Abstract
Background
Colorectal cancer (CRC) is a common malignancy, with mismatch repair deficient (dMMR) CRC comprising approximately 15% of non-metastatic cases. dMMR tumors generate neoantigens making them highly responsive to immune checkpoint inhibitors, this became the first-line treatment for metastatic dMMR CRC. Aim of this study is to evaluate the efficacy of single-agent Pembrolizumab for patients with locally advanced unresectable dMMR intestinal cancers.
Methods
We retrospectively reviewed patients with locally advanced unresectable dMMR/MSI-H CRC or small intestinal adenocarcinoma (SIA) who received PD-1 inhibitors between January 2022 and December 2023 at 1 University Medical Center and 3 regional hospitals and analyzed the treatment efficacy and survival outcomes.
Results
Response rate was 78% after at least one cycle of Pembrolizumab with conversion to resection in nearly 40%. Patients who underwent primary tumor resection had a two-year overall survival (OS) of 100%, whereas those without resection had significantly lower OS (42%), progression-free survival (PFS; 36%), and cancer-specific survival (CSS; 71%) at two years. In total, 22% of the patients discontinued the treatment due to toxicity.
Discussion
Although the observed response rates of Pembrolizumab are high, there is still room for improvement. Dual immune checkpoint inhibitors might be needed for these patients to improve outcomes.
Clinical Trial Registration
A selection of patients included in this study were part of the ATAPEMBRO study, a single-centre, open label, phase 1-2 study (NCT04014530).
Similar content being viewed by others
Background
Colorectal cancer (CRC) is one of the most common malignancies worldwide, and accounts for 9.6% of all cancer diagnoses [1]. Microsatellite instable (MSI) or mismatch repair deficient (dMMR) colorectal cancer accounts for nearly 15% of all non-metastatic CRCs and even up to 30% in small intestinal carcinoma (SIA). It is characterized by a defect in DNA mismatch repair proteins subsequently resulting in microsatellite instability-high (MSI-H) phenotype, leading to the accumulation of mutations and the generation of neoantigens. These neoantigens stimulate the anti-tumor immune response and make these tumors highly sensitive for immune checkpoint inhibition [2,3,4,5].
Immunotherapy has profoundly changed the treatment landscape for this patient group, that historically responds poorly to standard chemotherapy schedules. As shown in the Keynote 177 trial, treatment of dMMR CRC with checkpoint inhibitors resulted in high response rates with long duration of response. Based on the survival benefit with an acceptable toxicity profile, Pembrolizumab is now considered standard first line systemic treatment in patients with metastatic dMMR CRC. Currently, multiple ongoing studies are examining the effect of different treatment protocols for patients with microsatellite instability-high or mismatch repair-deficient metastatic CRC.
The COMMIT trial (NCT02997228) studied the benefit of the addition of chemotherapy or CTLA-4 blockade to PD-1 or PD-L1 inhibitors [6], whereas K-1308A-008 (NCT04895722) [7] and the recently published CheckMate 142 and CheckMate 8HW studied the effect of immune-checkpoint inhibitor-based combination immunotherapy in patients with metastatic dMMR CRC [8, 9].
In patients with localized dMMR CRC neo-adjuvant immunotherapy is potentially even more effective. The NICHE, NICHE 2 and NICHE 3 studies showed high rates of complete pathological responses after short treatment with dual checkpoint inhibitors with an acceptable safety profile, showing that this treatment strategy has promising results for this patient population [10,11,12]. However, unresectable and high-risk resectable dMMR CRCs were not part of the NICHE trials. Neoadjuvant chemotherapy is hardly effective in dMMR CRC, as shown in the phase 3 FOxTROT trial in colon cancer and the trial published by Cercek et al. in rectal cancer [13, 14]. Defining the optimal induction regime for patients with locally advanced unresectable dMMR intestinal cancer is an unmet clinical need.
Here, we describe a multi-center case series of patients with locally advanced, unresectable dMMR colorectal and small intestinal cancers (SIAs) that received Pembrolizumab, either as monotherapy or together with Ataluren as part of a clinical trial (NCT04014530), to explore the benefit of Pembrolizumab in these cancers.
