and entered into a tailored data collection tool using REDCap software, ensuring uniform data entry and secure storage of patient records. The data collection tool is available (see supplementary information). A pilot test was conducted on the REDCap form to validate its comprehensiveness. To enhance data consistency and accuracy, validation measures, such as multiple researchers entering patient data from the same record and checking for possible discrepancies, were implemented to ensure uniform data entry practices.
Basic descriptive statistics were analyzed using Microsoft Excel [28]. Mean, standard deviation, median and proportion are applied to describe our results.
Survival analysis was performed using R and RStudio software version 4.3.1. for Windows including packages “survival”, “survminer”, and “ggsurvfit” [29]. Two survival models were created. One for patients with a documented date of biopsy and one for all included patients with the compiled date of diagnosis. Results are presented in two separate Kaplan-Meier plots with risk tables. The compiled diagnosis model is composed of the following diagnosis dates: documented date of biopsy, estimated date of biopsy, estimated date of clinical diagnosis before enrollment and date of enrollment with clinical diagnosis.
For the “date of enrollment” group, the enrollment in RHHJ represents the first registered encounter with cancer-diagnosing healthcare professionals. Survival time was defined as the time from diagnosis to the event of all-cause mortality. Active, remitted, and LTFU patients all had censored events. Events were censored on the date of death. LTFU and remitted patients were censored on the date of the last contact they had with RHHJ. Active patients were also censored on the date of the last contact with RHHJ, which was either in January or February 2024.
A Cox proportional hazards model was applied to assess the association between patient outcomes and ten sociodemographic and clinical variables. These variables were selected to gauge their impact on BC survival. Schoenfeld residuals (see supplementary information) were evaluated to assess the variable’s variance over time at a significance level of p < 0.05. HRs with 95% CIs are reported at a significance level of p < 0.05.
Chi-square tests for goodness-of-fit were used to assess the sociodemographic and clinical variable differences between the LTFU patients and the other patients. The significance level was set to p < 0.05.
Results
Table 1 shows the sociodemographic characteristics of the 256 included BC patients. The patients were between 19 and 88 years old, with the median age being 50. The largest proportion of patients were from 30 to 49 years old. 54.3% of patients had 1 or more dependents, with the range being from 0 to 13.
Table 2 shows the descriptive statistical results of the enrolled patients. 65.6% had a critical clinical presentation suggesting advanced-stage disease at enrollment in the RHHJ program. 64.5% reported having had symptoms >1 year before enrollment and 22.3% >3 years. This needs to be considered in the context of a mean time from prior diagnosis (either clinical or biopsy-confirmed) to enrollment being 6 months. 68.8% were referred to treatment, 38.7% before enrollment, while 30.1% were referred after enrollment. Where 68.8% were referred within one month after enrollment, 28.6% were referred later than that. 31.3% were not referred to treatment. Only 49.6% received cancer-directed treatment such as chemotherapy, surgery and radiotherapy outside of the RHHJ palliative program, with a majority attending Mulago Uganda Cancer Institute. This constitutes 72.2% of patients referred for treatment. 22% started treatment within one month of enrollment, 61% within 1–6 months, and 12% started treatment more than 6 months after enrollment to the RHHJ program.
Table 3 shows the distribution of “events”. Of the 256 patients, 172 died, 8 went into remission, 41 were LTFU and 35 were alive, but with active disease by the end of our observation. Furthermore, it shows the distribution of starting points. 78 women in the cohort had a documented biopsy, 48 had a biopsy, but were not able to report an exact date, 49 were clinically diagnosed at another institution than RHHJ, and the rest were diagnosed at enrollment.
Figure 2 shows distinction in all-cause survival duration where patients with a documented date of biopsy have longer survival time compared to the compiled model cohort. The 3- and 5- year SRs in the biopsy (documented date) only model was the same, as no event happened after 3 years.
Figure 3 presents four variables significantly associated with patient survival. Age ≥50 years was shown to be a positive factor for survival compared to those aged <50 years. Having a higher educational attainment was associated with improved survival outcomes. Lastly, those with a clinical presentation of advanced disease: lymphadenopathy and/or ulcerating/infiltrative tumor, had a higher mortality risk, than those without. No association was found between HIV infection and BC survival.
