Abstract
Urbanization is increasingly shifting the ranges of vector-borne diseases, including in temperate regions. In China, scrub typhus (ST), a bacterial vector-borne disease, was endemic exclusively in southern, tropical and extratropical provinces, but has surged northward in recent years. Here we use a comparative framework and focus on Jiangsu Province to investigate how urbanization modulates ST risks in temperate urban settings. Since 2014, infections have surged and expanded spatially. Additionally, ST is disproportionately afflicting populations along the urban–rural gradient, yet greater urbanization and human impacts are associated with an increased urban fraction of cases. Conversely, aging populations are associated with a lower proportion of urban cases. Our findings collectively underscore that ST expansion can be rapid in temperate regions under urbanization, although population aging may partially counteract this effect. Informed surveillance and management of disease expansion in urban settings is crucial for informing public health strategies.
Similar content being viewed by others
Main
The accelerating process of urbanization and climate change presents many challenges, including risks of vector-borne disease (VBD) dynamics and their increasing threat to human populations1,2,3,4,5,6. An essential piece of building resilience to VBDs in the changing environment is to understand how sociodemographic factors synergistically modulate disease risk across empirical settings. This motivates us to integrate multifaceted datasets and make a detailed assessment of the impacts of urbanization on VBD epidemiology and its ultimate threat to human health.
Recent studies are increasingly highlighting the expanding spatial range of many VBDs, particularly in urbanized and warmer areas, amplifying the risk for human populations7,8,9,10,11,12,13. Despite its global threat, the epidemiology of locally transmitted VBDs on finer scales has not been rigorously analyzed. This is largely because local signals of disease emergence and transmission are context specific. This means that local transmissions are often mediated through complex processes that are not amenable to those on larger (for example, global and regional) scales10,11,12. Therefore, the implications of large-scale evidence for disease mitigation in local settings remain limited. The lack of progress makes it difficult for effective disease surveillance and targeted risk mitigation to operate locally in urban areas, leaving urban populations vulnerable to outbreaks.
Note that the determinants of urban VBDs have been mostly studied in tropical regions but neglected in temperate urban populations13,14. It is widely recognized that human infections of the VBDs endemic in temperate climates have substantially surged in most recent years10, such as scrub typhus (ST). Originating as a chigger-borne zoonosis caused by Orientia tsutsugamushi15, human infections of ST are characterized by non-specific febrile symptoms, typically with fever, headache, rash and lymphadenopathy16,17. The disease has been endemic in southern China but subsequently expanded to other provinces over past decades18,19,20,21. Although rural areas typically present a higher risk of infection due to proximity to habitats of rodents and mites that facilitate the infection, one of the changing epidemiology of ST is the increased risk of infection in urban settings22,23. In view of China’s ongoing urbanization and continuing epidemic threats, there is a persistent need to understand how VBDs disproportionately threaten populations across the urban–rural gradient.
Addressing these issues requires a nuanced examination to distinguish the influence of socioecological modifications and interventions influencing VBD transmission risks. In the absence of interventions24,25, ST presents a unique opportunity to assess VBD epidemiology under the interplay of urbanization and climate change. Using ST as a case study, we ground our study in Eastern China where counties are in diverse sociodemographic and climatic settings, as well as a variety of public health and environmental problems26,27,28.
We aim to explicitly investigate the impact of urbanization and climate condition on the fraction and occurrence of ST in urban settings. To do this, we incorporate data on sociodemographic status, human pressures and meteorological conditions to elucidate the factors that may be relevant to VBD transmissions on finer scales. We begin by characterizing the spatiotemporal diffusion of ST epidemiological dynamics in 2006–2023. Subsequently, we establish statistical models to investigate the determinants of ST expansion in urban areas. Our findings highlight the important role of urbanization and climate change in shaping VBD dynamics, offering a critical evidence base for disease surveillance and mitigation strategies.