Methods
Patients and treatment
We retrospectively reviewed all patients with dMMR/MSI-H CRC who received PD-1 inhibitors between January 2022 and December 2023 at Amsterdam UMC, Meander MC, Catharina MC and Haaglanden MC. Patients were eligible for inclusion if they were at least 18 years of age, known with histopathological confirmed dMMR/MSI-H CRC or SIA in which the primary tumor was deemed unresectable or high risk resectable, without distant metastases and who received at least one dose of Pembrolizumab. High-risk resectable tumors were defined as tumors with T4 tumors with invasion through the visceral peritoneum and/or directly invading adjacent organs or structures and/or presence of extensive lymphnode metastases needing induction treatment in order to reduce the complication risk of an intentional operation, as discussed in a multidisciplinary team meeting. The status of dMMR/MSI-H was determined by immunohistochemical analysis for MLH1, PMS2, MSH2, and MSH6 proteins, and deficiency was specified as the absence of staining of one or more proteins. If immunohistochemical analysis was not available, the status of dMMR/MSI-H was determined by PCR as per the clinical routine of the participating centers. The decisions to treat patients with PD-1 inhibitors instead of surgery were made by the primary surgeons of the participating centers, with the most common reason being to avoid a multivisceral resection. Patients who were included in the ATAPEMBRO study, a single-centre, open label, phase 1–2 study, were treated with Pembrolizumab 200 mg in 3 weekly cycles in combination with Ataluren, an orally administered drug that facilitates read-through-translation, continuously three times a day. Other patients received Pembrolizumab 200 mg in a three week cycle for three times followed by cycles every 6 weeks with a treatment evaluation every 3 months.
All patients signed the informed consent form, either through the ATAPEMBRO trial (NCT04014530) or under the approval of the institutional review board (IRB) of the Amsterdam UMC (IRB00013752).
This study was performed in accordance with the Declaration of Helsinki and approved to waive informed patient consent by the Medical Research Ethics Committee (MREC) of Amsterdam UMC due to the observational and noninterventional study. Therefore, the Medical Research Involving Human Subjects Act (WMO) did not apply to this study.
Data collection and statistic analysis
Demographic and clinicopathological data of patients were collected from the electronic patient files, including tumor staging, tumor location, MMR/MSI status, RAS/BRAF mutation status, serum CEA levels at baseline, tumor radiological response, pathological response, progression free survival (PFS) and overall survival (OS). Treatment characteristics were collected, including the number of cycles, treatment interruption and whether or not the tumor was resected. When resected, the pathological tumor response was recorded. Follow-up data were collected until June 2024, or until patient was lost to follow-up. Details on treatment toxicity were collected and classified using the Common Terminology Criteria for Adverse Events (CTCAE) score [15].
Descriptive statistics were used to analyze the demographic and clinicopathological data, using IBM SPSS statistics (version 28). A Kaplan-Meier plot was used to analyze the PFS and OS at 1 and 2 years. A swimmers-plot was made to graphicly illustrate the treatment response of all patients, using R studio (version 4.2.1).
Results
Patient characteristics
In total, 14 patients with locally advanced unresectable dMMR CRC and 4 patients with SIA (three duodenal and one jejunal) were identified. Of those patients, 10 received Pembrolizumab and ataluren combination therapy. Baseline characteristics are reported in Table 1. The included patients had a median age of 67 years and were predominantly female (61%). One of the patients was diagnosed with Lynch Syndrome. Two patients received systemic treatment consisting of CAPOX prior to treatment with Pembrolizumab. The median follow-up period was 14 months (IQR 5-34). All individual characteristics can be found in Table 1.
Treatment and outcome
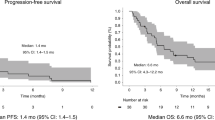
All 18 patients received at least one cycle of Pembrolizumab, with a mean of 9 cycles (range 2–34) and a median duration of 8.5 months (IQR 4–14). In total, ten patients received Pembrolizumab and ataluren combination therapy. The radiological response was available in 16 patients, one patient died before radiological response was measured. In total, 4 patients (22%) showed complete radiological response, 9 patients (50%) showed partial response, one patient (5.6%) showed stable disease and progressive diseases was observed in two (11%) of the patients (Fig. 1). Eight patients (44%) received a tumor resection after a mean of 10 cycles (range 6–36). A complete pathological response (pCR; ypT0N0) was found in 5 patients (63%), one patient (13%) had a complete pathological response of the primary tumor but had one tumor deposit (ypT0N1c), and two patients (25%) had a major pathological response (defined as ≤ 10% residual tumor cells) and were classified as ypT3N0 and ypT4N0 respectively. In total, five of the 17 patients (29%) with clinical benefit (response or stable disease) showed disease progression after a median of 14.5 months, of which three during treatment with Pembrolizumab and one after the resection. Three of these patients received additional treatment after progression. One patient was treated with radiotherapy on the primary tumor, one patient restarted nivolumab after progressive disease after Pembrolizumab with again a radiological response and one patient started chemotherapy consisting of FOLFOXIRI-B with also again a radiological response. The PFS of patients who underwent a resection was 100% at one year and 80% at two years. PFS in patients who did not undergo a primary tumor resection was 67% and 36% at one and two years, respectively. The overall survival (OS) in patients who underwent a resection was 100% at two years. The OS in patients who did not undergo a resection was 80% and 42% at one and two years, respectively. The cancer specific survival (CSS) in patients who did not undergo resection was 89% and 71% at one and two years, respectively (Fig. 2).