Contingency-tables and chi-square test results for LTFU compared to not LTFU patients, for differences in sociodemographic and clinical variables, found all results insignificant except that LTFU had significantly less lymphadenopathy than the patients who were not LTFU, with a chi-square value of 11.94 and p-value < 0.01. There was also a trend, though insignificant, toward a difference in the proportion of patients presenting with ulcerative/infiltrative tumors with a chi-square value of 3.20 and p-value = 0.07 (see supplementary information).
Discussion
Our compiled diagnosis model includes all patients in the cohort, providing as accurate starting points as possible. Our 1-, 3- and 5-year SRs are significantly lower than those found in comparable settings. Our 1-, 3- and 5-year BC SRs estimates are 57.7%, 19.1% and 16.3%, respectively, while other studies on BC SRs from SSA present 1-year SRs from 78 to 91% [16, 30,31,32], 3-year SRs from 50 to 72% [16, 30, 31, 33, 34] and 5-year SRs from 24 to 61% [30, 31, 35,36,37,38,39].
These differences in our results could be explained by our study included a larger proportion of clinical diagnoses but could also be indicative of the rural/urban inequality in access to healthcare. The study by Shita, A., et al. from 2023 estimated a 3-year survival of 27.5% in the rural group, which is closer to our result, and identified rural living as an independent predictor of mortality [34]. However, there are important aspects to consider when evaluating our results.
Information bias
Survival estimates from this study are based on data collected by RHHJ, whose primary field is palliative care. This gives rise to the procedure of estimating the date where the patient initially was biopsy-confirmed or clinically diagnosed. The proportion of estimated starting points constituting 37.9% of the cohort can induce inaccuracies in the survival estimates as they are based on memory and interpretations of clinical records. It is unclear whether this under- or overestimates the SRs.
When only including those with a documented date of biopsy, the survival increases, but this group might constitute women with higher health literacy, educational level or resources in general. Biopsies have a substantial cost, as a considerable number of patients stated in their clinical records not to have the financial resources to obtain a biopsy. Biopsies also have consequences for further treatment as many health facilities do not make mastectomies or give chemotherapy and radiotherapy before having biopsy results. This could lead to a positive selection of patients with biopsy-confirmed diagnoses.
Delay
We observed a general tendency towards delay in taking contact to health facilities (64.5% reported to have had symptoms >1 year before being enrolled) and some resistance in seeking further diagnosis and attending treatments, where only 49.6% of patients got treatment which constitutes 72.2% of referred patients. Table 2 presents various factors of delay in diagnosis and treatment but should be considered in the context of a hospice setting. For 40% of the patients, RHHJ was their initial contact with a diagnostic health facility, while the rest of the patients had been in contact with other health institutions before enrollment in RHHJ. We estimated the mean time from diagnosis to enrollment to be 6 months, which should be considered when examining delays in diagnosis and treatment. Still, it is worrying that 22.3% reported having symptoms more than 3 years before enrollment and 65.6% had a critical clinical presentation at enrollment, causing a reduced possibility of effective treatments. This corresponds with the 31.3% that were not referred for treatment. It may also explain the relatively low proportion of biopsy-confirmed diagnoses. A study on the African Breast Cancer-Disparities in Outcomes (ABC-DO) cohort found a negative association between late disease stage at diagnosis and treatment receipt [16, 40]. Other studies from rural SSA also found that the majority of BC patients were diagnosed at advanced stages, significantly reducing their survival [32, 34].
It is also notable that survival in our cohort decreases significantly from 1 to 3 years but appears to stabilize by year 5. This pattern may, in part, be due to the fact that many patients do not receive treatment, which substantially impacts their survival chances, while those who do receive treatment continue to survive beyond 3 and 5 years. As shown, just under half of the patients in the cohort received cancer-directed treatment. A similar trend was observed in another study, where SRs varied based on adherence to chemotherapy [34]. This pattern could also reflect the poor prognosis of breast cancer, especially given that many women are diagnosed at an advanced stage. The heterogeneity of breast cancer tumors, when considering aggressiveness, could also cause a steep initial decline. However, it is important to consider that the small sample size at year 5, may also influence this trend. Therefore, it is more accurate to focus on our survival results at year 1 and year 3.