Results
Study population
We retrieved a total of 16,775 records of ST human infections during 2006–2023 in Jiangsu Province, China. The overall age profile of the infections shows that disease occurs in all age groups; yet, people aged 60–69 years were associated with a larger number of infections (Fig. 1a). No significant difference was observed in the age profile between sexes (P = 0.755). We subsequently analyzed the median age of the documented patients, indicating an increase from 53 years in 2006 to 66 years in 2023 (Fig. 1b). Moreover, we observed a consistent increase in the median age of patients across different urbanicity levels of residence over the study period (Fig. 1c).
Spatiotemporal diffusion of human infections
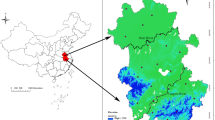
We observed distinct spatiotemporal heterogeneities of locally infected ST cases (Fig. 2a and Supplementary Fig. 1). Across the years, we showed that the ST incidence rates were higher in four prefectural-level cities (Yancheng, Taizhou, Suqian and Nantong; Fig. 2b). Additionally, ST dynamics exhibited strong seasonality, with an epidemic peak of cases typically occurring between October and November (Supplementary Fig. 2). In particular, there has been a significant surge of human infections since 2014, accounting for approximately 80% of the documented infections in 2006–2023. Importantly, this surge coincides with the spatial expansion of ST infections, with a growing number of infected counties (Fig. 2c).
a, Spatial heterogeneity of ST dynamics is presented by the county-stratified average incidence rates over the years. b, Overall epidemic profile is illustrated by the annual incidence rates by city. c, Spatial diffusion is characterized by the annual number of affected counties. The color of the circle represents the annual incidence rates in Jiangsu Province. Basemap[s] in panel a adapted from Tianditu (https://www.tianditu.gov.cn).
Disparities of epidemiological dynamics across urbanicity
Stratifying the counties by their urbanicity revealed disparities of ST epidemiology along the urban–rural gradient (Fig. 3a). The relative fraction of rural cases gradually attenuated and fell below the average fraction for all years since 2019; however, the fraction of the suburban and urban cases typically increases since 2019 and 2022, respectively, with urban cases dominating in 2022 and 2023. Of note, disparities were also seen in the duration between symptom onset and illness diagnosis (Fig. 3b). It was estimated that the overall onset-to-diagnosis duration over the study period is 5 days (interquartile range, 2–8 days). Compared with this, our estimates showed a longer duration for people living in the urban area, with a median (interquartile range) of 7 days (3–12 days) but 5 days (2–8 days) and 4 days (1–7 days) among suburban and rural patients, respectively.
Given the disparities in ST epidemiology along urbanicity, we sought to examine the determinates of ST expansion to urban areas. We identified a nonlinear but generally increasing association between the level of urbanization, measured by the proportion of residents living in urban areas, and the fraction of urban cases at the county level (F = 8.875, P < 0.05; Fig. 4a). Consistent with this, we found an approximately linear association between the human footprint index and the fraction of urban cases (F = 5.634, P < 0.05; Fig. 4b). These associations indicate that the overall higher urbanization and human impact results in a higher risk of ST human infections spreading into urban areas. Further assessment demonstrates disparities in the fraction of urban cases across counties with different levels of population aging. Compared with the aging counties in which people over 65 years account for 7%–14% of the population, our estimates show that the aged counties have around 65% lower odds of urban cases. The odds of urban cases decreases to around 23% for the super-aged counties (Table 1). These findings indicate that population aging may counteract the spread of ST into urban areas.
a,b, Partial effects from urbanization and human impact on the fraction of ST urban cases are quantified using a generalized additive model. The curves represent the model-predicted partial effects, and the shaded bands represent the 95% confidence intervals around the estimated effect. c, Estimates of urbanization and human impact on the occurrence of ST urban cases are presented. The points represent the estimated odds ratios, with the error bars indicating the 95% confidence intervals. A total of 24 counties over 10 years were included. All the statistical tests were two sided. Significant associations (P < 0.05) are marked with *.