Treatment toxicity
Immune-related adverse events of any grade were observed in six patients (33%). Four (22%) patients experienced toxicity and had to discontinue treatment early (after a maximum of 5 cycli). All four patients experienced hepatoxicity (100%) of which three experienced CTCAE grade 3 toxicity and one grade 4, two dermatitis (50%) both grade 2, two nephritis (50%) of which one grade 3 and one grade 4, and one encephalitis and myocarditis (25%) grade 4. All grade 4 toxicities were present in the same patient after two cycles of Pembrolizumab. This patient eventually experienced myasthenia gravis (grade 5). Despite treatment with prednisone, CellCept and Intravenous immunoglobulin (IVIG) the patient died after 10 days as a results of Pembrolizumab toxicity.
In total, ten patients received ataluren during treatment in the context of the ATAPMEBRO study. Of those ten patients, six patients (60%) observed Ataluren related adverse events. Four observed diarrhea (three CTCAE grade 2 and one grade 3) and three patients observed nausea (CTCAE grade 2).
Discussion
Immunotherapy plays a crucial role in treatment of patients with dMMR/MSI-high metastatic CRC, with first line Pembrolizumab as standard of care in metastatic disease [16]. Additionally very high response rates were shown with a short course of dual therapy with checkpoint inhibition in localized disease [10,11,12].
In this case series, we also showed high response rates of 78% after at least one cycle of Pembrolizumab in locally advanced unresectable dMMR intestinal cancers (SIAs and CRC), with conversion to resection in nearly 40% of the patients. However, also non-responders (11%) were observed and disease progression occurred in two patients during a median follow-up period of 14 months. Similar to the study by Morton et al., which investigated a neoadjuvant chemotherapy strategy in resectable locally advanced colon cancers, our study highlights the importance of systemic therapy in locally advanced tumors, with the potential of immunotherapy [13]. Pathological complete response (pCR) rates observed in immunotherapy studies in locally advanced dMMR CRC, such as the PICC trial including 34 patients with clinical T3 or T4 disease and randomized between neo-adjuvant toripalimab (anti-PD1) with or without celecoxib for a duration of 6 months [17], are higher than those reported in the study by Morton et al. with chemotherapy, the differences in treatment modality and tumor characteristics (resectable versus unresectable) underscore the potential complementary role of immunotherapy in this specific subgroup of patients with locally advanced dMMR intestinal cancer [17].
Our data are in line with two previous reports on immunotherapy in this setting. A phase II single-center trial on Pembrolizumab in localized unresectable or high-risk resectable dMMR gastro-intestinal cancers (27 out of 35 were CRC patients) found an ORR of 82% and a pCR rate of 65% after 6 months of treatment [18]. In a retrospective analysis of 73 patients with locally advanced disease, including T4a (26.0%) and T4b (39.7%) tumors, a radiological ORR of 84.9% and a pCR rate of 57.1% was seen after treatment with various PD-1 inhibitors. However, 21.5% of these patients also received other treatment modalities, such as radiotherapy, chemotherapy and targeted agents [19].
A phase II single-center study on 12 patients with stage II/III dMMR rectal cancer treated with dostarlimab reported a 100% complete radiological response after 6 months treatment, allowing for a watch-and-wait strategy [20]. Additionally, results with the outcomes of studies consisting of locally advanced tumors in the Niche trials, show similar high response rates [10,11,12].