Selection bias
The cohort had an LTFU proportion of 16%. As stated in the methods section, reasons for being LTFU are possibly associated with negative or positive disease states affecting survival time. Our chi-square test reveals that significantly fewer patients who were LTFU had lymphadenopathy. As presented in Fig. 3, a clinical presentation with lymphadenopathy had a significant effect on survival, why we must consider that patients who were LTFU had a higher survival probability than the rest of our patients, possibly skewing our results by underestimating the actual SRs.
Risk factors for breast cancer mortality
We identified young age as a risk factor for shorter BC survival. Notably, the majority of our participants were aged 30–49 years. A review also indicated a higher BC incidence in younger age groups in SSA compared to HICs [41]. However, demographic differences complicate this comparison: only 2% of Ugandan women are over 65, compared to 24% of European women, with Uganda’s median age at 15.9 years and Europe’s at 45.5 years [42, 43].
The ABC-DO cohort finds a U-shaped association between age and mortality, revealing the greatest risk for the youngest and oldest included patients, which is also found in large cohorts from HICs [16, 44, 45]. After adjusting for background mortality, the U-shape disappears in the ABC-DO cohort, leaving only the youngest at a higher risk. Reasons for this are presented in studies that indicate BC in young women is more aggressive, has a worse prognosis, and is often characterized by histological grade 3 tumors and negative hormone receptors [46,47,48,49].
We found no association between HIV infection and BC survival. Other studies have different results concerning HIV infection and the possible negative contribution to survival, e.g. Cubasch et al. do not find an association, but McCormack et al. do find a negative contribution of simultaneous HIV infection, with a HR of 1.48 [16, 30]. The limited number of patients with HIV in our cohort (n = 16) restricts the strength of any conclusions regarding the association between HIV infection and BC survival.
Assessing barriers to treatment
We found that 65.6% of BC patients are enrolled at an advanced disease stage. This high proportion corresponds with a meta-analysis from 2016 on BC stage at diagnosis in SSA, where the median proportion of late stage (stage III/IV) was 74.7% [50]. Moreover McCormack et al. finds that enhancements in early diagnosis are anticipated to yield the most significant survival gains among survival determinants for BC in SSA [16]. Where there are no nationwide or large-scale BC screening programs in SSA, very few asymptomatic or slowly evolving breast tumors are discovered, causing lead and length time bias when compared to SRs in HICs that do have screening programs [51]. A review on BC screening in SSA finds clinical BC examination to be more cost-effective than mammography [51]. So, implementation of clinical BC examination, or campaigns on manual BC screening, in SSA could be a viable alternative, though numerous sociocultural factors are found to hinder preventative health behaviors, and should therefore be accounted for [52,53,54].
The rural setting poses a variety of barriers in diagnosis and treatment, a frequently registered reason in our study being financial constraints. Other examples were fear of procedures, use of herbal medicine, husbands disapproving of patients seeking help and lack of biopsy needles at local hospitals. None of this information was systematically collected, and therefore only presented here as possible reasons for the delay and the low proportion of biopsy-confirmed diagnoses. These factors reduce the SRs but are part of the rural setting and points of action towards intervention.
Adding to limited healthcare facilities, healthcare knowledge, attitudes and practices also represent a barrier to the treatment of BC. Other studies find a positive association between higher education and positive attitudes and practices towards healthcare, and as rural areas in Uganda have lower educational levels this adds to the barriers to treatment [55, 56]. In our study, we observed that a higher educational level was associated with improved survival outcomes, potentially reflecting better health-seeking behaviors, higher socioeconomic status, and greater health literacy, which may facilitate earlier diagnosis and access to care.
Investigating the knowledge, attitudes and practices of women in SSA concerning BC will help to identify and uncover the behavioral obstacles concerning BC healthcare in specific settings. Finally, this area needs action from global institutions, local governments, NGO stakeholders and the research community to see our current and future scientific understanding turn into health care for some of the most vulnerable people of the world. This could lead the way for interventions concerning the World Health Organization’s Global Breast Cancer Initiative’s (GBCI) three pillars: health promotion for early detection, timely diagnosis, and comprehensive BC management [57].