Additionally, we observed similar significant associations between urbanization and odds of ST occurrence in urban areas (Fig. 4c). Per unit increase in urbanization was associated with 6.2% higher odds of ST occurrence. Similarly, per unit increase in the intensity of human pressure on the eco-environment brought up the odds of ST occurrence by 22.3% (Fig. 4c). Disaggregation of the levels of aging society indicated heterogeneity in the odds of occurrence within the population (Table 1). The overall higher population aging level results in lower odds of disease occurrence, with estimates suggesting the odds would decrease by 90.8% (62.1%–98.0%) and 82.1% (10.5%–96.8%) for the aged and super-aged counties, respectively, compared with that in the aging counties.
Discussion
Our study provides the essential evidence regarding the evolving epidemiology of the many VBDs in Eastern China in most recent years22,29,30. By comprehensively analyzing the newest and most complete records of ST human infections and sociodemographic covariates, we have contextualized the spatiotemporal expansion of ST in rapid urbanization over the past decades. Our findings typically shed light on the signature of urbanization on VBD dynamics in temperate urban areas, highlighting that ST may have disproportionately affected people residing in different urbanicity levels.
The finding of the substantial increase in human infections and the affected counties broadly aligns with studies done for other VBDs31,32,33. Therefore, our evidence emphasizes the need to investigate the potential changes in VBD dynamics across broader settings. Importantly, although ST cases have been mostly recorded in the rural area22, we illustrated the persistent increase in urban and suburban infections. Key questions arise that the process of ST expansion may be more complex than previously acknowledged. Note that urban areas often have specific vulnerabilities to infectious diseases. This largely explains the fact that the role of urbanization on ST and other VBD dynamics is highly diverse. Primarily, the process of urbanization promotes the exposure opportunity and interactions at the human–vector interface, which contribute to the increase in disease risk34,35,36,37. Additionally, the urban environment has proven to be favorable for the breeding of vectors. Although temperature and precipitation are associated with vector biology and VBD risk, unique microclimates (such as urban heat islands in temperate climates) can facilitate vector development, survival and feeding behavior. Invasion by these adapted vectors from rural settings may accelerate the spread of pathogens and disease, putting urban populations at increased risk3,38,39.
Population aging and human pressure on the eco-environment emerge as the primary concerns in the context of urbanization. The age dependency of ST epidemiology indicates that people aged 60–69 years are at a higher risk of infection compared with those in other age groups, aligning with previous nationwide research22. This increased risk in the age group could be attributed to the fact that people in this age group may still be actively doing agricultural work and, therefore, are at a higher risk of infection22. That is, these people have a higher opportunity of exposure to agriculatural areas, which are also the primary the habitats of vector mites24,25,40. Additionally, their immune systems may be weaker compared with younger people, making them more susceptible to infections. By disaggregating counties into varying levels of population aging, we show a higher fraction of urban cases in aging counties compared with aged and super-aged counties. This finding suggests that population aging may slow down the spread of cases into urban areas. One potential explanation for the slowed spread of cases into urban areas in aged and super-aged counties compared with aging counties could be the age structure of these regions. Aged and super-aged counties typically have a larger proportion of individuals aged 80 years and beyond, known as the oldest old41. This group is less likely to participate in farm work or other outdoor activities, which may reduce their exposure to infectious agents. By contrast, human pressure on the eco-environment aligns with that of urbanization. This result is not unexpected because intensified urbanization may bring about higher human pressure and collectively elevate the expansion and occurrence of ST.
Understanding the effect of urbanization provides a critical evidence base for the surveillance and mitigation of VBDs. It is noted that much attention has also been given to the importance of rural areas and farmers16,40. Our findings shift the focus of VBD surveillance to urban areas, emphasizing the urgency of targeting the urban environment for a quick response to public health threats. Accordingly, the disproportionate burden among urban and rural populations suggests the need for context-specific disease mitigation approaches. It is also worth highlighting the public health guidance of our study on the surveillance and intervention for VBDs, which has largely been absent in temperate regions until now42,43. The emphasis should be laid particularly on enhancing public knowledge to ST and other VBDs. We identified disparities in the interval between symptom onset and diagnosis among residents in different urbanicities. The urban population associated with a longer interval may experience delayed treatments and subsequent severe complications. This delay is largely attributable to the limited knowledge of ST among the urban population. We argue that campaigns will be necessary to improve the public knowledge of ST symptoms, promoting care-seeking and treatment.