Although the response rates observed in our study in patients with locally advanced dMMR intestinal cancers are encouraging, they do not reach the high response levels reported in studies involving resectable cases [10]. This discrepancy gives rise to a number of questions regarding the optimal management of locally advanced unresectable dMMR intestinal cancers. One potential explanation for the observed discrepancy in response rates is the level of resistance to immunotherapywhich seems higher in advanced cancer stages. as known from patients with liver metastases effectiveness of immunotherapy can be reduced due to macrophage-mediated, intratumoral T-cell elimination [21,22,23]. Furthermore, preclinical research suggests that liver metastases may impede the efficacy of immunotherapy by redirecting activated CD8 + T-cells away from the systemic circulation. Resistance to immune checkpoint inhibitors (ICIs) has been observed in patients with liver metastases across various cancer types, such as melanoma, kidney and urothelial carcinomas, and non-small cell lung cancer [21]. While these findings have not yet been investigated in patients with locally advanced disease, they may offer insights into the lower efficacy of immune therapy in locally advanced cancers compared to earlier stages. Further research is needed to investigate whether T-cell elimination is driving these variations in therapeutic outcomes or other, yet unknown, mechanisms are underlying these lower responses.
Nevertheless, this study calls for a reassessment of the current treatment protocols in locally advanced intestinal cancer. In particular, there is a need to investigate whether dual checkpoint inhibition might result in superior response rates, thereby increasing the likelihood of achieving response and thereby resectability. Previous studies have indicated that combining checkpoint inhibitors can enhance anti-tumour immune responses, and this approach merits further investigation in the context of locally advanced unresectable disease, recognizing the possibility of elevated toxicity.
An additional factor to be taken into account is the treatment duration. In resectable CRC short course neoadjuvant dual immunotherapy was highly effective [10,11,12]. In contrast, patients with locally advanced unresectable disease may require longer treatment durations to achieve comparable pathological response. However, while prolonging the duration of therapy could potentially enhance the primary response to immunotherapy, it concomitantly elevates the probability of treatment-related toxicities. In our study, 22% of patients discontinued Pembrolizumab due to severe adverse events, including hepatotoxicity, dermatitis, nephritis, encephalitis, and myocarditis. One patient ultimately deceased due to treatment-related toxicity. Nonetheless, these patients experienced toxicity in an early stage of the treatment, after a maximum of 5 cycles, and the grade 5 toxicity was observed after only two cylces of pembrolizumab.In the Keynote 177 study, a study analysing patients with advanced CRC receiving Pembrolizumab for a median period of 11.1 months, a lower rate percentage of patients discontinued the study treatment (14%) [16]. The difference in outcomes could be due to the retrospective nature of the study, the small sample size, the mix of patients with CRC and SIAs, or could be an effect of the addition of ataluren in x% of the cases. Either way, these findings underscore the necessity for a delicate balance between maximizing therapeutic efficacy and minimizing adverse effects, emphasizing the need for personalized treatment strategies that account for individual patient tolerability and risk profiles.
The principal strength of our study is the provision of real-world evidence to support the use of Pembrolizumab in a specific and challenging subset of patients with locally advanced dMMR CRC or SIA. The multicentre nature of the study enhances the generalisability of the findings, as the data were collected from a variety of institutions. Moreover, the comprehensive collection and analysis of clinicopathological data provide valuable insights into patient outcomes. Despite the study’s limited sample size, its findings align with existing literature, underscoring its potential to contribute valuable insights to the field of treatment with checkpoint inhibitor.Pembrolizumab in treatment of dMMR SIA and CRC
Nevertheless, it is important to acknowledge the limitations of our study. The retrospective design of the study restricts the ability to establish causality and introduces the potential for selection bias. Furthermore, some patients received Ataluren in combination with Pembrolizumab as part of a clinical trial, which may introduce difficulties in the interpretation of Pembrolizumab’s efficacy and safety profile as monotherapy. The relatively modest sample size further constrains the statistical power of our analyses and the robustness of our conclusions. It is recommended that future prospective studies with larger cohorts will be conducted in order to validate the findings of this study and to refine treatment protocols. Currently, the role of Pembrolizumab is further investigated in primary resectable (NCT05197322) [24] and unresectable non-metastatic disease (NCT05131919).
Despite the study limitations it remains highly important to report these outcomes to evaluate the role of immune checkpoint inhibitors for locally advanced dMMR intestinal cancers, as it is still not generally accessible for this indication.
Conclusion
Our study supports the use of single-agent Pembrolizumab as an effective induction therapy for patients with locally advanced unresectable dMMR intestinal cancers, offering a viable alternative to traditional chemotherapy. Although the observed response rates are high and better than generally observed with chemotherapy, there is still room for improvement. It is essential to weigh the potential risks of increased toxicity with increase efficiency, especially when considering dual therapy.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Diaz LA Jr., Le DT. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl J Med. 2015;373:1979.