Limitations
BC survival studies in SSA are prone to selection bias, as patients with low resources attend healthcare facilities less [58]. We have tried to avoid this selection bias by investigating a rural population. As this study relies on patient records from a healthcare provider rather than being strictly population-based, it cannot be categorized as such. Given the inclusive nature of patient enrollment at RHHJ, and as they serve as one of the few consistent providers of cancer care in the region, it is estimated a reasonable and representative source for understanding the rural population in Uganda. Furthermore, the study does not fully represent rural Uganda, as it also includes patients from urban settings enrolled in RHHJ’s program. As stated in the methods section, approximately 14% of Busoga’s population live in urban settings [26]. These people have easier access to healthcare services and could therefore constitute a greater proportion in our study. This is alleviated by RHHJ’s raison d'être which is to help those who live furthest away from healthcare services, as described in the methods section. We believe that compared to other survival studies conducted in SSA, our study is more representative of rural populations in SSA.
The World Bank’s definition of rural and urban is made by national statistical offices, where the Uganda Bureau of statistics define urban areas as gazetted cities and towns [59, 60]. Cross-country comparisons should therefore be made with caution, as estimates are based on national definitions. Of the BC survival studies from SSA that include rural variables, the ABC-DO cohort defines rural from patients’ self-declaration in their questionnaires, Eber-Schulz et al. uses the definition from the Central Statistical Agency of Ethiopia and Shita, A., et al. does not disclose the basis for the definition of rural from their included medical records [16, 32, 34]. Therefore, there is a need for a more standardized definition of rural and urban populations that can be adapted to different contexts, to facilitate comparability in global health research.
A limitation to our study is that our information is based on data from RHHJ, therefore we do not have exhaustive information on patients’ disease and treatment history. Furthermore, there is no information on disease staging as the charts do not provide a full record of diagnostic procedures. Access to diagnostic imaging for assessing metastases (e.g., liver, bone, or lung) was very limited, making systematic staging unfeasible. Instead, we relied on detailed clinical examinations to assess features such as lymphadenopathy and tumor ulceration or infiltration. The Essential TNM framework developed by the International Agency for Research on Cancer, is an approach for low-resource settings where full staging data are unavailable [61]. While we considered its retrospective use, inconsistent clinical documentation and missing diagnostic data prevented meaningful application in this cohort. Though we emphasize the value of simplified staging tools for future studies in similar settings.
As shown in Table 2 we only have information on time to referral and treatment for patients after enrollment, even though some were treated before enrollment. As RHHJ was not in all cases the first contact with a healthcare facility, our data on clinical presentation, lymphadenopathy and infiltrative tumors, is not necessarily representative of the clinical presentation at time of diagnosis.
A great limitation to this study is the small study size and the relatively short follow-up time, as few BC patients in rural SSA live longer than ≥3 years. As a natural consequence of fewer patients being at risk, the 3- and especially 5-year survival estimates are less reliable than the 1-year survival. Therefore, studies with larger sample size and longer follow-up are needed.
Conclusions
BC in SSA exhibits lower survival compared to HICs. Our results show that BC in rural Uganda exhibits an even lower survival.
BC barriers in SSA are multifaceted. A large proportion of our cohort presented with advanced-stage disease. We therefore need to understand factors contributing to delays in seeking care and promote early detection. We find a positive association between enhanced education and improved survival outcomes, potentially reflecting better health-seeking behaviors. However, further research is needed to explore the barriers and enablers for timely BC diagnosis in rural SSA.
When tailoring interventions the GBCI should stand as a guideline [57]. We found that advanced-stage disease, resulting from delayed detection, has a substantial impact on BC survival. Health promotion for early detection is the first pillar of the GBCI. We consider delayed presentation to be the primary barrier, and if addressed it could improve the possibility of timely diagnosis and, in turn, enhance BC management, which are the two other pillars of the GBCI.
Further BC research and interventions are imperative to improve survival of BC patients in rural Uganda and SSA. An improved survival of BC will affect numerous women, men and families, with extensive implications for future generations.
Data availability
The data set of this study is the common property of Aarhus University, Denmark and Rays of Hope Hospice Jinja, Uganda. The data set may be available upon request to the corresponding author.
Code availability
The data set and computer code of this study is the common property of Aarhus University, Denmark and Rays of Hope Hospice Jinja, Uganda. The data set and the computer code used may be available upon request to the corresponding author.