In conclusion, our findings provide crucial evidence about ST dynamics and determinates in temperate regions. ST expansion can be rapid and result in an increasing number of urban cases. The expansion is driven by both climatic conditions and urbanization in nature, making it difficult to predict future disease dynamics. The findings collectively underscore the significance of surveillance and interventions in the highly populated urban area, informing public health strategies to mitigate disease expansion in urban settings.
Methods
ST cases
ST is a nationally notifiable disease with standardized case definitions. A confirmed case is an infection confirmed through laboratory testing in accordance with the national diagnosis criteria and case classification guidelines44. Case-level records of confirmed ST human infections in Jiangsu Province from 2006 to 2023 were obtained from the Nationwide Notifiable Infectious Diseases Reporting Information System. The associated information documented for each case was also collected, including residential address, age, sex, occupation, date of illness onset and diagnosis. Restricting to records after 2014, we translated the residential address to urbanicity based on the official urban–rural code table (https://tj.jiangsu.gov.cn/), where residents are assigned to the urban, suburban or rural area.
Further, case-level records were aggregated annually and by city to characterize the overall epidemic profiles of ST and to identify endemic cities. These records were subsequently aggregated annually by county to examine disease expansion (see the “Epidemiological analyses” section). County-level administrative regions are below the prefecture-level cities, mainly including municipal districts, county-level cities and counties. For this finer-scale investigation, we stratified the fraction of cases in counties of various urbanicities. The fraction of urban cases was defined as the number of infections recorded in urban areas out of all the cases recorded. Consistent with this, the fraction of suburban and rural cases was designated by the number of cases in suburban and rural areas among all the cases, respectively. Additionally, the occurrence of urban cases was defined as the presence of infections in urban areas per county per year. These county-level fraction and occurrence of urban cases were used for subsequent epidemiological and statistical analyses (see the “Epidemiological analyses” and “Statistical model” sections).
Sociodemographic data
Sociodemographic factors combined information about the urbanization rate and the population aging level. Out of these factors, the urbanization rate was defined as the fraction of residents in urban areas. In line with WHO guidance45, we categorized counties into varying levels of population aging: aging (share of senior people is 7%–14%), aged (15%–20%) and super-aged (21% or higher) counties. Both urbanization rate and fraction of senior people (age over 65 years) were collected from the Jiangsu Provincial Bureau of Statistics (https://tj.jiangsu.gov.cn/). All missing data were imputed using linear interpolation.
Covariates
As urbanization and climate change continue to accelerate, human pressures imposed on the eco-environment are intensified. Given this, we incorporated human footprint as a covariate of our analyses. The human footprint incorporates eight pressure variables that reflect diverse aspects of human impact, including built environments, population density, nighttime light, croplands, pasture lands, roadways, railways and navigable waterways. The annual human footprint data from 2014 to 2020 were obtained from an updated global record of annual terrestrial dataset46. With the time-series datasets, we estimated the human footprint for the years 2021–2023 in each county using linear interpolation.
Precipitation and temperature were the most commonly used meteorological factors incorporated into the model inference of VBD transmission47. Therefore, we acquired daily climate data including the 2-m air temperature and total precipitation from the fifth-generation reanalysis from the European Center for Medium-Range Weather Forecasts48. Time-series data streams were extracted by identifying the nearest grid point to the center of each local authority. The data were then aggregated to derive the annual mean temperature and accumulated number of precipitation days for each county.