Germano G, Lamba S, Rospo G, Barault L, Magrì A, Maione F, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552:116–20.
Mandal R, Samstein RM, Lee KW, Havel JJ, Wang H, Krishna C, et al. Genetic diversity of tumors with mismatch repair deficiency influences anti-PD-1 immunotherapy response. Science. 2019;364:485–91.
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–91.
Overman MJ, Yothers G, Jacobs SA, Sanoff HK, Cohen DJ, Guthrie KA, et al. Colorectal Cancer Metastatic dMMR Immuno-Therapy (COMMIT) Study: A randomized phase III study of atezolizumab (atezo) monotherapy versus mFOLFOX6/bevacizumab/atezo in the first-line treatment of patients (pts) with deficient DNA mismatch repair (dMMR) or microsatellite instability high (MSI-H) metastatic colorectal cancer (mCRC)—NRG-GI004/SWOG-S1610. J Clin Oncol. 2021;39:TPS3618–TPS.
André T, Pietrantonio F, Avallone A, Gumus M, Wyrwicz L, Kim JG, et al. KEYSTEP-008: phase II trial of pembrolizumab-based combination in MSI-H/dMMR metastatic colorectal cancer. Future Oncol. 2023;19:2445–52.
Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol. 2022;40:161–70.
Andre T, Elez E, Van Cutsem E, Jensen LH, Bennouna J, Mendez G, et al. Nivolumab plus Ipilimumab in Microsatellite-Instability-High Metastatic Colorectal Cancer. N. Engl J Med. 2024;391:2014–26.
Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 2020;26:566–76.
Chalabi M, Verschoor YL, Tan PB, Balduzzi S, Van Lent AU, Grootscholten C, et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair-Deficient Colon Cancer. N. Engl J Med. 2024;390:1949–58.
de Gooyer PGM, Verschoor YL, van den Dungen LDW, Balduzzi S, Marsman HA, Geukes Foppen MH, et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: a phase 2 trial. Nat Med. 2024;30:3284–90.
Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J Clin Oncol. 2023;41:1541–52.
Cercek A, Dos Santos Fernandes G, Roxburgh CS, Ganesh K, Ng S, Sanchez-Vega F, et al. Mismatch Repair-Deficient Rectal Cancer and Resistance to Neoadjuvant Chemotherapy. Clin Cancer Res. 2020;26:3271–9.
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81.
André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl J Med. 2020;383:2207–18.
Hu H, Kang L, Zhang J, Wu Z, Wang H, Huang M, et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:38–48.
Ludford K, Ho WJ, Thomas JV, Raghav KPS, Murphy MB, Fleming ND, et al. Neoadjuvant Pembrolizumab in Localized Microsatellite Instability High/Deficient Mismatch Repair Solid Tumors. J Clin Oncol. 2023;41:2181–90.
Xiao BY, Zhang X, Cao TY, Li DD, Jiang W, Kong LH, et al. Neoadjuvant Immunotherapy Leads to Major Response and Low Recurrence in Localized Mismatch Repair-Deficient Colorectal Cancer. J Natl Compr Canc Netw. 2023;21:60–6.e5.
Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl J Med. 2022;386:2363–76.
Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med. 2021;27:152–64.
Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10:292–302.
Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5:eaba0759.
Shiu K-K, Jiang Y, Saunders M, Seligmann JF, Iveson T, Wilson RH, et al. NEOPRISM-CRC: Neoadjuvant pembrolizumab stratified to tumour mutation burden for high risk stage 2 or stage 3 deficient-MMR/MSI-high colorectal cancer. J Clin Oncol. 2024;42:LBA3504–LBA.
Funding
The author(s) received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Design of study: OF, IS, RG, AB, TB; Acquired data: OF, IS, RG, GC, FJ, GC, AB, TB; Data analyses: OF, IS, TB; Writing: OF, IS, RG, AB, TB; Reviewing manuscript: OF, IS, RG, GC, FJ, JC, JT, AB, TB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All patients signed the informed consent form, either through the ATAPEMBRO trial (NCT04014530) or under the approval of the institutional review board (IRB) of the Amsterdam UMC (IRB00013752). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Figaroa, O.J.A., Spaanderman, I.T., Goedegebuure, R.S.A. et al. Treatment with checkpoint inhibitors for unresectable non-metastatic mismatch repair deficient intestinal cancer; a case series. BJC Rep 3, 67 (2025). https://doi.org/10.1038/s44276-025-00171-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44276-025-00171-0