References
Breast cancer fact-sheet. (International Agency for Research on Cancer, World Health Organisation (WHO), Global Cancer Observatory, 2024).
Cancer fact-sheet World. (International Agency for Research on Cancer, World Health Organisation (WHO), Global Cancer Observatory, 2024).
Cancer fact-sheet Africa 2022. (International Agency for Research on Cancer, World Health Organisation (WHO), Global Cancer Observatory, 2024).
Guida F, Kidman R, Ferlay J, Schuz J, Soerjomataram I, Kithaka B, et al. Global and regional estimates of orphans attributed to maternal cancer mortality in 2020. Nat Med. 2022;28:2563–72.
Mailhot Vega RB, Balogun OD, Ishaq OF, Bray F, Ginsburg O, Formenti SC. Estimating child mortality associated with maternal mortality from breast and cervical cancer. Cancer. 2019;125:109–17.
Cumber SN, Nchanji KN, Tsoka-Gwegweni JM. Breast cancer among women in sub-Saharan Africa: prevalence and a situational analysis. Southern African. J Gynaecol Oncol. 2017;9:35–37.
Moodley J, Constant D, Mwaka AD, Scott SE, Walter FM. Mapping awareness of breast and cervical cancer risk factors, symptoms and lay beliefs in Uganda and South Africa. PLoS ONE. 2020;15:e0240788.
Odongo J, Makumbi T, Kalungi S, Galukande M. Patient delay factors in women presenting with breast cancer in a low income country. BMC Res Notes. 2015;8:467.
Moodley J, Constant D, Mwaka AD, Scott SE, Walter FM. Anticipated help seeking behaviour and barriers to seeking care for possible breast and cervical cancer symptoms in Uganda and South Africa. Ecancermedicalscience. 2021;15:1171.
Ilaboya D, Gibson L, Musoke D. Perceived barriers to early detection of breast cancer in Wakiso District, Uganda using a socioecological approach. Global Health. 2018;14:9.
Foerster M, McKenzie F, Zietsman A, Galukande M, Anele A, Adisa C, et al. Dissecting the journey to breast cancer diagnosis in sub-Saharan Africa: Findings from the multicountry ABC-DO cohort study. Int J Cancer. 2021;148:340–51.
Scheel JR, Giglou MJ, Segel S, Orem J, Tsu V, Galukande M, et al. Breast cancer early detection and diagnostic capacity in Uganda. Cancer. 2020;126:2469–80.
Togawa K, Anderson BO, Foerster M, Galukande M, Zietsman A, Pontac J, et al. Geospatial barriers to healthcare access for breast cancer diagnosis in sub-Saharan African settings: The African Breast Cancer-Disparities in Outcomes Cohort Study. Int J Cancer. 2021;148:2212–26.
McCutchan, G, Weiss, B, Quinn-Scoggins, H, Dao, A, Downs, T, Deng Y, et al. Psychosocial influences on help-seeking behaviour for cancer in low-income and lower middle-income countries: a mixed-methods systematic review. BMJ Glob Health 2021;6.
Mwaka AD, Walter FM, Scott S, Harries J, Wabinga H, Moodley J. Symptom appraisal, help-seeking and perceived barriers to healthcare seeking in Uganda: an exploratory study among women with potential symptoms of breast and cervical cancer. BMJ Open. 2021;11:e041365.
McCormack V, McKenzie F, Foerster M, Zietsman A, Galukande M, Adisa C, et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): a prospective cohort study. Lancet Glob Health. 2020;8:e1203–e1212.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Niksic M, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75.
Maajani K, Jalali A, Alipour S, Khodadost M, Tohidinik HR, Yazdani K. The global and regional survival rate of women with breast cancer: a systematic review and meta-analysis. Clin Breast Cancer. 2019;19:165–77.
Kantelhardt EJ, Cubasch H, Hanson C. Taking on breast cancer in East Africa: global challenges in breast cancer. Curr Opin Obstet Gynecol. 2015;27:108–14.
Joko-Fru WY, Griesel M, Mezger NCS, Hammerl L, Seraphin TP, Feuchtner J, et al. Breast Cancer Diagnostics, therapy, and outcomes in sub-saharan africa: a population-based registry study. J Natl Compr Canc Netw 2021;20.