Epidemiological analyses
We conducted comprehensive analyses of the epidemiological dynamics of ST human infections from 2006 to 2023. Initially, we examined the overall epidemic profiles of ST dynamics, focusing on identifying the endemic prefecture-level cities with typically higher incidence rates. We subsequently conducted finer-scale analyses by assessing the spatial expansion of the disease across counties. This involved examining the geographic spread of infections and identifying areas experiencing increasing incidence rates. We made further assessment of the disparities of epidemiological dynamics, taking into account variations in the fraction of human infections and the onset-to-diagnosis interval along the urban–rural gradient (that is, urban, suburban and rural areas). To facilitate comparison across regions and years, the annual fraction of infections was then rescaled as relative fractions compared with the overall fraction using \({{\rm{RF}}}_{{{c}}}=\frac{{F}_{{{c}}}}{\bar{{F}_{{{c}}}}}\), where Fc represents the fraction of rural, suburban or urban cases in county c, and \(\bar{{F}_{c}}\) is the average fraction of cases in the corresponding urbanicity over years from 2014 to 2023. This rescaling ensures that the relative fraction of urbanicity-stratified infections fluctuates around a mean of 1.
Statistical model
We used statistical models to investigate the association between ST dynamics in urban areas and various sociodemographic factor, human pressures and meteorological conditions of the counties. Specifically, the fraction and occurrence of urban cases were explicitly considered as the two key epidemiological indicators of disease dynamics for the regression analyses. We started by formulating a generalized additive model to examine the modification of multifaceted factors on the fraction of urban case. The model is described by the following equation:
where Yi, j represents the fraction of urban cases in year i and county j. The parameter αi, j denotes the overall intercept. b(Ui, j), c(Hi, j), d(Ti, j) and e(Pi, j) are smooth functions for the annual urbanization rate, human pressure indicator, mean temperature and number of precipitation days, respectively. Population aging levels are included as a categorical variable, Ai, j.
Following this, we further formulated a multivariate logistic regression to assess the impact of multifaceted factors on the odds of ST occurrence, that is,
where Pi, j is the probability of ST occurrence in year i and county j. β0 is the overall intercept, and βn (n = 1…5) are the coefficients for the multifaceted factors. The definition of the factors are the same as those in equation (1). Throughout our analyses, P values of less than 0.05 were considered to be statistically significant.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The minimum dataset analyzed in this study includes anonymized surveillance records and epidemiological case data archived within the surveillance system of the Jiangsu Provincial Center for Disease Control and Prevention. Due to legal and privacy restrictions associated with the use of personally identifiable health data, these datasets are not publicly available. The datasets used and/or analyzed in this study are available from the corresponding author on reasonable request.
Change history
14 July 2025
In the version of this article initially published, Zhe Lou and Jinjing Wu were not described as co-first authors. The Author Contributions section has been updated accordingly in the HTML and PDF versions of the article.
References
Rocklöv, J. & Dubrow, R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 21, 479–483 (2020).
Neiderud, C.-J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 5, 27060 (2015).
World Health Organization. Global Framework for the Response to Malaria in Urban Areas (2022); https://www.who.int/publications/i/item/9789240061781
Connolly, C., Keil, R. & Ali, S. H. Extended urbanisation and the spatialities of infectious disease: demographic change, infrastructure and governance. Urban. Stud. 58, 004209802091087 (2020).
Messina, J. P. et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 4, 1508–1515 (2019).
Ryan, S. J. et al. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Global Change Biol. 27, 84–93 (2020).
Ryan, S. J., Carlson, C. J., Mordecai, E. A. & Johnson, L. R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 13, e0007213 (2019).
Colón-González, F. J. et al. Projecting the risk of mosquito-borne diseases in a warmer and more populated world: a multi-model, multi-scenario intercomparison modelling study. Lancet Planet. Health 5, 404–414 (2021).
Paz, S. Climate change: a driver of increasing vector-borne disease transmission in non-endemic areas. PLoS Med. 21, e1004382 (2024).
