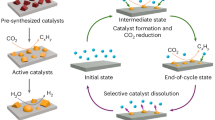
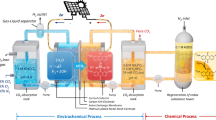
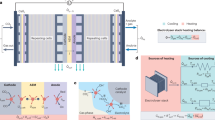
Abstract
Whereas chemical reactors can be run for years with limited maintenance, no reactor is inherently stable. Catalysts and components naturally degrade over time. If degradation is slow enough and understood, however, performance losses with time need not impede commercialization. For example, thermochemical reactions can be run at progressively increasing temperatures to compensate for degrading catalysts. In recent years, various electrochemical reactions have been investigated to support the renewable electrification of various sectors, with stability being a key necessity for future use. Unfortunately, in fields such as CO2 electrolysis, more efforts have been placed on achieving stability instead of characterizing degradation, which is a lost opportunity. This Perspective provides a critical reflection on stability—a flawed performance metric—and advocates for a switch in mindset toward characterizing pseudo-steady-state operation. A classification of transient versus pseudo-steady-state degradation mechanisms present in CO2 electrolysis is also provided, along with recommended characterization practices. Collectively, it is advocated that redefining stability is the best way to improve it.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Clark, E. L. et al. Standards and protocols for data acquisition and reporting for studies of the electrochemical reduction of carbon dioxide. ACS Catal. 8, 6560–6570 (2018).
Birdja, Y. Y. & Vaes, J. Towards a critical evaluation of electrocatalyst stability for CO2 electroreduction. ChemElectroChem 7, 4713–4717 (2020).
Ma, M. et al. Insights into the carbon balance for CO2 electroreduction on Cu using gas diffusion electrode reactor designs. Energy Environ. Sci. 13, 977–985 (2020).
Iglesias van Montfort, H.-P. et al. An advanced guide to assembly and operation of CO2 electrolyzers. ACS Energy Lett. 8, 4156–4161 (2023).
Dutta, N., Bagchi, D., Chawla, G. & Peter, S. C. A guideline to determine Faradaic efficiency in electrochemical CO2 reduction. ACS Energy Lett. 9, 323–328 (2024).
Schreiber, M. W. Industrial CO2 electroreduction to ethylene: main technical challenges. Curr. Opin. Electrochem. 44, 101438 (2024).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Li, M. et al. Local ionic transport enables selective PGM-free bipolar membrane electrode assembly. Nat. Commun. 15, 8222 (2024).
Lees, E. W., Bui, J. C., Romiluyi, O., Bell, A. T. & Weber, A. Z. Exploring CO2 reduction and crossover in membrane electrode assemblies. Nat. Chem. Eng. 1, 340–353 (2024).
Vavra, J. et al. Solution-based Cu+ transient species mediate the reconstruction of copper electrocatalysts for CO2 reduction. Nat. Catal. 7, 89–97 (2024).
Kok, J., de Ruiter, J., van der Stam, W. & Burdyny, T. Interrogation of oxidative pulsed methods for the stabilization of copper electrodes for CO2 electrolysis. J. Am. Chem. Soc. 146, 19509–19520 (2024).
Sahin, B. et al. Fine-tuned combination of cell and electrode designs unlocks month-long stable low temperature Cu-based CO2 electrolysis. J. CO2 Util. 82, 102766 (2024).
Han, J. et al. Structuring Cu membrane electrode for maximizing ethylene yield from CO2 electroreduction. Adv. Mater. 36, 2313926 (2024).
Deen, W. M. Analysis of Transport Phenomena 2nd edn (Oxford Univ. Press, 2013)
Pavel, C. C. et al. Highly efficient platinum group metal free based membrane-electrode assembly for anion exchange membrane water electrolysis. Angew. Chem. Int. Ed. 53, 1378–1381 (2014).
Ito, H. et al. Experimental investigation of electrolytic solution for anion exchange membrane water electrolysis. Int. J. Hydrogen Energy 43, 17030–17039 (2018).
Li, D. et al. Phenyl oxidation impacts the durability of alkaline membrane water electrolyzer. ACS Appl. Mater. Interfaces 11, 9696–9701 (2019).
Li, D. et al. Highly quaternized polystyrene ionomers for high performance anion exchange membrane water electrolysers. Nat. Energy 5, 378–385 (2020).
Lindquist, G. A. et al. Oxidative instability of ionomers in hydroxide-exchange-membrane water electrolyzers. Energy Environ. Sci. 16, 4373–4387 (2023).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Endrődi, B. et al. High carbonate ion conductance of a robust PiperION membrane allows industrial current density and conversion in a zero-gap carbon dioxide electrolyzer cell. Energy Environ. Sci. 13, 4098–4105 (2020).