Cancer fact-sheet Uganda, 2022. (International Agency for Research on Cancer, World Health Organisation (WHO), Global Cancer Observatory, 2024).
World Health Organisation, International Classification of Diseases 11th Revision. 2023.
Rays of Hope Hospice Jinja, RHHJ - Who We Are And What We Do. 2022.
Rays of Hope Hospice Jinja, Annual Report. 2023.
Busoga Health Forum, Baseline Survey Report for the Busoga Region. 2017.
Busoga Health Forum, Busoga Health Forum Profile. Vol. 2024.
UN Statistics Devision, SDG indicator metadata. International Poverty Line.
Microsoft Excel. (Microsoft Corporation, 2024).
Team, R.C. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, V.A., 2024).
Cubasch H, Dickens C, Joffe M, Duarte R, Murugan N, Tsai Chih M, et al. Breast cancer survival in Soweto, Johannesburg, South Africa: A receptor-defined cohort of women diagnosed from 2009 to 11. Cancer Epidemiol. 2018;52:120–7.
Joko-Fru WY, Miranda-Filho A, Soerjomataram I, Egue M, Akele-Akpo MT, N’da G. et al. Breast cancer survival in sub-Saharan Africa by age, stage at diagnosis and human development index: A population-based registry study. Int J Cancer. 2020.
Eber-Schulz P, Tariku W, Reibold C, Addissie A, Wickenhauser C, Fathke C, et al. Survival of breast cancer patients in rural Ethiopia. Breast Cancer Res Treat. 2018;170:111–8.
Ssentongo P, Oh JS, Amponsah-Manu F, Wong W, Candela X, Acharya Y, et al. Breast cancer survival in eastern region of Ghana. Front Public Health. 2022;10:880789.
Shita A, Yalew AW, Seife E, Afework T, Tesfaw A, Gufue ZH, et al. Survival and predictors of breast cancer mortality in South Ethiopia: a retrospective cohort study. PLoS ONE. 2023;18:e0282746.
Ntekim AI, Folasire AM, Ali-Gombe M. Survival pattern of rare histological types of breast cancer in a Nigerian institution. Pan Afr Med J. 2019;34:114.
Zingue S, Atenguena EO, Zingue LL, Tueche AB, Njamen D, Nkoum AB, et al. Epidemiological and clinical profile, and survival of patients followed for breast cancer between 2010 and 2015 at the Yaounde General Hospital, Cameroon. Pan Afr Med J. 2021;39:182.
Zongo N, Ouedraogo S, Korsaga-Some N, Some OR, Go N, Ouangre E, et al. Male breast cancer: diagnosis stages, treatment and survival in a country with limited resources (Burkina Faso). World J Surg Oncol. 2018;16:4.
Galukande M, Wabinga H, Mirembe F. Breast cancer survival experiences at a tertiary hospital in sub-Saharan Africa: a cohort study. World J Surg Oncol. 2015;13:220.
Makanjuola SB, Popoola AO, Oludara MA. Radiation therapy: a major factor in the five-year survival analysis of women with breast cancer in Lagos, Nigeria. Radiother Oncol. 2014;111:321–6.
Foerster M, Anderson BO, McKenzie F, Galukande M, Anele A, Adisa C, et al. Inequities in breast cancer treatment in sub-Saharan Africa: findings from a prospective multi-country observational study. Breast Cancer Res. 2019;21:93.
Anyigba CA, Awandare GA, Paemka L. Breast cancer in sub-Saharan Africa: The current state and uncertain future. Exp Biol Med. 2021;246:1377–87.
Population ages 65 and above, female (% of female population) - Uganda. in United Nations Population Division. World Population Prospects. Vol. 2024 (The World Bank).
Eurostat EU. Median age increased by 2.3 years since 2013. (European Union).
Freedman RA, Keating NL, Lin NU, Winer EP, Vaz-Luis I, Lii J, et al. Breast cancer-specific survival by age: Worse outcomes for the oldest patients. Cancer. 2018;124:2184–91.
Zhao XR, Tang Y, Wu HF, Guo QS, Zhang YJ, Shi M, et al. Influence of age as a continuous variable on the prognosis of patients with pT1-2N1 breast cancer. Breast. 2022;66:136–44.