Robert, M. A., Stewart-Ibarra, A. M. & Estallo, E. L. Climate change and viral emergence: evidence from Aedes-borne arboviruses. Curr. Opin. Virol. 40, 41–47 (2020).
Gibb, R. et al. Interactions between climate change, urban infrastructure and mobility are driving dengue emergence in Vietnam. Nat. Commun. 14, 8179 (2023).
Pfenning-Butterworth, A. et al. Interconnecting global threats: climate change, biodiversity loss, and infectious diseases. Lancet Planet. Health 8, e270–e283 (2024).
Bogoch, I. I. et al. Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: a modelling study. Lancet Infect. Dis. 16, 1237–1245 (2016).
Kraemer, M. U. G. et al. Spread of yellow fever virus outbreak in Angola and the Democratic Republic of the Congo 2015–16: a modelling study. Lancet Infect. Dis. 17, 330–338 (2017).
Bonell, A., Lubell, Y., Newton, P. N., Crump, J. A. & Paris, D. H. Estimating the burden of scrub typhus: a systematic review. PLoS Negl. Trop. Dis. 11, e0005838 (2017).
Zangpo, T. et al. Environmental, occupational, and demographic risk factors for clinical scrub typhus, Bhutan. Emerg. Infect. Dis. 29, 909–918 (2023).
Lewis, M. D. et al. Scrub typhus reemergence in the Maldives. Emerg. Infect. Dis. 9, 1638–1641 (2003).
Wu, Y. C. et al. Spatiotemporal dynamics of scrub typhus transmission in Mainland China, 2006-2014. PLoS Negl. Trop. Dis. 10, e0004875 (2016).
Zhang, S. et al. Scrub typhus in previously unrecognized areas of endemicity in China. J. Clin. Microbiol. 48, 1241–1244 (2010).
Liu, Y. et al. Clinical characteristics of the autumn-winter type scrub typhus cases in south of Shandong Province, Northern China. BMC Infect. Dis. 9, 82 (2009).
Hu, J. et al. Clinical characteristics and risk factors of an outbreak with scrub typhus in previously unrecognized areas, Jiangsu Province, China 2013. PLoS ONE 10, e0125999 (2015).
Li, Z. et al. Epidemiologic changes of scrub typhus in China, 1952–2016. Emerg. Infect. Dis. 26, 1091–1101 (2020).
Park, S. W. et al. Urbanization of scrub typhus disease in South Korea. PLoS Negl. Trop. Dis. 9, e0003814 (2015).
Elliott, I. et al. Scrub typhus ecology: a systematic review of Orientia in vectors and hosts. Parasit. Vectors 12, 513 (2019).
Xu, G., Walker, D. H., Jupiter, D., Melby, P. C. & Arcari, C. M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 11, e0006062 (2017).
Ren, Z., Zhao, H., Shi, K. & Yang, G. Spatial and temporal variations of the precipitation structure in Jiangsu Province from 1960 to 2020 and its potential climate-driving factors. Water 15, 4032 (2023).
Mi, L. et al. Evaluating the effectiveness of regional ecological civilization policy: evidence from Jiangsu Province, China. Int. J. Environ. Res. Public Health 19, 388 (2022).
Zhang, S. et al. Indicators for environment health risk assessment in the Jiangsu Province of China. Int. J. Environ. Res. Public Health 12, 11012–11024 (2015).
Ren, J. et al. The epidemiology of Aedes-borne arboviral diseases in Zhejiang, Southeast China: a 20 years population-based surveillance study. Front. Public Health 11, 1270781 (2023).
Wang, T. et al. Mapping the distributions of mosquitoes and mosquito-borne arboviruses in China. Viruses 14, 691 (2022).
Sun, J.-M. et al. Factors associated with spatial distribution of severe fever with thrombocytopenia syndrome. Sci. Total Environ. 750, 141522 (2021).
Miao, D. et al. Mapping the global potential transmission hotspots for severe fever with thrombocytopenia syndrome by machine learning methods. Emerg. Microbes Infect. 9, 817–826 (2020).