Biemolt, J., Singh, J., Prats Vergel, G., Pelzer, H. M. & Burdyny, T. Preventing salt formation in zero-gap CO2 electrolyzers by quantifying cation accumulation. ACS Energy Lett. 10, 807–814 (2025).
Hao, S. et al. Improving the operational stability of electrochemical CO2 reduction reaction via salt precipitation understanding and management. Nat. Energy https://doi.org/10.1038/s41560-024-01695-4 (2025).
Joensen, B. Ó. et al. Unveiling transport mechanisms of cesium and water in operando zero-gap CO2 electrolyzers. Joule 8, 1754–1771 (2024).
Samu, A. A., Szenti, I., Kukovecz, Á., Endrődi, B. & Janáky, C. Systematic screening of gas diffusion layers for high performance CO2 electrolysis. Commun. Chem. 6, 41 (2023).
Forner-Cuenca, A. et al. Engineered water highways in fuel cells: radiation grafting of gas diffusion layers. Adv. Mater. 27, 6317–6322 (2015).
El-Nagar, G. A., Haun, F., Gupta, S., Stojkovikj, S. & Mayer, M. T. Unintended cation crossover influences CO2 reduction selectivity in Cu-based zero-gap electrolysers. Nat. Commun. 14, 2062 (2023).
Plank, C. et al. A review on the distribution of relaxation times analysis: a powerful tool for process identification of electrochemical systems. J. Power Sources 594, 233845 (2024).
Ranz, M., Grabner, B., Schweighofer, B., Wegleiter, H. & Trattner, A. Dynamics of anion exchange membrane electrolysis: unravelling loss mechanisms with electrochemical impedance spectroscopy, reference electrodes and distribution of relaxation times. J. Power Sources 605, 234455 (2024).
Bohn, L. et al. Reference electrode types for zero-gap CO2 electrolyzers: benefits and limitations. Adv. Sci. 11, 2402095 (2024).
Hansen, K. U., Cherniack, L. H. & Jiao, F. Voltage loss diagnosis in CO2 electrolyzers using five-electrode technique. ACS Energy Lett. 7, 4504–4511 (2022).
Xu, Q. et al. Identifying and alleviating the durability challenges in membrane-electrode-assembly devices for high-rate CO electrolysis. Nat. Catal. 6, 1042–1051 (2023).
Disch, J. et al. High-resolution neutron imaging of salt precipitation and water transport in zero-gap CO2 electrolysis. Nat. Commun. 13, 6099 (2022).
Yang, S. et al. Halide-guided active site exposure in bismuth electrocatalysts for selective CO2 conversion into formic acid. Nat. Catal. 6, 796–806 (2023).
Choi, W. et al. Exploring the influence of cell configurations on Cu catalyst reconstruction during CO2 electroreduction. Nat. Commun. 15, 8345 (2024).
Larrazábal, G. O. et al. Analysis of mass flows and membrane cross-over in CO2 reduction at high current densities in an MEA-type electrolyzer. ACS Appl. Mater. Interfaces 11, 41281–41288 (2019).
Vavra, J., Shen, T.-H., Stoian, D., Tileli, V. & Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 60, 1347–1354 (2021).
Malkow, T., Pilenga, A. & Tsotridis, G. EU Harmonised Test Procedure: Electrochemical Impedance Spectroscopy for Water Electrolysis Cells EUR 29267 EN (Publications Office of the European Union, 2018).
Lazanas, A. C. & Prodromidis, M. I. Electrochemical impedance spectroscopy—a tutorial. ACS Meas. Sci. Au 3, 162–193 (2023).
Birkner, L. et al. Dynamic accelerated stress test and coupled on-line analysis program to elucidate aging processes in proton exchange membrane fuel cells. Sci. Rep. 14, 3999 (2024).
Kuhnert, E., Hacker, V. & Bodner, M. A review of accelerated stress tests for enhancing MEA durability in PEM water electrolysis cells. Int. J. Energy Res. 2023, 3183108 (2023).
Acknowledgements
T.B. acknowledges the Dutch Research Council (NWO) for providing the FlexEChem Grant (NWA.1237.18.002) via the NWA-themed call ‘Opslag en conversie’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Justin Bui, Guoxiong Wang and Haotian Wang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burdyny, T. Using pseudo-steady-state operation to redefine stability in CO2 electrolysis. Nat Chem Eng 2, 350–357 (2025). https://doi.org/10.1038/s44286-025-00210-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-025-00210-0
This article is cited by
-
Overcoming copper stability challenges in CO2 electrolysis
Nature Reviews Materials (2025)