Winchester DP, Osteen RT, Menck HR. The National Cancer data base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78:1838–43.
Chung M, Chang HR, Bland KI, Wanebo HJ. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996;77:97–103.
Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS ONE. 2009;4:e7695.
Han W, Kang SY.& Korean Breast Cancer Relationship between age at diagnosis and outcome of premenopausal breast cancer: age less than 35 years is a reasonable cut-off for defining young age-onset breast cancer. Breast Cancer Res Treat. 2010;119:193–200.
Jedy-Agba E, McCormack V, Adebamowo C, Dos-Santos-Silva I. Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2016;4:e923–e935.
Martei YM, Dauda B, Vanderpuye V. Breast cancer screening in sub-Saharan Africa: a systematic review and ethical appraisal. BMC Cancer. 2022;22:203.
Sarmah N, Sibiya MN, Khoza TE. The sociocultural influences on breast cancer screening among Rural African Women in South Africa. Int J Environ Res Public Health 2023;20.
Magwesela FM, Msemakweli DO, Fearon D. Barriers and enablers of breast cancer screening among women in East Africa: a systematic review. BMC Public Health. 2023;23:1915.
Tetteh DA, Faulkner SL. Sociocultural factors and breast cancer in sub-Saharan Africa: implications for diagnosis and management. Womens Health. 2016;12:147–56.
Cutler DM, Lleras-Muney A. Understanding differences in health behaviors by education. J Health Econ. 2010;29:1–28.
Uganda Bureau of Statistics (UBOS). The Uganda National Household Survey Report 2019/2020. 2021.
Global Breast Cancer Initiative Implementation Framework Assessing, strengthening and scaling up services for the early detection and management of breast cancer. (Worlds Health Orgaization, 2023).
Gbenonsi G, Boucham M, Belrhiti Z, Nejjari C, Huybrechts I, Khalis M. Health system factors that influence diagnostic and treatment intervals in women with breast cancer in sub-Saharan Africa: a systematic review. BMC Public Health. 2021;21:1325.
The World Bank. DataBank. Metadata Glossary. Rural population. 2024.
Uganda Bureau of Statistics (UBOS). Statistical Glossary. Urban population. 2024.
The International Agency for Research on Cancer. User’s Guide to Essential TNM. (ed. (WHO), W.H.O.) 2022.
Acknowledgements
The research team expresses gratitude to all participants for their dedicated effort and thoughtful contributions. We thank the whole Rays of Hope Hospice Jinja staff and the Research Unit for Global Health at Aarhus University for their immense support of the project.
Funding
The research team thank the funding organization William Demant Fonden under Grant 23–4381. The fund contributed with finances for travel expenses between Uganda and Denmark for the main author.
Author information
Authors and Affiliations
Contributions
The corresponding author, R.K., has had full access to the data in the study and has final responsibility for the decision to submit for publication. All authors contributed to the study's conception and design. Study preparation and data collection were performed by R.K., K.B.L. and T.A.E., under the consultancy of John M. and Joanita M. The analysis was performed by R.K., K.B.L. and T.A.E. The main manuscript was written by R.K. and K.B.L. under the consultancy of P.K. and T.A.E., and all other authors have reviewed previous versions of the manuscript. All authors have reviewed and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was granted by the Uganda Cancer Institute Research and Ethical Committee (UCI-2023-80). All patients of Rays of Hope Hospice Jinja are provided with a consent form, which is thoroughly explained to them before they consent to enroll. This form contains an option to allow for research to be performed based on their patient care sheet. Subsequently, at each visit, patients are asked to reaffirm their consent to ensure ongoing informed consent. All patients included in this study provided informed consent. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
All patient information has been individually revised and collected using the REDCap software program. REDCap is under the license of Aarhus University, and access to the database is restricted to the researchers only. Under the collection of data, no identifying, personal information has been collected, and patients have been separated using a random record ID for reference, ensuring anonymization of all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kallestrup, R., Lorentzen, K.B., Mwayi, J. et al. Breast cancer survival in a rural setting in the Busoga Region of Uganda. BJC Rep 3, 56 (2025). https://doi.org/10.1038/s44276-025-00166-x
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44276-025-00166-x