Wu, W., Huang, X. & Li, J. in Prevention and Control of Infectious Diseases in BRI Countries Vol. 85 (Springer, 2021).
Vanwambeke, S. O., Linard, C. & Gilbert, M. Emerging challenges of infectious diseases as a feature of land systems. Curr. Opin. Env. Sust. 38, 31–36 (2019).
Kernbach, M. E. et al. Light pollution affects West Nile virus exposure risk across Florida. Proc. R. Soc. B 288, 20210253 (2021).
Hassell, J. M., Begon, M., Ward, M. J. & Fèvre, E. M. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol. Evol. 32, 55–67 (2017).
Kweon, S.-S. et al. A community-based case-control study of behavioral factors associated with scrub typhus during the autumn epidemic season in South Korea. Am. J. Trop. Med. Hyg. 80, 442–446 (2009).
LaDeau, S. L., Allan, B. F., Leisnham, P. T. & Levy, M. Z. The ecological foundations of transmission potential and vector‐borne disease in urban landscapes. Funct. Ecol. 29, 889–901 (2015).
Mordecai, E. A., Ryan, S. J., Caldwell, J. M., Shah, M. M. & LaBeaud, A. D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 4, e416–e423 (2020).
Tran, H. T. D. et al. Ecological and behavioural risk factors of scrub typhus in central Vietnam: a case-control study. Infect. Dis. Poverty 10, 110 (2021).
Zeng, Y., Vaupel, J. W., Xiao, Z., Zhang, C. & Liu, Y. Sociodemographic and health profiles of the oldest old in China. Popul. Dev. Rev. 28, 251–273 (2002).
Marchi, S., Trombetta, C. M. & Montomoli, E. in Public Health—Emerging and Re-emerging Issues (InTech, 2018).
Huang, Y.-J. S., Higgs, S. & Vanlandingham, D. L. Arbovirus-mosquito vector-host interactions and the impact on transmission and disease pathogenesis of arboviruses. Front. Microbiol. 10, 22 (2019).
National Guideline of Scrub Typhus Control and Prevention (2009); http://www.jygcdc.com/html/col15/content15_1070.html
World Health Organization. Health at a Glance: Asia/Pacific 2020: Measuring Progress Towards Universal Health Coverage (OECD, 2020).
Mu, H. et al. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 9, 176 (2022).
Gage, K. L., Burkot, T. R., Eisen, R. J. & Hayes, E. B. Climate and vectorborne diseases. Am. J. Prev. Med. 35, 436–450 (2008).
Hersbach, H. et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 146, 1999–2049 (2020).
Acknowledgements
This study was supported by grants from the Start Fund for Specially Appointed Professors of Jiangsu Province, seeds funding of Nanjing Medical University (NMUR20220001), Jiangsu Provincial Medical Key Discipline (no. ZDXK202250) and the Scientific Research Project of Jiangsu Provincial Health Commission (no. DX202302). The funders of this study had no role in the study design, data collection, data analysis, data interpretation or writing of the paper.
Author information
Authors and Affiliations
Contributions
Z. Lou and J.W. are co-first authors of this paper. This study was conceptualized and supervised by R.L. The data analyses were carried out by Z. Lou, J.W. and X.W. Z. Lou, J.W., X.W., Z.W., Z. Li and H.W. were involved in the data interpretation. Z. Lou and J.W. prepared the paper. All authors reviewed and approved the final version of the paper. R.L. and J.H. had full access to the data and codes used in this study. All authors have final responsibility for the decision to submit the paper for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cities thanks Nam-Hyuk Cho, Michael Tong and Xinyu Zhang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Table 1 and additional textual information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lou, Z., Wu, J., Wang, X. et al. Urbanization and scrub typhus expansion in temperate settings. Nat Cities 2, 732–738 (2025). https://doi.org/10.1038/s44284-025-00262-6
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s44284-025-00262-6